Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.24 no.1 Santa Marta Jan./Dec. 1995
SUCCESSIONAL PATTERNS ON FOULING PLATES IN THE BAY OF SANTA MARTA, COLOMBIAN CARIBBEAN
Camilo B. Garcia1 and Horst Salzwedel2
1Instituto de Investigaciones Marinas y Costeras José Benito Vives D'Andreis, INVEMAR, A.A. 1016, Santa Marta, Colombia (C. B. G.)
2Corporación Autónoma Regional del Magdalena y de la Sierra Nevada, CORPAMAG, Calle 23 # 4-27, Santa Marta, Colombia (H.S.)
ABSTRACT
Successional progress was followed in fouling communities, developing under tropical conditions. Primary succession was simulated by immersing asbestos plates. The plates represent small, discrete, isolated parts of habitat. According with expectations, a trend of increasing space dominancy by colonial forms was detected as succession progressed. High within-habitat diversity but global persistency was confirmed for this tropical system. Several mechanisms of succession were found to operate simultaneously. In this Caribbean fouling system thestrong self-replacement tendencies of solitary forms and the low recruitment and growth rates of the potential space dominants (colonial forms) were mostly responsible for the successional patterns observed.
RESUMEN
Se siguió el progreso sucesional en comunidades de "fouling" bajo condiciones tropicales. La sucesión primaria fue simulada mediante el uso de placas de asbesto. Las placas representan partes pequeñas, aisladas y discretas de habitat. De acuerdo a lo esperado, se observó que las formas coloniales tienden a dominar el espacio a medida que la sucesión avanza. Se confirmó el patrón de alta diversidad dentro de habitats junto con persistencia global en este sistema tropical. Varios mecanismos de sucesión operaron simultáneamente. Las fuertes tendencias de auto-reeplazamiento de las formas solitarias y las bajas tasas de reclutamiento y crecimiento de las formas coloniales potencialmente dominadoras del espacio son responsables de la mayor parte de los patrones sucecionales observados.
INTRODUCTION
Studies of succession on fouling plates have a long tradition (e.g. Anonymous, 1952; Cairns, 1982). Like in other fields, however, the bulk of work has been conducted in temperate latitudes, while the number of fouling studies in tropical waters is rather scarce (for instance, Shoener et al. 1978). The study presented here follows the successional progress of such a particular system developing under tropical conditions. Substrata chosen for the study were asbestos plates which are thought to simulate a disturbance that has created space for colonization or has eliminated the biota totally (primary succession, sensu Clemens, 1904). They also represent type 2 patches (sensu Sousa, 1985 and Connell and Keough, 1985), i.e. discrete small parts of habitat isolated from existing assemblages as opposed to type 1 patches, i.e., non-isolated parts of habitat.
The definition of type 2 patches has a number of implications as to the modes of occupancy, particularly in two aspects: (1) isolation, which implies that occupancy can only occur from the plankton and hence reproductive activity of remote parental populations, larval dispersal potential and regional hydrodynamics will determine the availability of competent larvae (Connell and Keough, 1985) and, (2) small size (but also shape and orientation of the substrata) that will influence settlement, which is, in turn, a function of the interaction between physical (transport regime at the boundary layer) and biological (larval behavior) agents (Vandermeulen and Dewreede, 1982; Keough, 1984; Jackson, G. A., 1986). Based on these implications it is possible to predict a number of patterns as to how succession will proceed (see below). Findings in this work will be compared with such predicted patterns, in particular with regard to three aspects: (1) functional groups, (2) effect of duration and date of immersion, and, (3) the mechanisms of succession.
(1) Jackson (1977a and b; 1979) introduced the concept of functional groups for epibenthic organisms on the basis of body plan, which has implications as to how resources are used. He distinguished between solitary and colonial animals, between determinate (mostly solitary animals) and indeterminate growth (mostly colonial animals) and, for colonial animals, between a number of growth forms. In general, he suggested that colonial animals are superior competitors for space with respect to solitary by virtue of their spread capacity (indeterminate lateral growth) on the substratum without needing sexual reproduction or recruitment, and by being less susceptible to fouling. Solitary animals do not disappear from local systems thanks to behavioral adaptations like gregariousness or escape in size, and selective disturbance events. He postulated that solitary and colonial animals with poor space competitive abilities will have higher recruitment rates and tend to be opportunistic strategists, appearing early in succession.
Jackson (1977b), Kay and Keough (1981) and Keough (1984) found that the occupation of isolated patches of hard substrata by different functional groups was strongly size-dependent. Small substrata tended to select poor space competitors such as solitary animals, notably serpulid polychaetes, and inferior colonial space competitors, notably sheet bryozoans, while larger substrata were more likely to collect better colonial space competitors, notably tunicates and sponges.
Thus, since in this study the plates represent small, discrete and isolated patches and taking into account the differential biology of potential colonists, plates immersed for a short time and early in succession are expected to show a greater incidence of solitary and colonial species with poor competitive abilities. As succession progresses and eventually better competitors recruit (in general growth indeterminate colonial organisms), this pattern is expected to reverse (Connell and Keough, 1985; but see Greene et al. 1983).
(2) With respect to effect of duration and date of immersion the following scenario may be expected (based on Moore, 1972, Sutherland, 1974, Sutherland and Karlson, 1977, Sutherland, 1980 and 1981, Greene et al. 1983 and Hatcher et al. 1989): in regions where recruitment is more or less continuos and of low intensity, like the tropics, chance differences in initial occupation of patches will persist for long periods, specially for small isolated habitats. This will produce high within habitat diversity, i.e., individual patches differing from each other, but global persistency of the system, i.e., similar species composition and relative abundances for extended periods of time, the rate of change depending on the invasibility and longevity of the resident assemblages. In consequence, the plates are expected to exhibit assemblage heterogeneity. The system, in contrast, is expected to be globally persistent.
(3) As for the mechanisms of succession, several types of interspecific interactions operating simultaneously are expected. Sutherland (1978), Sousa (1979) and Dean and Hurd (1980), for instance, have documented cases both of inhibition and facilitation interactions between invaders and residents. It is expected, however, that inhibitory interactions between invaders and residents increase, as well as competition between residents, as the assemblages become saturated and space becomes scarce (initial non-interactive stage followed by a longer, interactive stage in terminology of Simberloff, 1974; see also Sutherland and Karlson, 1977 and Greene et al., 1983).
MATERIALS AND METHODS
A description of the study site (the NW Pier of the Santa Marta port in front of the Instituto de Investigaciones Marinas y Costeras José Benito Vives D'Andreis, INVEMAR, 11°15'08"N: 74°13'13"W, Bay of Santa Marta, Colombian Caribbean) and of the environmental setting can be found in Garcia and Salzwedel (1993, in particular their figures 2-3, and references therein).
Asbestos plates (10 cm x 10 cm) were used to simulate primary succession in discrete, small, isolated patches of habitat. The plates were fixed to a PVC frame tied to the bottom under a pier and maintained up right by means of buoys so that the plates were placed at aproximately 9 m depth. Only the side of the plates facing outside the pier was studied.
The sampling was done biweekly starting April 30, 1981 and ending April 29, 1982. On each sampling date two plates were taken out, one of which had been inmersed at the start and the other two weeks before sampling, and replaced in the frame by new ones. Plates were also regularly photographed in situ (35 mm slides). Thus, three series of plates and a photographic record of different stages of development were obtained. Here we report results of the first series of plates, i.e., the series that reflects successional progress (called here after successional plates), and of the photographic record, which describes the interplay of date and duration of immersion. Garcia and Salzwedel (1993) reported results on the second series of plates (called here after recruitment plates, see that work for more details).
There is a potential bias in gathering information from the slides, where species were distinguished on the base of color and texture. Since we tried to be rather conservative in deciding to add a new species (a new color and texture) we expect the bias in the direction of underestimating the actual number of species. A total of 192 slides were assessed (see Table 1).
On retrieved plates solitary sessile organisms, i.e., mostly, serpulid polychaetes and barnacles were counted and measured when feasible, and their percentage cover estimated. Abundances and smaller size-classes were underestimated for those species whose juvenile tubes could not be distinguished. The polychaete serpulid worms Hydroides cf, brachiacanthus, Hydroides parvus and Protula sp (herein collectivelly called Hb/Hp/P) were considered together and quantified only in terms of percentage cover, as were Salmacina sp, Filograna sp and Josephella marencelli (called S/F/J), in both cases on account of their similar tubes, which for each group can hardly be distinguished. Spirorbinae polychaetes 1, 2 and 3 (species not identified) were considered together when percentage cover was estimated on account of the similarity of their growth form and low cover. Only percentage cover was estimated for serpulid Pseudovermilia occidentalis and colonial organisms (encrusting bryozoans). Since the only adult barnacle found was Balanus trigonus, we assumed that all juvenile barnacles belong to this species. Percentage cover of bare space on plates was estimated as well, corresponding to real bare primary space and primary space previously occupied by soft-bodied organisms which were lost after plates were dried (see Garcia and Salzwedel, 1993). On slides percentage cover of colonial organisms (encrusting bryozoans and sponges, mainly) was estimated. Rarely occurring species (in general less than 1 % accumulated cover at the end of the year) were not considered in the analysis.
The carino-rostral distance and the diameter of the aperture was meassured for B. trigonus and serpulid polychaetes, respectively. The latter because the tubes are readily overgrown (personal observation) making it impossible to estimate their length. Length measurements were done using a computerized image analyzer system, not directly on the hard structures, but on the respective distances translated to paper by means of a camera lucida.
Percentage cover was estimated using point sampling techniques (e.g. Bohnsack, 1979 on slides; Sutherland, 1981 on plates). For plates two arrays of 100 randomly positioned points each were used. First a transparent sheet with the points on it was superimposed on the plate under study and the identity of the organism (or bare space) at each point was registered. In the case of epizoism the point was counted two times, thus giving a potential of more than 100 % cover. The same procedure was performed on the same plate with the second array of points and the results pooled. Percentage cover on slides was estimated by projecting the slide vertically onto a 23 x 23 point grid on a digitizing table which was connected to a computer. Then with an electronic pencil the number of occurrences of the selected item at the points was entered into the computer. The output of the program written for this purpose provided the percentage cover for the different items (Garcia, 1990). Numerical analysis is described on presentation of results.
RESULTS AND DISCUSSION
General faunal and colonization patterns
A complete list of species is presented in Table 2. Three main groups constitute the bulk of colonists in terms of occupancy of space: serpulid polychaetes (16 species considering rare ones), which showed the highest level of percentage cover; cirripedes, represented by only one species; and sponges (7 species). Bryozoans (5 species, all encrusting forms) and tunicates (only 1 colonial species) played mostly a secondary role. Algae were probably excluded from the plates by adverse light conditions under the pier at 9 m depth. Interestingly, hydrozoans, which are well represented in the area (Bandel and Wedler, 1987), were not detected on the slides.
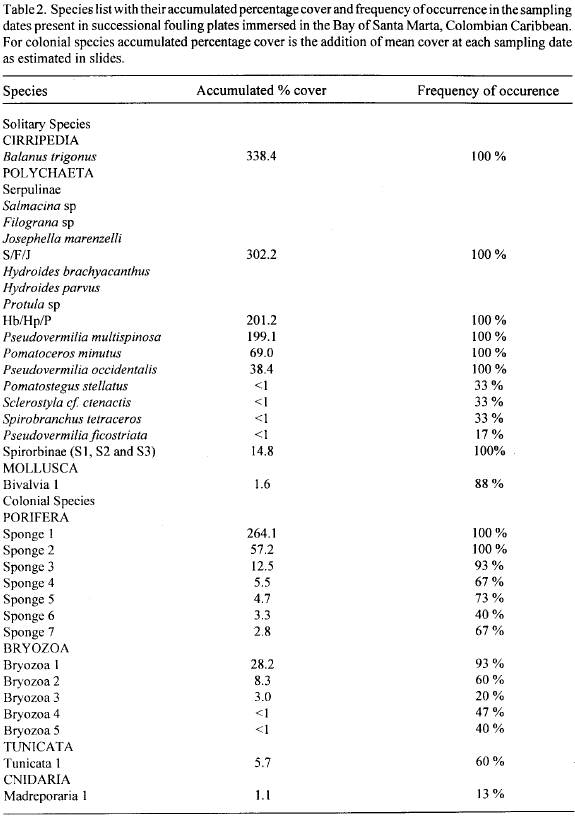
The comparison of forms (solitary versus colonial) as space occupiers shows a similar pattern with solitary percentage cover developing faster and at consistently higher levels than colonial percentage cover (Fig. 1). Figure 2 shows colonization curves for the plates, i.e. the number of species plotted against sampling date. The curve of the total number of species rises rapidly, then it levels off after about week 18 (Fig. 2). There is a slow decline in the total number of species for the next three months but a subsequent rise to a new peak in week 44, suggesting perhaps the beginning of an oscillatory phase. When the curve of total number of species is decomposed into its solitary and colonial species components a number of patterns can be seen. The solitary species number curve (number of species per plate) rises much faster and reaches its highest level much sooner than the corresponding curve for colonial species (accumulated number of species per sampling date on slides, Fig. 2). Thus, solitary species showed higher colonization rates (more species immigrating onto the plates per unit time) than colonial species. After the maximal level in both curves is reached, they tend to run parallel.
Community structural change with time
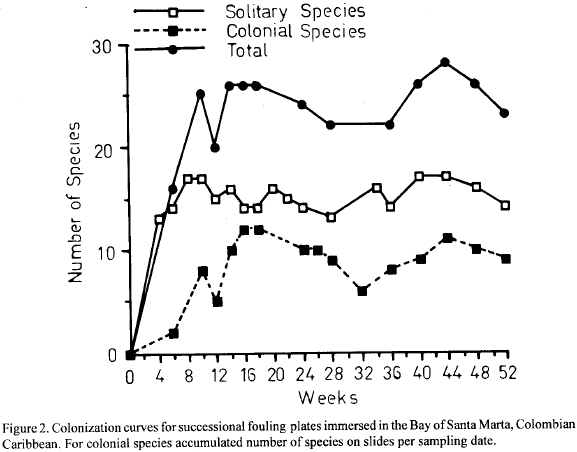
Temporal evolution of the fouling communities is depicted in Fig. 3a and 3b in the form of two-dimensional nonmetrical multidimensional scaling plots (NMDS ordination) performed on sampling dates (weeks missing in the graph were not considered). The data set consisted of arc-sine transformed (1/sin(symbol 214 \f "Symbol" x)) percentage cover values (individual values for plates, mean values for slides) as recommended by Underwood (1981) for this type of data.
In order to assess the possible impact on the analysis of equating in the slides texture and color forms to species, ordination of sampling dates was performed twice, once with all species treated individually, and once with sponges and bryozoans as two items (Fig. 3a and 3b, respectively). Results are very similar suggesting that the bias introduced by not being able to identify colonial species does not compromise the analyses.
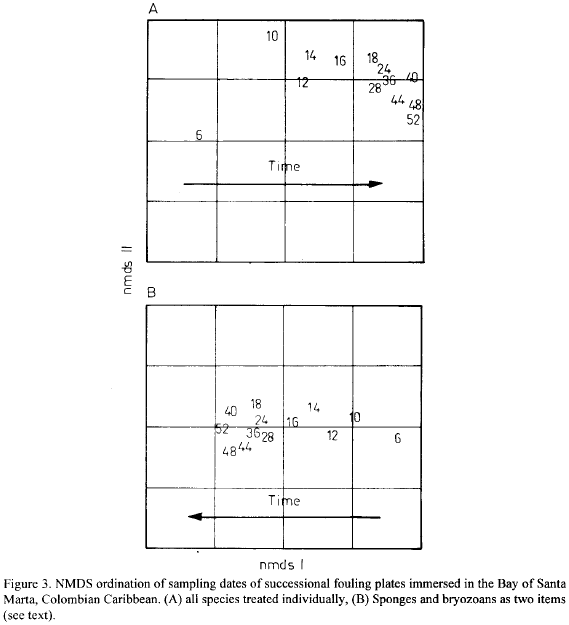
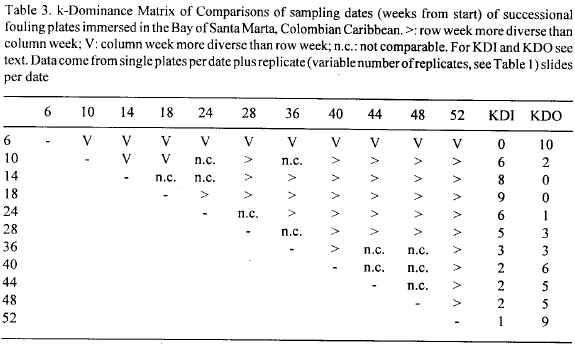
Succession in the plates was gradual and with a well defined direction in time. NMDS plots clearly show a temporal gradient of development with changes slowing down after about week 18 (Fig. 3a and 3b). Structural change was investigated by means of plots of diversity and evenness indices, based on percentage cover data, plotted against sampling dates. Fig. 4a and b shows the plots for the N1, N2 Hill's diversity numbers (Ludwig and Reynolds 1988) and the k-diversity index (KDT) proposed by Garcia (1993) (Fig. 4a), and for the modified Hill's ratio E5 (Ludwig and Reynolds, 1988) and the k-dominance index (KDO, Garcia, 1993) (Fig. 4b). Table 3 shows the k-dominance Matrix of Comparisons (Garcia, 1993) between sampling dates.
Diversity rose rapidly to a maximum level in week 14 for N1 and N2, and 18 for the KDI (Fig. 4a), concomitantly with the achievement of the first peak in number of species (see Fig. 2). Evenness closely followed this pattern (Fig. 4b) suggesting similar dynamics of space occupancy between colonists for that initial period. Afterwards diversity decreased continually at a low rate to the end of the immersion year, except a short leveling off between weeks 40 to 48 (Fig. 4a), probably caused by a strong recruitment event of the barnacle Balanus trigonus (see below). However, this recruitment event does not seem to have improved the distribution of percentage cover between species as it was barely reflected in eveness as expressed by E5 (Fig. 4b). Dominance, on the other hand as expressed by the KDO index, showed perhaps a clearer response as it inverted its increasing tendency for that periods (Fig. 4b). This pattern of decreasing diversity was due to differential space occupancy rates between residents, with some species losing and others gaining space, rather than to loss of species (see section below). As shown in Table 2, most of the species occur more than 60 % of sampling dates, i.e. basically, the complete pool of species, including the space dominants, are present most of the time.
The evenness curve (E5) shows an oscillatory phase after the first peak which coincided with the maximal diversity and minimal dominance (Fig. 4a), until week 36 (Fig. 4b). Afterwards evenness decreased rapidly suggesting an accelerated space monopolization trend by some species. This is confirmed by the behavior of dominance (KDO), which increased simultaneously (Fig. 4b).
Performance of species and groups during succession and Recruitment Pressure
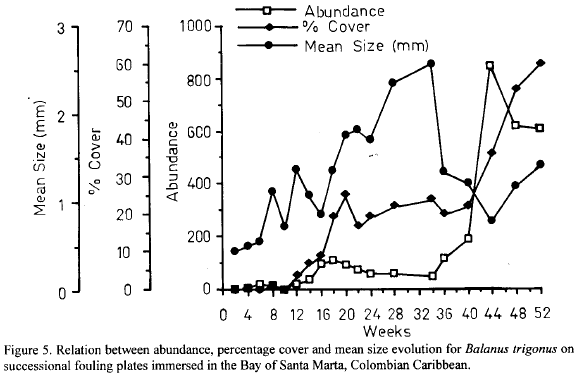
The cirripede Balanus trigonus showed well-defined patterns of space occupancy. From week 0 to week 34 individuals accumulated at low rates and mean size of individuals rose (Fig. 5), suggesting low levels of recruitment to the fouling communities. This pattern is partially explained by low availability of larvae in this period as reflected in the recruitment plates (Garcia and Salzwedel, 1993). Thus, in this first period increasing occupancy of space by this species, i.e. increasing percentage cover (Fig. 5), is attributable to growth of residents. Between weeks 38 and 46, however, a strong recruitment event occurred as indicated by the dramatical fall in mean size, while no less dramatically abundance rose (Fig. 5). In response to this recruitment event, percentage cover also increased steeply (Fig. 5) indicating that recruitment was strong enough to account for space occupancy patterns during that period. Afterwards abundance showed a decreasing pattern due probably to mortality, while mean size increased (Fig. 5). Thus, the further increase in percentage cover (Fig. 5) is due to growth. The main period of recruitment of this species as identified in the recruitment plates (Garcia and Salzwedel, 1993) coincides with this strong recruitment event in the succession plates. The implication is that the fouling assemblages, although in an advanced stage of development after about 8-9 months of immersion, were no barrier to the "rain" of B. trigonus larvae.
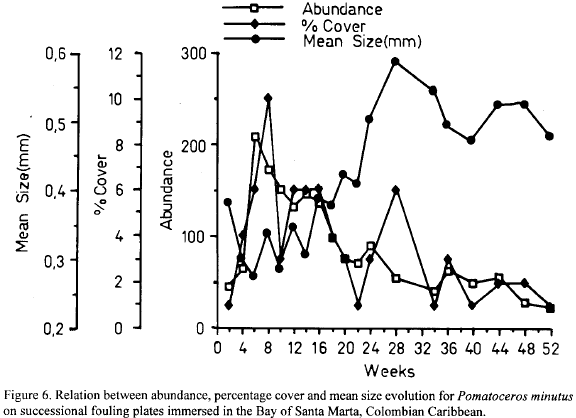
The serpulid polychaete Pomatocerus minutus also displayed a defined pattern of space occupancy like B. trigonus but of a different sign. The highest abundance was reached very rapidly by week 6. Concomitantly percentage cover reached its maximal values in week 8, while mean size remained low (Fig. 6). These patterns suggest that space occupancy was accounted for mostly by recruitment in that period. Afterwards both abundance and percentage cover declined to the end, while mean size showed the opposite trend (Fig. 6). Two conclusions can be drawn from these patterns. First, that after the first recruitment event at the beginning of the inmersion time, coincident with the main period of recruitment for this species as identified in the recruitment plates (Garcia and Salzwedel, 1993), new recruitment onto the succesional plates was negligible, either because the assemblages constituted an effective barrier to the larvae, or, because of the low availability levels of ready-to-settle larvae (Garcia and Salzwedel, 1993). Second, the growth of residents did not compensate as mechanism of space occupancy, probably on account of the small size (no massive tubes) of this species.
Pseudovermilia multispinosa, another serpulid polychaete, showed somewhat different patterns of development compared with P. minutus. Abundance increased rapidly to peak in week 18 and then fell at slow rate until the end of the immersion time (Fig. 7). Mean size and percentage cover, in turn, increased continually to the end (Fig. 7). Thus, occupancy of space was for this species until about week 18 a function of both recruitment and growth. Up to this point recruitment lost its importance as suggested by the decreasing pattern of abundance and the increasing pattern of mean-size. However, contrasting with P. minutus, this species continued gaining space (Fig. 7), suggesting that growth of residents prevented the decline of this species as space occupier, probably on account of its massive tube. Relations with recruitment patterns in the recruitment plates are obscured by the fact that counting included Pseudovermilia occidentalis (tubes not distinguishable on the recruitment plates, Garcia and Salzwedel, 1993). Nevertheless, the strongest recruitment event for these two species on the recruitment plates coincides with the highest abundance level on the succesional plates for P. multispinosa (compare Fig. 4 in Garcia and Salzwedel, 1993 with Fig. 7). Thus, the decline in abundance for this species seems related rather to lower recruitment pressure than to inhibitory interactions by the developing fouling assemblages.
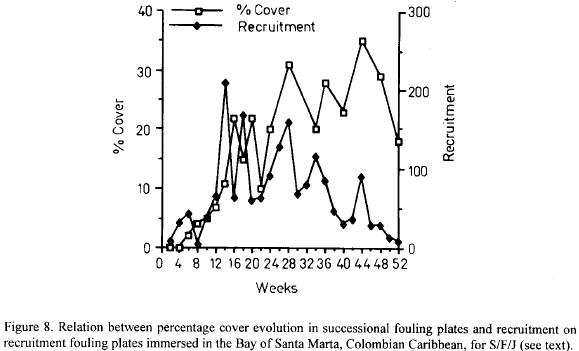
For S/F/J there seems to be a consistent relation between percentage cover in succesional plates and recruitment in recruitment plates. After a first period of similar increasing trends, recruitment on the recruitment plates started decreasing at about week 28, while percentage cover in succesional plates continued its increasing trend (Fig. 8). New subsidiary peaks in recruitment in the recruitment plates seem to be reflected in percentage cover oscillations on the succesional plates (Fig. 8). Thus, it would seem that space occupancy patterns before about week 28 were controlled both by recruitment and growth, but afterwards growth became more important, even though, recruitment was not totally obstructed. Moreover, Salmacina, Filograna and Josephella are capable of asexual reproduction by transverse fission or scissiparity, which may lead to chains of individuals (Ten Hove, 1979). The impact of this phenomenon in the occupancy patterns of this groups is unknown but probably not unimportant as such chains were common on the plates.
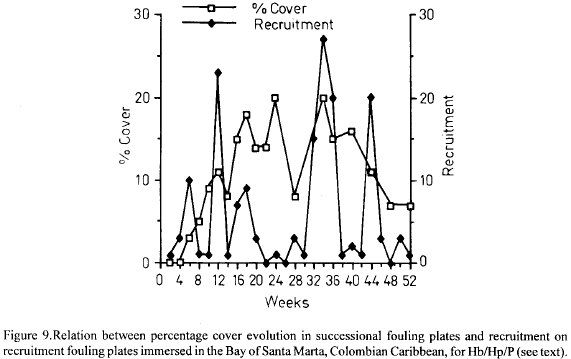
For Hb/Hp/P, in contrast, there seem to be no relation between recruitment on recruitment plates and percentage cover on succesional plates (Fig. 9). This might be an indication that growth rather than recruitment is responsible for space occupancy patterns in this group, and that in advanced stages of community development, the larvae of this group, even when abundant, are not able to invade. After about week 34 this group lost space as the decreasing percentage cover curve indicates (Fig. 9).
Of the seven species of sponges registered only two were important space occupiers and of this only Sponge 1 consistently gained space during the year of immersion (see Table 2). The other sponge species tended to enter the fouling communities slightly later (or, were detected later on the slides) and fluctuated at low levels of percentage cover (less than 10 % for a given date). The last assertion is also valid for all the other colonial organisms (Table 2). Since recruitment pressure could not be assessed for colonial organisms, except bryozoans, its impact on the developing fouling assemblages is unknown (but most likely small). Bryozoans were found to recruit at similar high intensities most of the year in the recruitment plates (Garcia and Salzwedel, 1993). This high recruitment level, however, was not reflected in the succesional plates as bryozoans were found to be marginal space occupiers (Table 2). Therefore at least for this group recruitment pressure can be considered unimportant as a mechanism of space occupancy. The general impression for colonial organisms is one of low recruitment and growth rates but high variability in the succesional plates.
Effect of duration and date of immersion on assemblage development
This analysis is based exclusively on information gathered from the slides (see Materials and Methods). Consequently only the colonial fraction of the fauna is examined here. Nonmetrical multidimensional scaling (NMDS) was performed on arc-sine transformed percentage cover data as before.
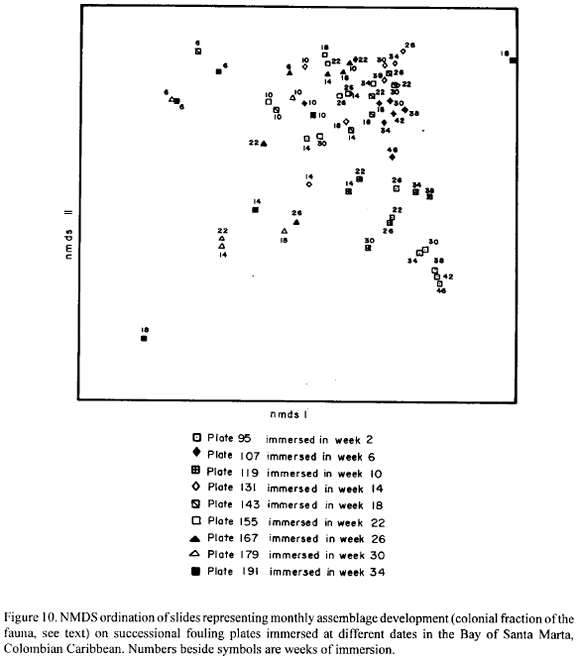
Figure 10 shows a two-dimensional NMDS plot of slides, which portrays the evolution of 9 plates photographed repeatedly during the year of immersion, the plates having different immersion dates. No clear grouping pattern emerges from the plot (Fig. 10). However, there seem to be two tendencies in the ordination of the slides: (1) slides representing early stages of development (6 and 10 weeks from immersion date) tend to segregate from slides representing later stages of development, which is to be expected, but in a random fashion, i.e. regardless of the immersion date of the plates; (2) for later stages of development, the identity of the plate appears to be more important as a grouping factor than date or duration of immersion. Some plates also developed in very different directions due to space dominance of rare species. On plate 95, for instance, Tunicate 1 covered 54.6 % of the surface after 46 weeks of immersion, which may explain its ordering in the plot (Fig. 10).
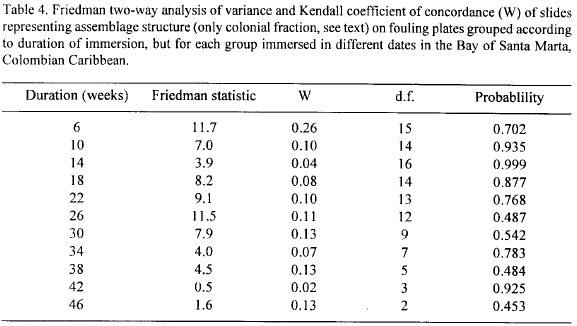
In order to test the null hypothesis that the development of the fouling assemblages (the colonial fraction) is independent of the date of immersion of the plates, i.e., that for any species it is a matter of chance for which immersion date the highest and the lowest percentage cover occur, the slides were grouped according to duration of immersion (Table 4), on each group a Friedman two-way analysis of variance was conducted and the Kendall coefficient of concordance (Siegel, 1956) was calculated. Since the Friedman test requires the same number of cases (species) in the samples (slides) drawn under the different conditions (immersion dates), missing values (in any one group of slides species present on some slides but not in others) were given a score of 0.
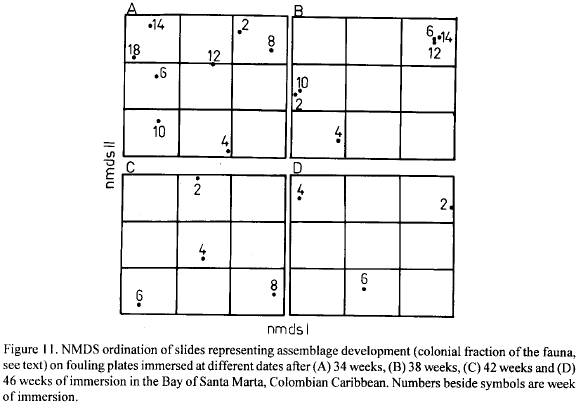
Table 4 shows that in no case could the null hypothesis be rejected (p>0.05). The Kendall coefficient of concordance, which takes values between 0 and 1 (Siegel, 1956), shows only very low values. Both results suggest that historical events on individual plates are of prime importance to the dynamics of the colonial fraction of the colonists. The fact that the results are the same for long and short durations of immersion indicates the lack of any homogenizing process, at least for the time scale of this study. NMDS plots of slides (Fig. 11) depicting the evolution of groups of plates immersed in different dates for 34, 38, 42 and 46 weeks confirms this assertion. The slides show no definite grouping pattern.
Predictions and syntesis
A number of predictions were made as to how succession would proceed in this Caribbean system concerning: (1) Solitary and colonial forms as competitors with the latter being superior than the former. (2) Within-habitat diversity versus global persistency. (3) Simultaneus operation of several mechanisms of succession (see Introduction for more details).
The first prediction was only partly met in this study. Both accumulation of species and, occupancy of space proceeded much more rapidly for the solitary fraction than for the colonial fraction of the colonists during early succession.
However, later in succession, instead of the predicted decline and exclusion of the solitary forms as a consequence of competition with the colonial forms, both continued to develop in a somewhat parallel fashion. Moreover, there is evidence that, although at much lower rate, the solitary forms were not prevented from conquering primary space in later stages in the succession.
This behavior contrary to expectations may be explained through a combination of several factors: (1) Life-history strategy features of the solitary forms, notably gregariousness (Burke, 1986), which tend to prevent extinction from local systems. (2) The continuous creation of free primary space in the form of the calcareous tubes of serpulid polychaetes and the shells of barnacles, which would favor new settlement of solitary forms (e.g. Keen and Neill, 1980; Quian and Lui, 1990) probably also retarding succession. (3) The ability of certain solitary forms to gain space by growing, notably the serpulid polychaete Pseudovermilia multispinosa and the barnacle Balanus trigonus, or by invading the assemblages from the plankton even at advanced stages of development, notably by B. trigonus. (4) The time scale of this study (one year), which was probably too short for competitive exclusion to be completed in this system as potential space dominants although present, are slow growing forms (Porifera, colonial tunicates) and, hence, the disturbance regime of the system (sloughing off events, for instance) is likely to work in longer time scales. So the adyacent pier with more than a decade in its place is dominated by colonial organisms (Sanchez, 1984).
An alternative explanation that the observed patterns are the product of a higher number of solitary than colonial species in the location of the study seems unlikely, because the total number of solitary species found in the year (18) is comparable with the total number of colonial species (14). A more convincing argument is the report by Zea (1987) of 67 porifera species for the Santa Marta area. The faunal dynamics on the plates is mediated by their size. Bigger plates would probably have been conductive to a more rapid displacement of solitary species by colonial species in this system, as postulated by Jackson (1977a,b) and Keough (1984).
An artifact influencing the resulting pattern of space occupancy is the fact that due to epizoism solitary forms will tend to receive more points during the estimation of percentage cover (on the plates) than colonial forms (mostly on the slides but for bryozoans on the plates as well). However, initial stages of development, when epizoism is reduced, showed even more markedly this difference in space occupancy rate (due mostly to serpulid colonists, see Fig. 3); and, even though at lower rates after about week 20, percentage cover of solitary forms continued increasing to the end (mostly due to cirripede colonists, Figs. 3 and Fig. 4). Both facts suggest that this pattern is real. An inspection of the primary space curve (Fig. 4) provides additional evidence as to the role of solitary forms as space occupiers. The curve's trend indicates that occupancy of primary space contributed substantially to this increasing pattern of percentage cover by solitaries, also after week 20. The implication of this is that although colonial forms were present and growing, they could not prevent solitary forms from colonising primary space on the plates.
As to the colonization dynamic of the plates the possibility of overestimation of the number of solitary species in some sampling dates can not be ruled out, since, as mentioned before, certain serpulids were treated together. Thus, when the tube form S/F/J (see above) was registered it counted for three species, but there is not certainty that the three species were actually on the plate. The effect would be to "inflate" the importance of solitary species in analysis involving number of species. Moreover, the number of colonial species is thought to be underestimated as adding new species based on color and texture on the slides was done in a conservative manner. However, even in the worst case (when for the tube forms Hb/Hp/P and S/F/J six species are computed being only two actually present on the plate for a sampling date) the conclusion that solitary species displayed a higher colonization dynamics than colonial species still holds. For example Fig. 5: if for each sampling date in the solitary species curve four species are subtracted, it still runs over the colonial species curve most of the time, and this although the information for solitary species originates from nonreplicated plates whereas the information for colonial species originates from replicated slides and accumulated number of species is plotted in the colonial species curve instead of mean number of species per sampling date, which, in turn, should compensate for the presumed underestimation in the number of colonial species.
The second prediction which was tested only for the colonial forms, was met as the colonial species settled independent of the date of immersion and persisted on the plates, regardless of the duration of immersion within the time scale of this study. In addition, the reasons adduced to explain this pattern seem to apply in the system under study (see Introduction): recruitment intensity was probably low and unpredictable, growth rate was also low (no plate was monopolized) and the species were long-lived (again in the time-scale of this study). The small size of the plates probably discouraging settlement by colonial species also contributed to the aleatority in their settlement.
It is interesting to speculate which picture would have emerged if the solitary forms could have been included in this part of the analysis. As showed and discussed elsewhere (Garcia and Salzwedel, 1993), the solitary forms did respond to local climate changes, although in no uniform fashion. Different dates of immersion would have favored the recruitment of some species over others, though without automatically excluding any. Therefore, although solitary forms appeared to be in general more uniformly distributed in time than colonial forms, their recruitment patterns would have also contributed to the within-habitat diversity. For how long this effect would have lasted, would have depended on the fate of the residents and on the ability of the larvae to invade from the plankton. On the other hand, at least in one instance within-habitat diversity would have been reduced: during and immediately after the main recruitment period of Balanus trigonus (the dry season). This species was found able to invade the assemblages in great numbers even at an advanced developmental stage ("swamp" effect, Sutherland, 1978) and to gain space by growing. Such an event would have tended to homogenize the assemblages, i.e. to reduce within-habitat diversity.
The third prediction was concerned with the mechanisms of succession. Structural change with time in the system can be divided in two stages. The first is non-interactive in that all the colonists were gaining space regardless of the presence of other species, and the number of species was increasing. This stage lasted until about week 20. In the second stage, some colonists continued to gain space, whereas others were loosing territory. Among the former were Balanus trigonus, Pseudovermillia multispinosa, Salmacina sp/Filograna sp/Josephella marenzelli (S/F/J), Porifera as a group, and particularly Sponge 1 (encrusting yellow form) and the colonial tunicate; among the later were Pomatoceros minutus, Spirorbinae polychaetes 1, 2 and 3, and Hydroides cf brachyacanthus/Hydroidesparbus/Protula sp (Hb/Hp/P), whereas bryozoans as a group and Psudovermillia occidentallis stabilized at low and very low space occupancy, respectively. The question here is to what extent is this patterns due to interspecific interactions among residents and between residents and invading larvae. The observations give no indication that predation played a role in this system. Therefore this aspect will be ignored. Three other relevant interactions in such a system have been postulated: (1) competition for space between residents, (2) degree of resistance to invasion from the plankton (inhibition) and its counterpart, (3) grade of enhancement of recruitment from the plankton by residents (facilitation).
Interspecific competition for space in hard substrata is assumed whenever physical contact between organisms occurs, particularly in situations of limited available space (Osman, 1977; Sutherland, 1978). The interaction has basically two outcomes, either growth at the contact edges stops (not necessarily permanently), or one organism physically overgrows the other, totally or partially (Russ, 1982; Sebens, 1982). The consequence for the overgrown organism is generally death of the overgrown tissue. Death of the whole organism does not necessarily follow, not even when the overgrown organism is totally covered (Ayling, 1983; Todd and Turner, 1988).
Such interference competition did occur in the system studied. In the slides, for instance, for a given plate photographed consecutively, increase of percentage cover by some colonial residents at the expense of other residents was a common observation. In the plates, tubes of serpulid polychaetes oriented perpendicular to the substratum, which has been postulated as a mechanism for escaping competition (Jackson, 1977a; b), were numerous in later stages. Furthermore, totally covered tubes of small serpulids and barnacles were observed under the crust of bryozoans.
Inhibition in fouling systems functions in three ways: settling larvae are consumed by the residents or are killed by larvotoxic substances produced by the residents (Dyrynda, 1983; Young and Gotelli, 1988; Young and Cameron, 1989) and in a third, indirect mechanism, larvae actively avoid sites occupied by predators or future competitors (Grosberg, 1981; Young, 1990). It is difficult to judge to what extent inhibition took place here. In any case if inhibition did take place, its effect was clearly modulated by recruitment pressure, i.e. by the number of ready-to-settle larvae as estimated from the recruitment plate series (for solitary forms, Garcia and Salzwedel, 1993). Abundance patterns in the recruitment plates series tended to be reflected in the succesional plates series for most of the species, the most conspicuous case being the barnacle Balanus trigonus. As for the colonial forms whether inhibition played a role in their recruitment patterns is an open question. The only group for which something on recruitment pressure can be said, are the bryozoans (sheet forms), which were abundant in the recruitment plates series (Garcia and Salzwedel, 1993) but not in the succesional plates series. Two explanations are possible: either they did not recruit in the succesional plates at similar levels as in the recruitment plates due to inhibition by residents, or they did but were overgrown. There is no reason to prefer one explanation over the other.
The third type of interaction, enhancement of recruitment (facilitation), has been associated in hard bottom systems with increasing physical structuring of the assemblages through the colonists (Brault and Bourget, 1985; Okamura, 1986; Navarrete and Castilla, 1990), not to mention the known phenomenon of gregariousness (Chia, 1990; Gotelli, 1990). The first mechanism is not species-specific, although at least one exception is known (Turner, 1983), and is not strictly biological as mimetic artificial structures have the same effect (Dean, 1981; Bros, 1987).
In this system, apparently no species was particularly dependent on biogenically produced structure in order to colonize. That is not to say that the appearance of serpulid tubes and barnacle shell walls on the plates was to no effect. Rather the (assumed) effect was diffuse and non-discriminatory. The very fact of producing calcareous exoskeletons has as a consequence that new bare space is created that can be settled by larvae, thus probably favoring the further recruitment of solitary species in general in view of their life-history strategy. Such an effect might delay decline of solitary species when competing for space with colonial species and retard succession. This diffuse effect together with gregariousness, however, would rapidly lead to crowded conditions (as the case was, epizoism was a common observation) augmenting the probability of being overgrown by other solitary species, and perhaps inhibition (more feeding openings per area). In conclusion, facilitation operated here as a self-replacement mechanism for solitary forms as a group hence retarding succession but it probably also augmented competition between solitary species accelerating the decline of some of them, an example being Pomatoceros minutus and the Spirorbinae, which possess no massive tubes.
Thus, it seems that for this system no single mechanism of succession was dominant in the time scale of the study, i.e., no clear hierarchy emerged. The prediction that several mechanism would operate simultaneously was met. This succession was not followed enough time to test the prediction that inhibition would dominate in later stages, i.e., in this system, when abundance recruitment patterns in the recruitment plates series are not longer reflected in the succesional plates series. But as the space dominants gain space, this would presumably have been the case, especially with regard to the effect of the colonial species.
How then did succession proceed in this tropical system and time scale? First, not by replacement of species. Extinction events in this systems were probably rare, although the decline of a number of species was apparent. With more time allowed the assemblages would have become poorer in species. Thus, change with time (in the time-scale of this study) was instead a function of differential space occupancy rates which, in turn, responded to both differential recruitment and growth rates, morphological features, and the ability to invade from the plankton.
Differential recruitment rates were observed between colonial (low, patchy recruitment) and solitary forms (high more uniform, although fluctuating recruitment, Garcia and Salzwedel, 1993). Differential growth rates were observed between colonial (slow growth) and solitary (rapid growth) forms. Morphological features include, apart from the dichotomy colonial/solitary with the afore mentioned advantages in competition for space for colonial forms, massive exoskeletons (tubes of Pseudovermilia multispinosa and Pseudovermilia occidentalism shell walls of Balanus trigonus) which permitted their possessors to smother smaller forms. Ability to invade from the plankton was, in turn, a function of both diffuse facilitation by residents as they produced new bare space (calcareous tubes of serpulid polychaetes and walls of barnacles) favoring further colonization, specially by solitary forms (and perhaps gregarism played a role in this context), and recruitment pressure, i.e., the abundance of ready-to-settle larvae, an abundance that at least for solitary species was correlated with the local climatic regime (see Garcia and Salzwedel, 1993).
ACKNOWLEDGEMENTS
This paper is based on a Dr. rer. nat. dissertation by C.B. Garcia, Biology/Chemistry Faculty, University of Bremen, Germany. The first author was finantially supported by a scholarship of the Deutscher Akademischer Austausch Dienst, DAAD. Field work was financed by the Gesellschaft fur Technische Zusammenarbeit, GTZ and supported by the Instituto de Investigaciones Marinas de Punta de Betín, INVEMAR. Laboratory work and data analysis was carried out at the Alfred Wegener Institut fur Polar und Meeresforschung. Dr. Harry A. Ten Hove (Institut of Taxonomic Zoology, Amsterdam) kindly identified the serpulid polychaetes. Comments by two anonymous reviewers are gratefully acknowledged.
REFERENCES
1 Anonymus 1952. Marine fouling and its prevention. U.S. Naval Institute, Annapolis, 368 p. [ Links ]
2 Ayling, A.L. 1983. Factors affecting the spatial distrinutions of thinly encrusting sponges from temperate waters. Oecologia, 60: 412-418. [ Links ]
3 Bandel, K. and E. Wedler 1987. Hydroid, Amphineuran and Gastropod zonation in the littoral of the Caribbean Sea, Colombia. Senckenbergiana maritima 19(1/2): 1-129. [ Links ]
4 Bohnsack, J. 1979. Photographic quantitative sampling of hard-bottom benthic communities. Bull. Mar. Sci., 29(2): 242-252. [ Links ]
5 Brault, S. and E. Bourget 1985. Structural change in an estuarine subtidal epibenthic community: biotic and physical causes. Mar. Ecol.-Prog. Ser., 21: 63-73. [ Links ]
6 Bros, W. 1987. Effects of removing or adding structure (barnacle shells) on recruitment to a fouling community in Tampa Bay, Florida. J. Exp. Mar. Biol. Ecol., 105: 275- 296. [ Links ]
7 Burke, R.D. 1986. Pheromones and the gregarious settlement of marine invertebrate larvae. Bull. Mar. Sci., 39(2): 323-331. [ Links ]
8 Cairns, J. (Ed). 1982. Artificial substrates. Ann Harbor Science, Michigan, 279 p. [ Links ]
9 Chia, F. 1990. Differential larval settlement of benthic marine invertebrates: 3-12. In Reproduction, genetics and distribution of marine organisms (ed. by J. Rylandand P.J.Tayler).Olsen and Olsen, Viborg. [ Links ]
10 Clemens, F.E. 1904. Plant succession: an analysis of the development of vegetation. Carnagie Inst. Washington Publ. 242: 512 p. [ Links ]
11 Connell, J.H. and M.J. Keough 1985. Disturbance and patch dynamics of subtidal marine animals on hard substrata: 125-152. In The ecology of natural disturbance and patch dynamic (ed. by S.T.A. Pickett and P.S. White). Academic Press. London. [ Links ]
12 Dean, T.A, 1981. Structural aspects of sessile invertebrates as organizing forces in an estuarine fouling community. J. Exp. Mar. Biol. Ecol., 53: 163-180. [ Links ]
13 Dean, T.A. and L.E. Hurd 1980. Development in an estuarine fouling community: the influence of early colonists on later arrivals. Oecologia, 46: 295-301. [ Links ]
14 Dyrinda, P.E. 1983. Modular sessile invertebrates contain larvotoxic allelochemicals. Develop. Compar. Immun., 7: 621-624. [ Links ]
15 García, C.B. 1990. Two simple methods for recording points when using photography and point sampling techniques. An. Inst. Invest. Mar. Punta de Betín, 19-20: 205-208. [ Links ]
16 García, C.B. 1993. Comparing k-dominance curves: yet another suggestion. An. Inst. Invest. Mar. Punta de Betín, 22: 122-128. [ Links ]
17 García, C.B. and H. Salzwedel 1993. Recruitment patterns of sessile invertebrates onto fouling plates in the Bay of Santa Marta, Colombian Caribbean. An. Inst. Invest. Mar. Punta de Betín, 22: 30-44. [ Links ]
18 Gotelli, N.J. 1990. Stochastic models of gregarious larval settlement. Ophelia, 32(1.2): 95-108. [ Links ]
19 Green, C.H.; A. Schoener and E. Corets 1983. Succession on marine hard substrata: the adaptive significance of solitary and colonial strategies in temperate fouling communities. Mar. Ecol.- Prog. Sen, 3: 121-129. [ Links ]
20 Grosberg, R.K. 1981. Seasonal, annual and spatial variation in the development of hard-bottom communities. Helgol. wissenschaft. Meeresuntersuch., 36: 137-150. [ Links ]
21 Hatcher, B.G; R.E. Johannes and H.I. Robertson 1 989. Review of research relevant to the conservation of shallow tropical marine ecosystems. Oceanog. Mar. Biol. Ann. Rev., 27: 337-414. [ Links ]
22 Jackson, G.A. 1986. Interaction of physical and biological processes in the settlement of planktonic larvae. Bull. Mar. Sci., 39(2): 202-212. [ Links ]
23 Jackson, J.B.C. 1977a. Competition on marine hard substrata: the adaptive significance of solitary and colonial strategies. Amer. Nat., 111: 743-767. [ Links ]
24 Jackson, J. B.C. 1977b. Habitat area, colonization, and development of epibenthic community structure: 349-357. In Biology of benthic organisms (ed. by B.F. Keegan, P.O. Cidigh and P.J.S. Boaden). Pergamon Press. London. [ Links ]
25 Jackson, J. B.C. 1979. Morphological strategies of sessile animals: 499-553. In Biology and systematics of colonial organisms, (ed. by G. Larwood and B.R. Rosen). Academic Press. London. [ Links ]
26 Kay, A.M. and M.J. Keough. 1981. Occupation of patches in the epifaunal communities on pier pilings and the bivalve Pinna bicolor at Edithburg, South Australia. Oecologia, 48: 123-130. [ Links ]
27 Keen, S.L. and W.E. Neill. 1980. Spatial relationships and some structring processes in benthic intertidal communities. J. Exper. Mar. Biol. Ecol., 45: 139-155. [ Links ]
28 Keough, M.J. 1984. Effects of patch size on the abundance of sessile marine invertebrates. Ecology, 65: 423-437. [ Links ]
29 Ludwig, J.A. and J.F. Reynolds 1988. Statistical ecology. A primer on methods and computing. John Wiley and Sons, 337 p New York. [ Links ]
30 Moore, H.B. 1972. Aspects of stress in the tropical marine environment. Advan. Mar. Biol., 10: 217- 269. [ Links ]
31 Navarrete, S.A. and J.C. Castilla 1990. Barnacle walls as mediators of intertidal mussel recruitment: effects of patch size on the utilization of space. Mar. Ecol.-Prog. Ser., 68: 113-119. [ Links ]
32 Okamura, B. 1986. Formation and disruption of aggregations of Mytilus edulls in the fouling community of San Francisco Bay, California. Mar. Ecol.-Prog. Ser., 30: 275-282. [ Links ]
33 Osman, R.W. 1977. The stablishment and development of a marine epifaunal community. Ecol. Monogr., 47: 37-63. [ Links ]
34 Quian, P.Y. and L.L. Lin 1990. Recruitment of barnacles into empty adult test. J. Exp. Mar. Biol. Ecol., 142: 63-74. [ Links ]
35 Russ, G.R. 1982. Overgrowth in a marine epifaunal community: competitive hierarchies and networks. Oecologia, 53: 12-19. [ Links ]
36 Sanchez, H. 1984. Poriferengesellschaften an einer Hafenmole in Santa Marta, Kolumbien, unter dem Einflussunterschiedlicher Lichtexposition. Dr.rer.nat.Thesis,Universität Giessen, Deutschland, 210 zeiten. [ Links ]
37 Schoener, A., E.R. Long and J.R. DePalma 1978. Geographic variation in artificial island colonization curves. Ecology, 59(2): 367-382. [ Links ]
38 Sebens, K.P. 1982. Competition for space: growth rate, reproductive output and scape in size. Amer. Nat., 120(2): 189-197. [ Links ]
39 Siegel, S. 1956. Nonparametric statistics for the behavioral sciences. McGraw-Hill, 312 p. Tokio. [ Links ]
40 Simberloff, D.S. 1974. Equilibrium theory of island biogeography and ecology. Ann. Rev. Ecol. System., 5: 161-182. [ Links ]
41 Sousa, W.P. 1979. Experimental investigations of disturbance and ecological succession in a rocky intertidal algal community. Ecol. Monog., 49: 227-259. [ Links ]
42 Sousa, W.P. 1 985. Disturbance and patch dynamics on rocky intertidal shores: 101 -124. In The ecology of natural disturbance and patch dynamics, (ed. by S.T.A. Pickett and P.S. White). Academic Press. London. [ Links ]
43 Sutherland, J.P. 1974. Multiple stable points in natural communities. Amer. Nat., 108: 859-873. [ Links ]
44 Sutherland, J.P. 1978. Functional roles of Schizoporella and Styela in the fouling community at Beafort, North Carolina. Ecology, 59: 257-264. [ Links ]
45 Sutherland, J.P. 1980. Dynamics of the epibenthic community on roots of the mangrove Rhizophora mangle, at Bahía de Buche, Venezuela. Mar. Biol., 58: 75-84. [ Links ]
46 Sutherland, J.P. 1981. The fouling community at Beaufort, North Carolina: a study in stability. Amer. Nat., 118(4): 499-519. [ Links ]
47 Sutherland, J.P. and R. Karlson 1977. Development and stability of the fouling community at Beaufort, North Carolina. Ecol. Monog., 47: 425-446. [ Links ]
48 Ten Hove, H.A.1979. Different causes of mass occurrence in serpulids: 281-298. In: Biology and Systematics of Colonial Organisms (ed. by G. Larwood and B.R. Rosen). Academic Press. London. [ Links ]
49 Todd, CD. and S.J. Turner 1988. Ecology of intertidal and sublittoral cryptic epifaunal assemblages. II. Nonlethal overgrowth of encrusting bryozoans by colonial ascidians. J. Exp. Mar. Biol. Ecol., 115: 113-126. [ Links ]
50 Turner, T. 1983. Facilitation as a successional mechanism in a rocky intertidal community. Amer. Nat., 121(5): 729-738. [ Links ]
51 Underwood, A. J. 1981. Techniques of analysis of variance in experimental marine biology and ecology. Oceanog. Mar. Biol. Ann. Rev., 19: 513-605. [ Links ]
52 Vandermeulen, H. and R.E. Dewreede 1982. The influence of orientation of an artificial substrate (transite) on settlement of marine organisms. Ophelia, 21(1): 41-48. [ Links ]
53 Young, CM. 1990. Selection of predator-free settlement sites by larval ascidians. Ophelia, 30(2):132-140. [ Links ]
54 Young, CM. and N.J. Gotelli 1988. Larval predation by barnacles: effects on patch colonization in a shallow subtidal community. Ecology, 69(3): 624-634. [ Links ]
55 Young, CM. and J.L. Cameron 1989. Differential predation by barnacles upon larvae of two bryozoans: spatial effects at small scales. J. Exp. Mar. Biol. Ecol., 128: 283-294. [ Links ]
56 Zea, S. 1987. Esponjas del Caribe Colombiano. Editorial Catalogo Científico, 286 p. Bogotá. [ Links ]