Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.32 no.1 Santa Marta Jan./Dec. 2003
PLANKTONIC PRIMARY PRODUCTION AND COMMUNITY RESPIRATION IN SEVERAL COASTAL LAGOONS OF THE OUTER DELTA OF THE RIO MAGDALENA, COLOMBIA
PRODUCTIVIDAD PRIMARIA Y RESPIRACIÓN DE LA COMUNIDAD PLANCTÓNICA EN VARIAS LAGUNAS COSTERAS DEL DELTA EXTERIOR DEL RÍO MAGDALENA, COLOMBIA
Klaus Gocke1, José Ernesto Mancera Pineda2, Luis Alfonso Vidal3 and Diana Fonseca3
1Institut fuer Meereskunde, D-24105 Kiel, Germany. Fax: +49-431-6001515, e-mail: kgocke@ifm.uni-kiel.de (K. G.).
2Department of Biology, University of Louisiana at Lafayette, USA. P.O. Box 41297 Lafayette, LA 70504 - USA. e-mail: emancera@louisiana.edu (J. E. M. P.).
3Mundo Marino, Universidad Jorge Tadeo Lozano. Santa Marta, Colombia.e-mail: lavidalve@yahoo.com (L. A. V.), dipifo@hotmail.com (D. F.).
ABSTRACT
To understand the responses of the plankton community to the negative large-scale human impacts in a tropical coastal lagoon complex, we carried out a regional survey on primary productivity (PP), community respiration (CR), and contribution of different size fractions of the phytoplankton to overall PP. This comparative study was undertaken in several coastal lagoons within the Outer Delta of the Río Magdalena (Colombia) during the dry season(February/March). PP was measured using the 14C-method with in situ-incubations and CR was determined as oxygen demand, using the Winkler technique. According to their salinities the lagoons were separated into a brackish water group (salinity range 5.9 - 21.8) and a freshwater group, the latter being influenced directly by the Río Magdalena. In all of the lagoons the productive layer did not surpass 1 m in depth due to selfshading by the high density of plankton (brackish lagoons) or due to high amounts of suspended inorganic sediment particles introduced into the freshwater lagoons from the Río Magdalena. The brackish lagoons contained high chl a concentrations (62 - 130 µg/l) and were extremely productive (0.72 - 1.25 mg C/l/h in the most productive depth, usually at 0.1 m). The concentrations of chl a in the freshwater lagoons were much lower (5.5 - 19 µg/l), also the PP (0.073 - 0.32 mg C/l/h). In all of the studied lagoons the photosynthetic active algae were very small, algae >20 µm (microalgae) played only a very insignificant role with respect to PP. The assimilation index (AI) was quite high (11.6 - 18.5 mg C/mg chl a /h). Only in two of the lagoons the relatively low AI (6.9 - 7.4 mg C/mg chl a /h) was probably due to senescent phytoplankton algae. The depth-integrated PP rates in the brackish lagoons ranged from 1.40 - 5.76 g C/m2/d. Especially the enormous rate of 5.76 g C/m2/d which was representative for the central part of Ciénaga Grande de Santa Marta demonstrated that this aquatic system belongs to the most productive ones world-wide. In the freshwater lagoons the daily PP measured 0.24 - 0.80 g C/m2. In the brackish lagoons the highly significant correlation between chl a and CR showed that the phytoplankton dark respiration was the dominant component, whereas the absence of a significant correlation in the freshwater lagoons demonstrated that here the heterotrophic microorganisms contributed more to community respiration. Only in three of the seven studied lagoons (among these Ciénaga Grande) planktonic primary production surpassed pelagic respiration, in the remaining four lagoons the deficit of organic material is probably compensated from allochthonous sources such as the mangrove fringes and/or river input.
KEY WORDS: Primary production, Planktonic respiration, Coastal lagoons, Caribbean coast, Magdalena River.
RESUMEN
Para entender las respuestas de los organismos planctónicos a los impactos humanos negativos a gran escala en un complejo costero lagunar tropical, se llevó acabo un estudio regional sobre productividad primaria (PP), respiración de la comunidad planctónica (RC), y contribución de diferentes tamaños de fitoplancton a la productividad total. Este estudio comparativo fue realizado en varias lagunas costeras del Delta Exterior del Río Magdalena durante la estación seca (febrero/marzo). La PP fue medida usando el método 14C con incubaciones in situ y la RC fue determinada por demanda de oxígeno usando la técnica Winkler. De acuerdo con las salinidades, las lagunas fueron separadas en el grupo de agua salobre (rango de salinidad entre 5.9 - 21.8) y en el grupo de agua dulce, este último directamente influenciado por el Río Magdalena. En todas las lagunas la zona productiva no soprepasó 1m de profundidad debido al autosombreo por la alta densidad planctónica (lagunas de agua salobre) o debido a la alta cantidad de sedimentos inorgánicos suspendidos, introducidos por el Río Magdalena a las lagunas de agua dulce. Las lagunas de agua salobre presentaron altas concentraciones de chl a (62 - 130 µg/l) y fueron extremadamente productivas (0.72 - 1.25 mg C/l/h en la profundidad más productiva, generalmente a 0.1 m). Las concentraciones de chl a en las lagunas de agua dulce fueron mucho más bajas (5.5 - 19 µg/l) y también la PP (0.073 - 0.32 mg C/l/h). En todas las lagunas estudiadas las algas fotosinteticamente activas fueron muy pequeñas, las algas >20 µm (microalgas) jugaron un papel insignificante con respecto a la PP. El índice de asimilación (IA) fue muy alto (11.6 - 18.5 mg C/mg chl a /h). Solamente en dos de las lagunas el relativamente bajo IA (6.9 - 7.4 mg C/mg chl a /h) fue debido probablemente a fitoplancton senescente. La tasa de PP integrada en las lagunas de agua salobre tuvo un rango de 1.40 - 5.76 g C/m2/d. En especial la enorme tasa de 5.76 g C/m2/d, la cual fue representativa de la parte central de Ciénaga Grande de Santa Marta, demuestra que este sistema acuático pertenece a los más productivos del mundo. En las lagunas de agua dulce la PP diaria estuvo entre 0.24 y 0.80 g C/m2. En las lagunas de agua salobre la correlación altamente significativa entre chl a y RC mostró que la respiración fitoplanctónica fue el componente dominante, mientras que la ausencia de una correlación significativa en las lagunas de agua dulce, demostró que allí los microorganismos heterotróficos contribuyeron más a la respiración de la comunidad. En tres de las siete lagunas (entre estas Ciénaga Grande), la producción primaria planctónica sobrepasó la respiración pelágica. En las otras lagunas la carencia de material orgánico es probablemente compensada por fuentes alóctonas tales como el manglar y/o las descargas riverinas.
PALABRAS CLAVE: Producción primaria, Respiración planctónica, Lagunas costeras, Costa Caribe, Río Magdalena.
INTRODUCTION
Since the introduction of a method to determine the primary production (PP) in aquatic environments by Gaarder and Gran (1927) much information was collected about this parameter. The authors used the liberation of oxygen by photosynthesising algae (oxygen method) to measure the magnitude of PP. For analytical reasons the application of this method was limited to eutrophic biotopes. When Steemann-Nielsen (1952) developed the much more sensitive 14C-method which measures the uptake of 14CO2 by algae, PP could be studied even in very oligotrophic systems. From that time on the information about primary production in marine and limnic environments grew almost exponentially. Primary production measurements were included in many studies for obvious reasons, since the formation of organic matter by green plants is the most decisive factor which governs the flux of organic material through the ecosystem. In spite of the importance of the knowledge of PP, however, even up to the last decades most of the respective studies were restricted to temperate regions.
A good example for such a lack of studies outside of the temperate regions is the system of coastal lagoons east of the Río Magdalena mouth in northern Colombia. The largest lagoon in this "Delta Exterior del Río Magdalena" (DERM) is Ciénaga Grande de Santa Marta, an aquatic ecosystem of utmost ecological and economical importance for the adjacent region (Botero and Mancera, 1996; Polanía et al., 2001). Between 1964 and 1995 more than 430 papers and reports were published about Ciénaga Grande (Mancera et al., 1996), only eight of them dealt with problems related to the primary production of the system. Probably the most comprehensive one is the work of Hernández and Gocke (1990), who studied the annual cycle of the PP at four stations in 1987 using the oxygen method. The authors state that Ciénaga Grande is a hyperproductive system showing a well marked seasonal variation with highest PP-values at the end of the rainy season when the salinity of the lagoon was lowest. Annual primary production amounted to 1690 gC/m2 (gross PP) and community respiration (CR) in the pelagial had a magnitude of 700 gC/m2/year. Thus, about two fifth of the produced organic material is mineralized in the water column. A probably significant part is exported into the adjacent coastal region of the Caribbean Sea and the rest is further degraded at the lagoon's bottom or remains there as a permanent sediment.
Even though general knowledge of primary production of Ciénaga Grande might exist, there is still a substantial lack of information about the PP under special conditions. To determine whether the enormous human impact (hypersalinization, contamination, eutrophication,etc.) that the whole wetland had suffered during the last four decades (Botero and Mancera, 1996; Polanía et al., 2001) has lead to a deterioration of its pelagic primary productivity, we carried out a regional survey of several coastal lagoons within the DERM, comparing the primary production, community respiration, and the contribution of different size fractions of the phytoplankton to overall PP. Those lagoons were chosen which differ markedly with respect to hydrological and biological properties.
STUDY AREA
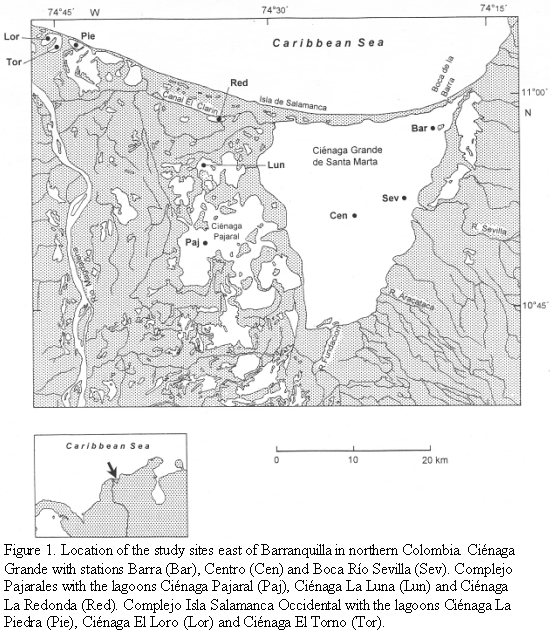
The study area forms part of the "Outer Delta of the Río Magdalena" which is located at the northern coast of Colombia (Figure 1). This enormous wetland area is bordered on the western side by the Río Magdalena and on the eastern side by the high mountain region Sierra Nevada de Santa Marta. The northern boundary is the Caribbean Sea. The study area lies between 10o 45‘-11o 05‘ N and 74o 16‘-74o 46‘ W. Since a detailed description of the study area is given by Palacio (1983) and especially by Botero and Mancera (1996) and Polanía et al. (2001), the dates presented here will concentrate on those geographical, hydrographical and biological features which are important for understanding our results.
Several lagoons are lying in this delta lagoon complex, the largest one is the brackish water system Ciénaga Grande de Santa Marta. It forms an enormous triangle (450 km2) bordered to the north by the Isla Salamanca, a long and narrow sand barrier. On its eastern side a fertile plain area mostly used for banana and oil palm plantations separates Ciénaga Grande from the Sierra Nevada de Santa Marta. Three sampling sites were selected in Ciénaga Grande. 1) Boca de La Barra: the inlet that connects Ciénaga Grande with the Caribbean Sea; 2) Centro: the central portion of the lagoon; 3) Boca Río Sevilla: the mouth of the second largest river that discharges into Ciénaga Grande (Figure 1).
On the southern and western side of Ciénaga Grande a huge inundation and accumulation area exists with large swamps and numerous lagoons. This so-called "Complejo Pajarales" represents a mangrove area, where Avicennia germinans, Rhizophora mangle and Laguncularia racemosa predominate. Three of our studied lagoons, Ciénaga Pajaral, Ciénaga La Luna and Ciénaga La Redonda, are situated within this area. A system of channels connects these lagoons with Ciénaga Grande and Río Magdalena.
The three remaining lagoons (Ciénaga La Piedra, Ciénaga El Loro, Ciénaga El Torno) are situated near the mouth of the Río Magdalena. This area, called "Complejo Isla Salamanca Occidental", is influenced by the Río Magdalena via an artificial channel. These lagoons are not directly connected with Ciénaga Grande or the lagoons within the Complejo Pajarales.
According to its geomorphological and geophysical characteristics the DERM can be classified as a river-dominated, arid, low tidal delta lagoon complex (Thom, 1982). The area is situated within the tropical dry life zone with a total of six to seven dry months a year. The annual precipitation varies from about 500 mm in the north to 1000 mm in the south. The annual water deficit is 1030 mm (IGAC, 1973). The main dry season lasts from late December to April and the rainy season from May to mid December. The latter generally has a short dry interval in July and August. The mean duration of the daily sunshine varies between 7.6 (wet season) and 8.8 hours (dry season). According to Sánchez et al. (1993) the monthly means of the solar radiation vary between 4.5 (October) and 5.5 kWh/m2 (from January to May). The temperature is isomegathermic with annual means from 27 - 28 oC and daily amplitudes of 8 - 9 oC (Wiedemann 1973).
All lagoons within the Outer Delta of the Río Magdalena, even the enormous Ciénaga Grande, are extremely shallow, nearly nowhere measuring more than 1.5 - 1.8 m. The few exceptions are of small scale. The mean annual water temperature is around 30 oC (Hernández, 1986). Since tidal ranges at the Caribbean coast are small (around 30 cm), the only part of the DERM which is directly influenced by salt-water intrusion due to tidal currents, is the north-eastern corner of Ciénaga Grande. Salinity fluctuations in the lagoons of the DERM are caused by seasonally varying amounts of intruding salt-water respectively freshwater discharge of the rivers and channels. During the last half year before the present study, the monthly means of the water discharge of the Río Magdalena were close to the means over the last 20 years. In Ciénaga Grande a total salinity range from 0 to 40 was observed (Hernández, 1986). In the lagoons of the Complejo Pajarales the salinity varies seasonally between 0 and 76 and in the Complejo Isla Salamanca Occidental between 0 and 10 (unpublished data).
The whole Outer Delta of the Río Magdalena is subjected to severe environmental stress factors. Many of them are associated with freshwater diversion (Mancera and Vidal, 1994; Botero and Salzwedel, 1999). Alterations of the hydrological regime of the DERM started when a highway constructed in 1956 interrupted most of the connections between the Caribbean Sea and Ciénaga Grande. During the seventies of the last century freshwater from the Río Magdalena was diverted from the mangrove forests west of Ciénaga Grande and from the lagoon itself by a road without culverts along the river. These alterations lead to soil hypersalinization resulting in a mass mortality of more than 70% of the original mangrove forest coverage (510 km2) in approximately 40 years.
In the late nineties some of the channels were re-opened as the most important correctional measure to rehabilitate the degraded mangrove forests and aquatic systems within the Outer Delta of the Río Magdalena. Today the Almendros and Torno channels supply again the lagoons of the Complejo Isla Salamanca Occidental with freshwater. The Caño Clarin connects the Río Magdalena with the north-west corner of Ciénaga Grande and influences several lagoons of the Complejo Pajarales. The Caño Aguas Negras enters the central part of the Complejo Pajarales. Its water discharge influences Ciénaga Pajaral and Ciénaga Mendegua. The Caño Renegado enters the southern part of the DERM and flows into the most southern part of Ciénaga Grande via the Río Fundación. In some areas these correctional measures have caused already a slight improvement of the environmental conditions. Much long-lasting and expensive effort, however, is still needed to improve the situation, since in addition to the salinity problems the whole DERM area and especially Ciénaga Grande are severely affected by eutrophication process, pesticide discharge, and microbial contamination (Botero and Mancera, 1996).
The study was performed in February and March 1997 which fall into the dry season. During these months the water discharge of the Río Magdalena and the rivers from the Sierra Nevada usually reaches its lowest level. North-easterly trade winds are dominant during the dry season with speeds of 3.2 - 5.4 m/s (Polanía et al., 2001). In the afternoons the wind freshens markedly up, thus preventing a stratification of the water column of the lagoons.
MATERIALS AND METHODS
Sampling. The samples were taken with a Niskin sampler just below the surface. Since the sampler has a length of 50 cm, the sample represented the upper half meter of the water column. Samples from each group of lagoons were processed together, the time lapse between sampling and beginning of the incubations for primary production and community respiration measurements never exceeded one hour, during which the samples were kept at in situ-temperature in an insulation box.
Physical, chemical and meteorological variables. Temperature, salinity and dissolved oxygen were measured with a TS- and an Oxygen probe (WTW, Weilheim, Germany). Secchi depth was obtained with a white 20 cm diameter disk. Dissolved inorganic carbon (DIC) was calculated from temperature, salinity, pH and alkalinity. The latter was obtained by titration with 0.1 N HCl to a pH of 4.3 (Wetzel and Likens, 1991). Incident radiation was measured with a Quantum Radiometer (LI-COR Inc.). Since these radiation measurements were impossible at the study sites, they had to be done at the Instituto de Investigaciones Marinas in Santa Marta, about 34 and 61 km respectively north-east off the incubation sites.
Phytoplankton composition. Identification and enumeration of phytoplankton organisms were mostly done with a standard microscope using phase contrast objectives. Since phytoplankton density or inorganic sediment loading were extremely high, Sedgwick Rafter counting cells (Sweetwater Products, GB) filled with 1 ml of the thoroughly mixed sample were used. For counting, size measurement and identification of the marine and brackish water species the procedure given by Vidal (1995) was followed. Freshwater unicellular algae were identified according to Yacubson (1969, 1972, 1974).
Chlorophyll a and primary production. For chlorophyll a determinations samples were filtered onto glass fiber filters (GF/F, Whatman). Pigments were extracted with 90% acetone and measured by HPLC (Mantoura and Llewellyn, 1983). Primary production was determined with the 14C-method according to Steemann-Nielsen (1952). From each station or lagoon 14 clear polycarbonate vials (30 ml) were filled with the sample and inoculated with 4 µCi of NaH14CO3. Pairs of vials were incubated in six depths for three hours around noon. The remaining two vials served as dark bottles and were incubated in light-tight PVC-tubes. The incubation depths were chosen according to the Secchi depth, the deepest being 90 cm for the stations in Ciénaga Grande, 60 cm for the lagoons in the Complejo Pajarales and 75 cm for the lagoons in the Complejo Isla Salamanca Occidental. Since always three sampling sites or lagoons were studied on the same day, the incubations were made together at one of the respective study sites. These were station Barra in Ciénaga Grande, Ciénaga Pajaral in the Complejo Pajarales and Ciénaga El Torno in the Complejo Isla Salamanca Occidental. For calculating the vertical profiles of the primary production of the respective lagoons which were studied on the same day, depths corrections were made as shown in the following example: The Secchi depths (Ds) of the sampling sites Barra, Centro and Boca Río Sevilla were 30, 28 and 27 cm respectively. The incubations were made together at station Barra in 0, 10, 20, 30, 60 and 90 cm. The corresponding depths of the other sites would be: (Ds of study site/Ds of site Barra) multiplied with the incubation depths of site Barra. Thus, the corresponding incubation depths of station Boca Río Sevilla would be 0, 9, 18, 27, 54 and 81 cm. After incubation the vials (not fixed) were brought in a light-tight insulation box to the laboratory and filtered immediately. Due to the high amount of organic and inorganic seston only small aliquots of 3 ml (Ciénaga Grande and lagoons of the Complejo Pajarales) or 5 ml (Complejo Isla Salamanca Occidental) could be filtered through 0.2 µm cellulose acetate membrane filters for determining the overall primary production. For assessing the contribution of different phytoplankton size classes to PP, aliquots of 5 ml were filtered through 2 and 5 µm polycarbonate filters and through disks made from 10 and 20 µm nylon nets. After exposing the filters and disks to HCl fumes they were placed in scintillation vials containing Lumagel. Radioactivity incorporated by photosynthetic organisms was determined in a Packard Tri-Carb-1500 liquid scintillation counter.
Community respiration (CR): Sets of 4 glass flasks of 50 ml (nominal volume) were cautiously filled with sample water. Two of them served to determine the initial oxygen concentration, the remaining two were incubated for 6 hours at in situ-temperature in a light-tight insulation box. Oxygen measurements were made using the Winkler technique. An RQ value of 0.83 was employed to convert O2-units into µg of organic carbon respired by the suspended organisms (Vollenweider, 1974).
RESULTS
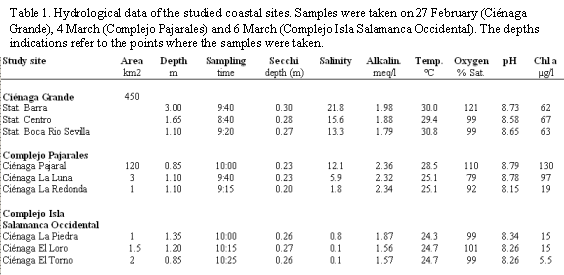
Physical, chemical and meteorological variables. In the following description a distinction will be made between brackish water and freshwater lagoons. As table 1 and figure 2 show, the group of brackish water lagoons consists of Ciénaga Grande with its three sampling sites Barra, Centro and Boca Río Sevilla. Additionally, Ciénaga Pajaral and Ciénaga La Luna fall into this group. The group of freshwater (or almost freshwater) lagoons of the study area includes Ciénaga La Redonda, Ciénaga La Piedra, Ciénaga El Loro, and Ciénaga El Torno.
In table 1 several hydrological variables of the lagoons are listed. The brackish water lagoons showed salinities ranging from 21.8 observed near the inlet of Ciénaga Grande, to 5.9 in Ciénaga La Luna. The "highest" salinity found in the group of freshwater lagoons was 1.8 in Ciénaga La Redonda, whereas the respective values in the other lagoons of this group ranged from 0.1 to 0.8.
The water temperature of the two groups of lagoons did not differ as clearly as did their salinity. Whereas the temperature of the freshwater lagoons was quite uniform ranging from 24.3 to 25.1 oC, the temperature of the brackish water lagoons was somewhat higher with the exception of Ciénaga La Luna. At the three sampling sites of Ciénaga Grande the water temperature was between 29.4 to 30.8 oC. Ciénaga Pajaral showed a temperature relatively similar to Ciénaga Grande (28.5 oC). Ciénaga La Luna, even though belonging to the brackish water lagoons, had a temperature almost identical with the freshwater lagoons (Table 1).
Alkalinity ranged from 1.56 to 2.36 meq/l. The lowest values were observed in those freshwater lagoons which were most bly subjected to riverine influences. The highest alkalinity was found in the three lagoons of the Complejo Pajarales (Table 1). Here obviously the type of the surrounding soil influences the alkalinity. The pH was related to the trophic status of the systems, since "low" values (8.15 - 8.34) were observed in the lagoons with relatively low chl a concentrations and "high" pH values (8.58 - 8.79) in the lagoons with high amounts of chl a.
Secchi depths of the study sites were very small and ranged only from 20 to 30 cm (Table 1) thus indicating an extremely high turbidity of the water. The greatest Secchi depth and hence lowest turbidity was found at station Barra near the connection of Ciénaga Grande with the Caribbean Sea. It shows a certain, albeit reduced influence of the clear Caribbean water that enters during high tide.
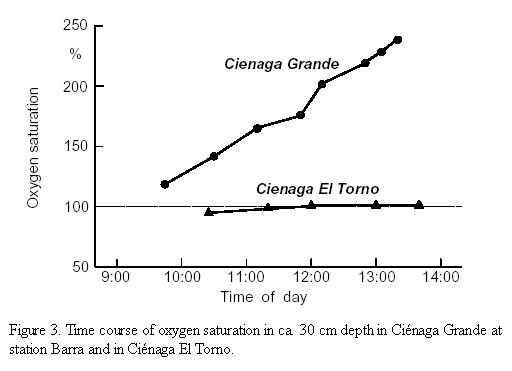
Oxygen saturation of the upper part of the water columns of the study sites is depicted in Table1. The four freshwater lagoons had an O2 content near the saturation value (92 - 101%), whereas the brackish water lagoons showed a much broader range with values from considerably below (79% in Ciénaga La Luna) to values much above the saturation value (121% at station Barra in Ciénaga Grande). The time course of oxygen saturation was followed over about three hours at station Barra (brackish water) and in Ciénaga El Torno (freshwater). In figure 3 it is shown that at station Barra the O2 saturation rose almost linearly from 121% at 9:45 to 238% at 13:20. In Ciénaga El Torno at 10:25 an oxygen saturation of 94% was measured. At 12:00 the saturation reached 101% and remained there to the end of the observation at 13:40.
The study took place on cloudless days with bright sunshine. The photosynthetic available radiation (PAR) of the three days was almost the same. It ranged from 45.48 to 47.77 x 106 µE/m2/d. Maximum flux values of 2000 µE/m2/s were observed during noon. The incubation periods for the primary production measurements lasted for 3 h more or less symmetrically around noon. Between 41.0 and 43.6% of the daily radiation fell into the incubation periods.
Phytoplankton composition. Microscopic analysis of the phytoplankton revealed that in Ciénaga Grande cyanobacteria were the dominant fraction of planktonic algae with respect to cell numbers. In the lagoons of the Complejo Pajarales diatoms became more important at the expense of cyanobacteria, and in the lagoons of the Complejo Isla Salamanca Occidental only diatoms were found. It should be mentioned, however, that additionally high numbers of minute algae belonging to the size class of picophytoplankton were detected which, due to their reduced size and lack of microscopic characteristics, could not be identified microscopically.
Chlorophyll a and primary production. Regarding chlorophyll a concentration, the two groups of lagoons differed considerably. In the brackish water lagoons the amounts of chl a ranged from 62 to 130 µg/l, whereas in the freshwater lagoons it amounted to values between 5.5 and 19 µg/l (Table 1 and Figure 2).
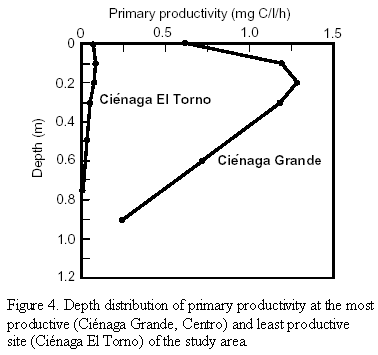
Figure 4 shows the magnitude and depth distribution of primary productivity at the most and least productive study sites. Maximum primary productivity was found mostly at the second incubation depth (10 cm), with the exception of the sites Centro and Boca Río Sevilla of Ciénaga Grande (20 cm depth). Compared with maximum PP, surface PP was on average 47% lower at the sites in Ciénaga Grande and 6% lower in the lagoons of the Complejo Pajarales and the Complejo Isla Salamanca Occidental. On the other hand, a measurable primary production occurred still down to the lowest incubation depths. On average it amounted to 13% of the maximum value for the Ciénaga Grande sites, 2% for the lagoons of the Pajarales Complex and 13% for those of the Complejo Isla Salamanca Occidental.
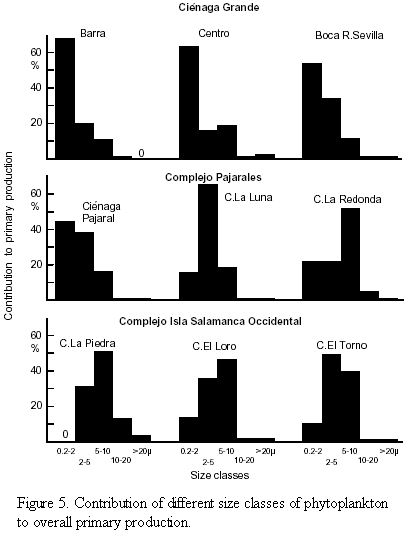
The size spectrum of photosynthetically active phytoplankton is presented in figure 5. In Ciénaga Grande and Ciénaga Pajaral the smallest size fraction (0.2-2 µm) was most important and contributed from 44 to 68% to overall primary production, whereas the contribution of this size class in the freshwater lagoons was much smaller and amounted from 0 to 22% only. In Ciénaga La Luna which belongs to the brackish water lagoons the size class of 2 - 5 µm was most important for primary production. In none of the study sites did the largest size class of phytoplankton (>20 µm) play any considerable role. The weighted mean size of the photosynthetic phytoplankton in the brackish water lagoons ranged from 2.51 to 4.06 µm and averaged 3.23 µm. In the freshwater lagoons the weighted mean ranged from 5.24 to 8.32 µm and averaged 6.3 µm.
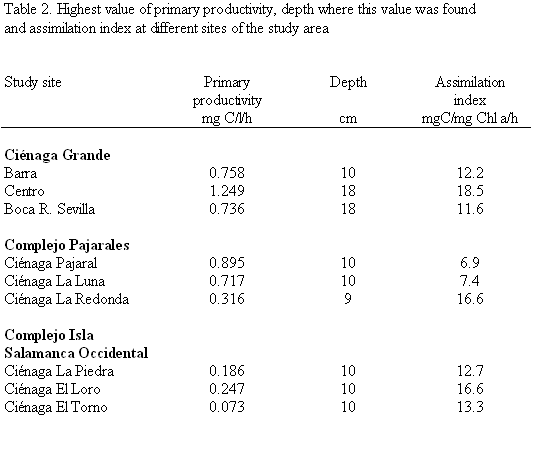
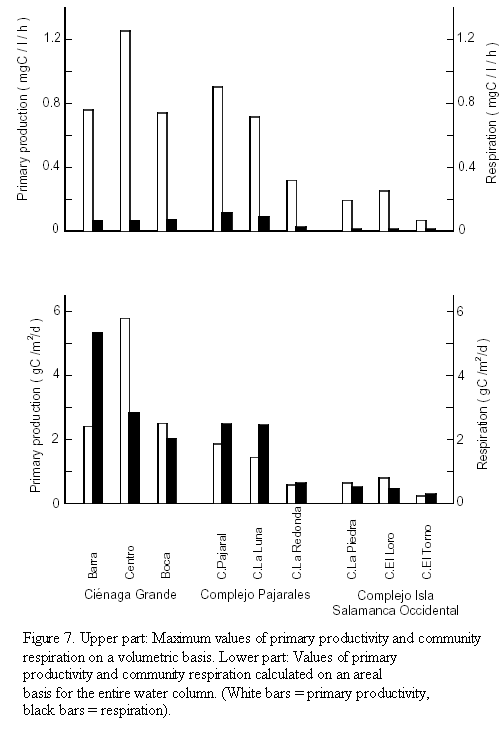
Maximum values of primary productivity per volume ranged from 0.72 to 1.25 mg C/l/h in the brackish water lagoons and from 0.073 to 0.32 mg C/l/h in the freshwater lagoons (Figure7, upper part). The lower part of figure 7 shows the depth-integrated primary productivity per unit area of the studied lagoons. Values ranged from 1.40 to 5.76 g C/m2/d in the brackish water lagoons and from 0.24 to 0.80 g C/m2/d in the freshwater lagoons. The assimilation index, which is the amount of carbon produced per unit chlorophyll a and unit time, ranged from 6.9 to 18.5 mg C/mg chl a/h in the brackish water and from 12.7 to 16.6 mg C/mg chl a/h in the freshwater lagoons (Table 2).
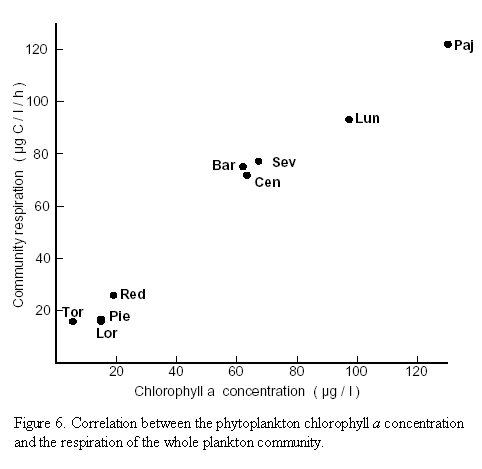
Community respiration. As can be deduced from figure 6 a highly significant correlation (p = 0.001) between chl a concentration and community respiration was observed in the brackish lagoons, whereas no significant correlation existed in the freshwater lagoons. Figure 7 (upper part) shows the community respiration rates (CR) per unit volume. The rates ranged from 0.072 to 0.122 mg C/l/h in the brackish water lagoons and from 0.016 to 0.026 mg C/l/h in the freshwater lagoons. On an areal basis (lower part of figure 7) the depth-integrated CR of the entire water column ranged from 2.07 to 5.37 g C/m2/d in the group of brackish water lagoons. In this group at sites Barra in Ciénaga Grande as well as in Ciénaga Pajaral and Ciénaga La Luna the amount of organic material which was respired (decomposed) in the water column, surpassed the organic material produced. In the group of freshwater lagoons community respiration per unit area ranged from 0.31 to 0.69 g C/m2/d. In this group in Ciénaga La Redonda and in Ciénaga El Torno slightly more organic material was decomposed than produced (Figure 7).
DISCUSSION
Generally, the primary productivity of freshwater lakes and coastal lagoons increases from higher to lower latitudes. A series of factors is responsible for this fact, the most important being the higher and more evenly distributed light energy throughout the year. The light conditions permit a longer duration of the productive phase which in the tropics lasts throughout the whole year. Superimposed on the increase of PP towards lower latitudes several factors may exist which can mask this general trend. Striking examples of a different trophic status between neighbouring aquatic systems are the coastal lagoons of our study area, the outer delta of the Magdalena River.
Already a short visit of the study area gives the impression that the brackish coastal lagoons Ciénaga Grande, Ciénaga Pajaral and Ciénaga La Luna are very productive aquatic systems, whereas the freshwater lagoons Ciénaga La Redonda, Ciénaga La Piedra, Ciénaga El Loro and Ciénaga El Torno are much less so. This assumption originates on the one hand from the very turbid waters with their greenish colour caused by high concentrations of phytoplankton and detritus in the brackish lagoons and on the other hand from the yellowish waters containing clay and silt in the freshwater lagoons. A further good indication for the anticipated differences in productivity is the time course of the dissolved oxygen concentration. At station Barra in Ciénaga Grande O2 supersaturation in the near surface water rose up to 238% at 13:20. From the almost linear increase of the curve up to this hour (Figure 3) a further increase of supersaturation could be expected later in the afternoon. This will probably happen to a certain degree, but much higher values are surely prevented by the onset of the afternoon breeze which mixes the water column and facilitates the outgasing of oxygen from of the water. Gocke et al. (1990) observed similar high supersaturations in the very productive coastal waters of Costa Rica during the formation of a dense red tide bloom. Melack and Kilham (1974) found even much higher saturation values (up to 340%) in extremely productive Lake Nakuru in Eastern Africa. On the other hand, in the freshwater lagoon Ciénaga El Torno oxygen concentration and hence percentage saturation changed only slightly during the observation period. We may deduce from the shape of the curve that the O2 concentration remains near the 100% saturation level during the day and probably also during the night. This indicates a relatively low intensity of oxygen liberating (hence primary productivity) and oxygen consuming (hence respiration) processes. Otherwise a larger day-night variation would have occurred.
Our primary production measurements corroborated this first impression of the trophic status of both types of lagoons within the DERM. Primary productivity in the very productive brackish lagoons ranged between 0.72 and 1.25 mg C/l/h and the depths where these high values were found lay between 10 - 20 cm. Severe light inhibition occurred at the water surface (Figure 4) which is to be expected considering the high light intensity of up to 2000 µE/m2/s measured during the incubation time. On the other hand, a rapid decrease of PP values below 20 cm indicates that light limitation occurs already almost directly below the water surface. Thus, the productive zone is limited to a thin layer of only about one meter thickness. The main reason for this is selfshading by the large amount of phytoplankton itself. In the freshwater lagoons the productive layer too was restricted to less than the uppermost meter. The highest values of PP within this layer (always found at 10 cm depth) ranged between 0.037 and 0.32 mg C/l/h. As in the brackish lagoons, an inhibition of PP occurs also at the surface of the freshwater lagoons due to high incident radiation, but the degree of inhibition seems to be much less than in the brackish ones. The reason for this may have resulted from an inadequate resolution of the primary productivity rates in the upper 10 - 20 cm. In general, even if the total light intensity in the uppermost meter of the highly productive brackish and the low productive freshwater lagoons may be roughly comparable, the light quality is surely very different. In the first group the light is reduced due to a more or less specific absorption mainly by phytoplankton pigments (selfshading). In the second group light scattering by high amounts of suspended fine inorganic sediment particles is the most important reason for the rapid light reduction.
Daily primary production of the brackish lagoons on an areal basis amounted to 1.40 5.76 g C/m2. The PP value of 5.76 g C/m2/d found in the central part of Ciénaga Grande is very similar to the value obtained in 1987 by Hernández and Gocke (1990) for the same location and time of year. These authors estimated an annual gross primary production of 1690 g C/m2 which makes this coastal lagoon one of the most productive coastal systems of the world (Knoppers, 1993). The high areal PP of 5.76 g C/m2/d is probably representative of the largest part of Ciénaga Grande. Deviations from this value such as those observed at sites Barra and Boca Río Sevilla are probably restricted to smaller "subregions". Lower PP values near the inlet of Ciénaga Grande were also found by Hernández and Gocke (1990) especially during the dry season. They were due to a certain degree of "dilution" of the lagoon water with clear Caribbean water with low sediment and plankton loads which affects this part of Ciénaga Grande during high tide. PP of the third sampling site near the mouth of Río Sevilla was quite similar to the central site during the annual survey of the above-mentioned authors. The large deviation encountered by us in the present study is difficult to explain, especially since the chl a concentrations of the three sampling sites were almost identical (Table 1) and the incubations were made under the same conditions (see under Methods). It may be due to a different composition of the phytoplankton, although the composition of the identifiable phytoplankton does not seem to corroborate this assumption. It may also reflect a better nutrient supply in the central part of the lagoon, which enabled a higher assimilation index to be reached (Table 2). The areal PP of the two other lagoons of the brackish group (Ciénaga Pajaral and Ciénaga La Luna) is comparable to sampling sites Barra and Boca Río Sevilla of Ciénaga Grande, whereas the areal PP of the freshwater lagoons ranging from 0.24 to 0.80 g C/m2/d was much lower. The latter is probably due to the special light conditions in these systems.
Yearly oscillations of PP are very much dampened in the tropics. According to Hernández and Gocke (1990) the lowest PP observed during their annual study in 1987 in the central part of Ciénaga Grande, was found in March and amounted to 2.7 g C/m2/d, whereas the highest value was measured in October and amounted to 8.8 g C/m2/d. Thus, the total range was only slightly larger than a factor of three. About the same annual variation between the least and most productive months was observed by Gocke et al. (2001) in the Golfo de Nicoya (Pacific coast of Costa Rica) and by Smayda (1966) in the Gulf of Panama. On the other hand, seasonal variations in temperate aquatic systems may run up to two orders of magnitude as shown e.g. by Moigis (1983) in the Kiel Fjord, Germany. The highest daily rates of PP in tropical coastal lagoons probably do not surpass significantly those in comparable aquatic systems in the temperate zone. It is especially the relative constancy of high rates throughout the year which is responsible for the high annual primary production of the tropical coastal lagoons.
In addition, the b radiation in the tropics causes high water temperatures which allows a higher Pmax (assimilation index) to be reached per unit of chlorophyll a. Harrison and Platt (1980) conclude that temperature accounted for 40% of the observed variation in the assimilation index of coastal marine phytoplankton. A thorough literature survey by Vedernikov (1985) revealed a total range of 0.1 - 35.0 mg C/mg chl a/h for natural populations of phytoplankton, usually, however, the values were from 0.5 - 15.0 mg C/mg chl a/h. Lalli and Parsons (1997) cite literature data which indicate an assimilation index of 9 - 17 mg C/mg chl a/h for high nutrient and high temperature regions such as tropical coastal lagoons. Our findings with values between 11.6 and 18.5 mg C/mg chl a/h for the three sampling sites of Ciénaga Grande and between 12.7 and 16.6 mg C/mg chl a/h for the freshwater lagoons (Table 2) coincide nicely with the literature data of the latter authors. The relatively low AI (6.9 and 7.4 mg C/mg chl a/h) encountered in Ciénaga Pajaral and Ciénaga La Luna, where very high PP rates and chl a concentrations were found, are difficult to explain. They may be due to senescent algae during the phase of an already declining phytoplankton bloom.
A further reason which adds to explain the high primary productivity of the brackish lagoons of the study area is the fact that smaller size classes of algae are especially active in relation to their size. A striking example is given by Pollinger and Berman (1982), who found in Lake Kinneret, Israel, that the nanoalgae were ten-fold more active in terms of C-assimilation per unit biomass than the dominant large dinoflagellates which they were accompanying. In the present study the overwhelming importance of the smallest size class of 0.2 - 2.0 µm which alone was responsible for 44 - 68% of total primary production in the brackish lagoons (with the exception of Ciénaga La Luna) is clearly depicted (Figure 5). In the freshwater lagoons the smallest algae became less important in favour of the 2-5 and 5-10 µm size classes. However, even if the weighted mean size of the algae with respect to primary production is about twice as large in the freshwater lagoons than in the brackish ones (6.30 compared to 3.23 µm), it is obvious that the fraction of pico and nanoalgae are the dominant primary producers in both groups of lagoons. In none of the lagoons the microalgae (>20 µm) played a significant role. The highest share of these larger algae was found in Ciénaga La Piedra and even here they contributed to only 3% of total PP.
The overwhelming importance of pico and nanoalgae in all coastal lagoons of the DERM (especially in the highly eutrophic and hyperproductive brackish systems) contrasts with the results of Iriarte and Purdie (1994) obtained in the temperate marine environments of southern England where the importance of the smallest algae diminished from offshore to estuarine waters. Similar observations were made by Owens et al. (1993) in a transect from the oligotrophic gyre in the central northern Indian Ocean to the highly productive upwelling region off Oman. Studies off Brazil by Teixeira and Gaeta (1991) also indicated that the importance of the smallest size fraction (0.45 - 1 µm) was less in estuaries than in oceanic regions. Although the many results reported in the literature are somewhat difficult to compare due to the lack of coincidence between the size classes, it is obvious that usually the contribution of the smallest algae of the phytoplankton to the overall primary production diminishes with increasing eutrophication. In this respect the lagoons of the DERM (especially the brackish ones) behave completely the other way round.
The highly significant correlation between chl a concentration and community respiration in the brackish water lagoons and the lack of a significant correlation in the freshwater lagoons indicates that, at high concentrations of chl a, the phytoplankton dark respiration is the dominant component of the community respiration (Figure 6). At low chl a concentrations phytoplankton respiration is less important because the respiration of heterotrophic microorganisms (bacteria and zooplankton) contribute proportionately more to community respiration. Similar observations were made by Iriarte et al. (1991) in marine coastal waters. Since in the freshwater lagoons of the DERM relatively low chl a concentrations paralleled high amounts of inorganic turbidity it is likely that many of the microheterotrophs were associated with fine sediment particles introduced by the Río Magdalena.
The amount of organic material respired per unit volume was quite small compared to the amount produced (Figure 7). It ranged from 4.9% (Ciénaga La Redonda) to 21.9% (Ciénaga El Torno) with a mean value of 10.6% if only the daytime and the depth of the highest PP rates are considered. Thus, during daytime a large excess of organic material is produced at least in the upper part of the euphotic layer. If, however, the whole water column (which is aphotic below around 1 m) and night time CR too are taken into consideration, mass balance calculations suggest that, in some lagoons or stations, more organic material is degraded than produced. The high areal respiration of 5.4 g C/m2/d at station Barra may be somewhat misleading. It is easily explained by the exceptionally "large" depth (3 m) of this sampling site. At "normal" depths in the vicinity of station Barra the amount of organic material respired is probably much less than the produced one. Thus, in the whole, Ciénaga Grande is an autotrophic system in which - as stated already by Hernández and Gocke (1990) - more organic material is produced than decomposed. The results are in line with the classical outwelling hypothesis which states that coastal embayments like estuaries and coastal lagoons usually act as a source of organic carbon for the adjacent coastal areas (Winter et al., 1996).
In Ciénaga Pajaral and Ciénaga La Luna the respiration rates per unit volume were much higher than in the Ciénaga Grande (Figure 7). This was paralleled by higher amounts of phytoplankton as indicated by higher concentrations of chl a (Table 1). As already stated, the assimilation index in both systems was relatively low (Table 2) probably indicating a senescent phytoplankton community. This lead to a comparably low primary production which in turn resulted in a situation where respiration in the whole water column surpassed depth-integrated primary production. Hence, Ciénaga Pajaral and Ciénaga La Luna were heterotrophic at the moment of the study, but this situation is probably restricted to short time intervals. Ciénaga La Redonda and Ciénaga El Torno were also heterotrophic, supply and demand of organic substances, however, were almost balanced. The demand of organic material in excess over the produced material must be compensated from allochthonous sources, such as the mangrove fringes present in all of the lagoons and the input of dissolved and particulate organic material by the Río Magdalena into the freshwater lagoons.
The question, whether the primary productivity of Ciénaga Grande has changed during the last 10 - 20 years or not, is difficult to answer. (About the other lagoons no statements can be made since previous data are not established yet). Mancera et al. (in prep.) stated that the concentrations of dissolved inorganic nitrogen and orthophosphate increased in the central part of Ciénaga Grande. It should be pointed out that in this aquatic system the inorganic fraction of nutrients is small compared to their total concentrations (Hernández and Gocke,1990). Instead of the magnitude of the inorganic fraction the high dynamics between uptake and release processes are more decisive for the high PP rates. Mancera et al. (in prep.) also report an increase in chlorophyll a concentration in Ciénaga Grande. The few measurements of PP rates made during the present study indicated almost exactly the same rate for the central part but significantly lower ones for sampling sites Barra and Boca Río Sevilla as were found by Hernández and Gocke (1990) at the end of February 1987. At the beginning of March in 1987 a drastic shift of primary productivity occurred. The rates decreased rapidly to about half of their earlier values and remained at this level till about June. Taking into consideration this fast change of PP we hesitate to draw conclusions. If at the moment of our study the lagoons were still in the phase of high PP, our values would indicate no change in the center and a decrease in the peripheral parts. If it were already in the low PP phase our data would indicate an increase in primary productivity. More measurements are urgently needed since primary production governs the magnitude of all processes which depend on the organic material delivered directly or indirectly by the photosyntheticaly active organisms.
ACKNOWLEDGEMENTS
This study was supported by a grant to the first author from a bilateral scientific interchange program between COLCIENCIAS (Colombia) and DAAD (German Academic Exchange Service). The field trips were financed by the Instituto de Investigaciones Marinas and Costeras (INVEMAR) in Santa Marta, Colombia. We thank the director, the scientific and technical board of the INVEMAR for constant encouragement during the study. The students Adriana Garavito and Liliana González are gratefully acknowledged for field and laboratory assistance. We also thank the reviewers for their most valuable hints to improve our manuscript. Last, but not least, we are especially indebted to Sr. Martin Montaño for his most skilful aid as boat helmsman during the field trips.
REFERENCES
1 Botero, L. and J. E. Mancera. 1996. Síntesis de los cambios de origen antrópico en los últimos años en la Ciénaga Grande de Santa Marta (Colombia). Rev. Acad. Colom. Cienc. Vol. 20, No. 78: 465-474. [ Links ]
2 Botero, L. and H. Salzwedel. 1999. Rehabilitation of the Ciénaga Grande de Santa Marta, a mangrove-estuarine system in the Caribbean coast of Colombia. Ocean & Coastal Management 42: 243-256. [ Links ]
3 Gaarder, T. and H. H. Gran. 1927. Investigations of the production of plankton in the Oslo Fjord. Rapp. et Proc. Verb., Cons. Internat. Explor. Mer 42: 1-48. [ Links ]
4 Gocke, K., J. Cortés and C. Villalobos. 1990. Effect of red tides on oxygen concentration and distribution in the Golfo de Nicoya, Costa Rica. Rev. Biol. Trop. 38: 401-407. [ Links ]
5 Gocke, K., J. Cortés and M.M. Murillo. 2001. The annual cycle of primary productivity in a tropical estuary: The inner regions of the Golfo de Nicoya, Costa Rica. Rev. Biol. Trop. (Suppl. 2): 298-306. [ Links ]
6 Harrison, W. G. and T. Platt. 1980. Variations in assimilation number of coastal marine phytoplankton: effects of environmental co-variates. J. Plankton Res. 2: 249-260. [ Links ]
7 Hernández, C. 1986. Producción primaria and dinámica del fitoplankton en la Ciénaga Grande de Santa Marta, Colombia. Tesis de Maestría, Universidad Nacional de Colombia, Bogotá, Colombia, 177 p. [ Links ]
8 Hernández, C. A. and K. Gocke.1990. Productividad primaria en la Ciénaga Grande de Santa Marta, Colombia. An. Inst. Invest. Mar. Punta de Betin 19-20: 101-119. [ Links ]
9 Hoppe, H.-G., K. Gocke, D. Zamorano and R. Zimmermann. 1983. Degradation of macromolecular organic compounds in a tropical lagoon (Ciénaga Grande, Colombia) and its ecological significance. Int. Rev. Ges. Hydrob. 68: 811-824. [ Links ]
10 IGAC. 1973. Monografía del Departamento del Magdalena. Instituto Geográfico Agustin Codazzi, Bogotá, 164 p. [ Links ]
11 Iriarte, A., G. Daneri, V. M. T. Garcia, D. A. Purdie and D. W. Crawford. 1991. Relations entre chlorophyll a et taux de respiration du plancton dans les eaux littorales. Oceanol. Acta 14: 379-388. [ Links ]
12 Iriarte, A. and D. A. Purdie. 1994. Size distribution of chlorophyll a, biomass and primary production in a temperate estuary (Southampton Water): The contribution of photosynthetic picoplankton. Mar. Ecol. Prog. Ser. 115: 283 - 297. [ Links ]
13 Knoppers, B.1993. Aquatic primary production in coastal lagoons. p. 219-260.In: B. Kjerfve ( Ed.). Coastal Lagoon Processes. Elsevier Science Publisher B. V. [ Links ]
14 Lalli, C. M. and T. R. Parsons. 1997. Biological oceanography. An introduction. 2nd edit. Butterworth-Heinemann, Oxford, 314 p. [ Links ]
15 Mancera Pineda, J. E., O. L. Baena Parra and J. C. Diez Grisales. 1996. Referencias bibliográficas publicadas e ineditadas de la Ciénaga Grande de Santa Marta, Caribe Colombiano. Ciencias naturales, Vol 1, 1964 - 1995. INVEMAR - CORPAMAG - GTZ, Santa Marta, Colombia. [ Links ]
16 Mancera Pineda, J. E. and A. Vidal.1994. Florescimiento de microalgas relacionado con la muerte masiva de peces en el complejo lagunar Ciénaga Grande de Santa Marta, Caribe Colombiano. An. Inst. Invest. Mar. Punta de Betín 23: 103-117. [ Links ]
17 Mancera, J.E., R.R. Twilley, R. Giraldo and W. Troncoso. (in prep.) Trends in the water quality of a tropical estuarine ecosystem along the Caribbean coast of Colombia: Ciénaga Grande de Santa Marta. Submitted to Wetlands Ecology and Management. [ Links ]
18 Mantoura, R. F. C. and C. A. Llewellyn. 1983. The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reverse-phase high-performance liquid chromatography. Analyt. Chim. Acta 151: 297-314. [ Links ]
19 Melack, J. M. and K. Kilham. 1974. Photosynthetic rates of phytoplankton in East African alkaline, saline lakes. Limnol. Oceanogr. 19: 743-755. [ Links ]
20 Moigis, A. G. 1983. Zur Grössenstruktur und Ökologie des Phytoplanktons in der Kieler Förde unter dem besonderen Aspekt einer möglichen Ölverschmutzung. Tesis Doct., Kiel, Germany, 171 p. [ Links ]
21 Owens, N. J. P., P. H. Burkill, R. F. C. Mantoura, E. M. S. Woodward, I. E. Bellan and J. Aiken. 1993. Size fractionated primary production and nitrogen assimilation in the northwestern Indian Ocean. Deep Sea Res. 40: 697-709. [ Links ]
22 Palacio, J. A. 1983. Die benthische Makroinvertebratenfauna der tropischen Ästuarregion Ciénaga Grande de Santa Marta (Kolumbien) und ihre Aktivität im Wechsel zwischen Trocken- und Regenzeit. Tesis de Doctorado, Ruhr-Universität Bochum,Germany. 248 p. [ Links ]
23 Parsons, T. R., M. Takahashi and B. Hargrave. 1984. Biologic oceanographic processes. 3rd edit. Pergamon Press, London, 330 p. [ Links ]
24 Polanía, J., A. Santos-Martínez, J. E. Mancera-Pineda and L. Botero Arboleda. 2001. The coastal lagoon Ciénaga Grande de Santa Marta. In : U. Seeliger and B. Kjerfve (eds.): Coastal marine ecosystems of Latin America. Springer Verlag Berlin Heidelberg. [ Links ]
25 Pollingher, U. and T. Berman. 1982. Relative contributions of net and nanno phytoplankton to primary production in Lake Kinneret (Israel). Arch. Hydrobiol. 96: 33 - 46. [ Links ]
26 Sánchez, C. E., F. Rodriguez, E. Collante and O. Simbaqueva. 1993. Atlas de radiación solar de Colombia. INEA, IDEAM, HIMAT, Bogotá, Colombia. 85 p. and anexos. [ Links ]
27 Smayda T. J. 1966. A quantitative analysis of the phytoplankton of the Gulf of Panama. III. General ecological conditions and the phytoplankton dynamics at 8o 45'N, 79o 23'W from November 1954 to May 1957. Bull. Inter-Am. Trop. Tuna Comm. 11: 353-612. [ Links ]
28 Steemann-Nielsen, E. 1952. The use of radioactive carbon (14C ) for measuring organic production in the sea. J. Cons. Perm. Int. Explor. Mer 18: 117-140. [ Links ]
29 Teixeira, C. and S. A. Gaeta. 1991. Contribution of picoplankton to primary production in estuarine, coastal and equatorial waters of Brazil. Hydrobiologia 209: 117-122. [ Links ]
30 Thom, B. G. 1982. Mangrove ecology - a geomorphological perspective. In : B. F. Clough (Ed.): Mangrove ecosystems in Australia. Australian National University Press, Canberra. [ Links ]
31 Vedernikov, V. I. 1985. The assimilation number and its variations in cultures and natural populations of marine phytoplankton. Can. Trans. Fish. Aquat. Sci. No. 5112, 35 p. [ Links ]
32 Vidal, L.A. 1995. Estudio del fitoplancton en el sistema lagunar estuarino tropical Ciénaga Grande de Santa Marta, Colombia, durante el año 1987. Tesis M. Sc. Univ. Nacional de Colombia, 207 p., 62 laminas. [ Links ]
33 Vollenweider, R. A. 1974. Methods for measuring production rates. In : R. A. Vollenweider (Ed.): Primary production in aquatic environments. IBP Handbook No 12, Blackwell Scientific Publications, Oxford, London. [ Links ]
34 Wetzel, R. G. and G. E. Likens. 1991. Limnological Analyses. Springer Verlag, New York, 391 p. [ Links ]
35 Wiedemann, H. U. 1973. Reconnaissance of the Ciénaga Grande de Santa Marta, Colombia: physical parameters and geological history. Mitt. Inst. Colombo-Alemán Invest. Cient. Santa Marta, Invemar 7: 85-119. [ Links ]
36 Winter, P. E. D., T. A. Schlachter and D. Baird. 1996. Carbon flux between an estuary and the ocean: A case of outwelling. Hydrobiologia 337: 123- 132. [ Links ]
37 Yacubson, S. 1969. Algas de ambientes acuáticos continentales nuevas para Venezuela (Cyanophyta, Chlorophyta). Bol. Cent. Invest. Biol. Univ. Zulia. Venezuela. 3, 87 p., 18 laminas. [ Links ]
38 Yacubson, S. 1972. Catálogo e iconografia de las Cyanophyta de Venezuela. Bol. Cent. Invest. Biol., Univ. Zulia. Venezuela. 5, 78 p., 17 laminas. [ Links ]
39 Yacubson, S. 1974. Catálogo e iconografia de las Cyanophyta de Venezuela. Bol. Cent. Invest. Biol., Univ. Zulia. Venezuela. 11, 143 p., 31 laminas. [ Links ]
DATE RECEIVED: 13/12/01 DATE ACCEPTED: 11/03/03