Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.32 no.1 Santa Marta Jan./Dec. 2003
PHYTOPLANKTON COMPOSITION IN COASTAL LAGOONS OF DIFFERENT TROPHIC STATUS IN NORTHERN COLOMBIA DETERMINED BY MICROSCOPE AND HPLC-PIGMENT ANALYSIS
DETERMINACION DE LA COMPOSICION DEL FITOPLANCTON EN LAGUNAS COSTERAS DE DIFERENTES ESTADOS TROFICOS EN EL NORTE DE COLOMBIA; A TRAVES DE ANÁLISIS MICROSCOPICOS Y ANÁLISIS DE PIGMENTOS POR HPLC
Klaus Gocke1, Michael Meyerhöfer1, José Ernesto Mancera-Pineda2 and Luis Alfonso Vidal3
1Institut fuer Meereskunde, D-24105 Kiel. Fax: +49-431-6001515 (K. G. and M. M.) e-mail: kgocke@ifm.uni-kiel.de.
2Department of Biology, University of Louisiana at Lafayette, USA. P.O. Box: 41297 Lafyette, LA 70504 - USA. (J. E. M. P.) e-mail: emancera@louisiana.edu.
3Mundo Marino, Universidad Jorge Tadeo Losano. Santa Marta, Colombia. (L. A. V.) e-mail: lavidalve@yahoo.com
ABSTRACT
The regional differences in the composition of the phytoplankton assemblages in several coastal lagoons of the "Outer Delta of the Río Magdalena" (Caribbean coast of Colombia) were determined using microscopic counting and HPLC-pigment measurements. The study sites can be classified as 1) a group of limnic lagoons with relatively low (5.5-19 µg/l) chlorophyll a (chl a)concentrations and high inorganic suspension loading, 2) a group of brackish lagoons with high (62-90 µg/l) chl a concentrations and high amounts of organic seston, (two further lagoons form a transition between group 1 and 2, and 3) a fully marine Caribbean bay with very low (0.3 µg/l) chl a and seston concentrations. The regional variations in salinity of the lagoons are due to inflow of water from the Río Magdalena or to inflow of Caribbean Sea water. All lagoons are very shallow (less than 2 m) with small Secchi depths (20 to 30 cm). With the exception of the eutrophic brackish lagoons, where both methods indicated cyanobacteria to be the prevalent algal group, great discrepancies were encountered in all other study sites between the compositions of the algal communities obtained with both approaches. According to the microscopic analysis the phytoplankton of the marine Caribbean bay and the limnic and transient coastal lagoons consisted mainly (in several cases exclusively) of diatoms. The pigment analysis, on the other hand, indicated that cyanobacteria were the dominant algal group in all coastal lagoons, their percentage increased from the limnic over the transient to the brackish lagoons. Fractionated filtration of 14C-labelled phytoplankton revealed that algae of <5µm size in the coastal lagoons contributed 32-88% of the total radioactivity and that algae of >20µm size played only an insignificant role in primary production. Only in the Caribbean bay 14% of the 14CO2 was taken up by organisms >20µm. We assume that the small forms are greatly underrepresented in microscopic observations due to extreme difficulties in identification and counting caused by detritus and/or sediment loadings of the samples. We therefore believe that these small forms (probably mostly picoplanktonic cyanobacteria) are better represented by pigment analysis.
KEY WORDS: Phytoplankton, Pigment analysis, Trophic status, Coastal lagoons, Caribbean coast.
RESUMEN
Las diferencias regionales en la composición de las agregaciones de fitoplancton en varias lagunas costeras del Delta Exterior del Río Magdalena (costa caribeña colombiana) fueron determinadas utilizando el conteo microscópico y mediciones de pigmentos por HPLC. Los sitios de estudio pueden ser clasificados como 1) un grupo de lagunas límnicas con concentraciones de chl a relativamente bajas (5.5-19 µg/l) y una alta carga de materia inorgánica, 2) un grupo de lagunas salobres con una alta concentración de clorofila a (chl a) (62-90 µg/l) y una gran cantidad de seston orgánico, (dos lagunas en transición entre el grupo 1 y 2, y 3) una bahía completamente marina con una muy baja concentración de chl a (0.3 µg/l) y de seston. Las variaciones regionales en la salinidad de las lagunas se deben a la incursión de agua proveniente del Río Magdalena o a la entrada de agua de mar desde el Caribe. Todas las lagunas son de baja profundidad (menos de 2 m) con profundidades de disco Secchi pequeñas (20 a 30 cm). Con excepción de las lagunas salobres eutróficas, en donde ambos métodos mostraron a las cianobacterias como el grupo algal prevalente, se encontraron grandes discrepancias en la composición de las comunidades algales según el método aplicado.De acuerdo con el análisis microscópico, el fitoplancton de la bahía marina y el de las lagunas límnicas y transientes estuvo constituído principalmente (en varios casos exclusivamente) de diatomeas. Por otra parte, el análisis de pigmento indicó a las cianobacterias como el grupo algal dominante en todas las lagunas costeras, con un incremento porcentual desde las lagunas límnicas a través de las en transición hasta las lagunas salobres.La filtración fraccionada de fitoplancton marcado con 14C reveló que las algas con tamaño <5 µm en las lagunas costeras contribuyeron entre un 32-88% al total de radioactividad y las algas con tamaño >20 µm jugaron un rol insignificante en la producción primaria. Solamente en la bahía del mar Caribe el 14% del 14CO2 fue asimilado por organismos >20 µm. Asumimos que los organismos pequeños están poco representados en las observaciones microscópicas debido a las grandes dificultades en su conteo e identificación de, debido al detritus y/o sedimento presente en las muestras. Por esto, creemos que estos pequeños organismos (probablemente cianobacterias picoplanctónicas) son representadas de una mejor manera a través del análisis de pigmentos.
PALABRAS CLAVE: Fitoplancton, Análisis de pigmentos, Estado trofico, Lagunas costeras, Costa Caribe.
INTRODUCTION
Biomass concentration and species composition of phytoplankton are important parameters in aquatic ecology. Identification and counting of planktonic algae are difficult, time consuming and expensive tasks requiring the collaboration of skilled specialists. This is the case especially when minute algae make up an important percentage of the phytoplankton. Stockner (1988) states that this fraction of the algal community (the so-called algal picoplankton) can be responsible for up to 80 - 90% of the total carbon production. In many cases, however, identification down to the species level is not necessary; instead, knowledge of the proportion of different algal classes is sufficient. Such information can be gained via chemical analysis of special compounds of the organisms. A current technique is the analysis of photosynthetic and photoprotective pigments using HPLC (High Performance Liquid Chromatography). It has been shown that some pigments, the so-called "marker pigments" (or "signature pigments"), are restricted to one or only two classes of planktonic algae. Thus, their presence can be used for the identification of phytoplankton classes. Furthermore, special algorithms have been developed which do not only permit the qualitative analysis of the phytoplankton but are aimed to allow also the determination of their quantitative composition (Gieskes and Kraay, 1986; Letelier et al., 1993; Mackey et al., 1996).
The present study was undertaken to determine the phytoplankton composition of several coastal aquatic systems in northern Colombia via microscopic analysis and by HPLC pigment measurements. These systems differ widely with respect to salinity, trophic status, detritus and/or sediment loading. The latter made the microscopic observations extremely difficult and it was therefore hoped that the pigment determinations would be a more feasible way for qualitative and quantitative phytoplankton studies.
STUDY AREA
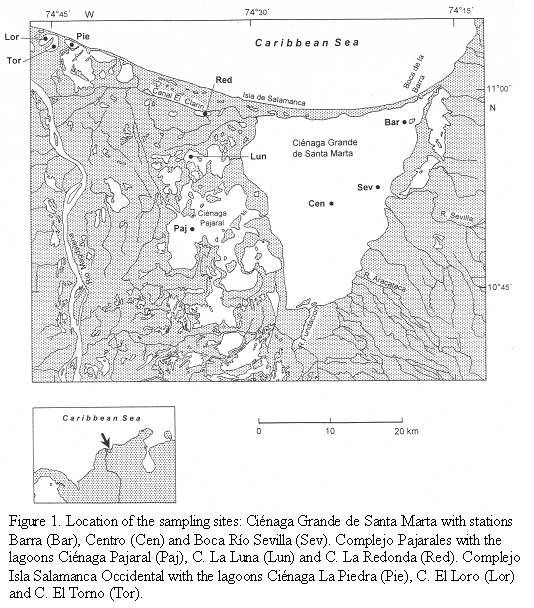
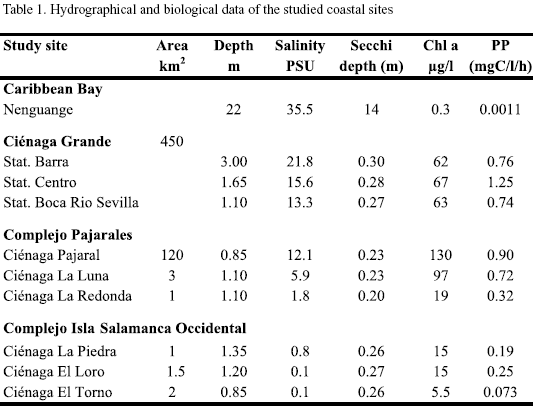
The study site is located on the Caribbean coast of Colombia and forms part of the so-called "Outer Delta of the Río Magdalena" (DERM). This large wetland is bordered to the east by the Sierra Nevada de Santa Marta (>5000m high) and to the west by the Río Magdalena, the largest river of Colombia (10o 45‘-11o 05‘ N, 74o 16‘-74o 46‘ W). The wetland consists of mangrove forests and several lagoons, the largest lagoon of these being the Ciénaga Grande de Santa Marta. Several smaller lagoons are situated between the Ciénaga Grande and the Río Magdalena (Figure 1). The hydrological regime of the wetland, which lies in a semi-arid region, depends to a regionally and temporally varying degree on freshwater input mainly from the Río Magdalena and saltwater inflow from the Caribbean Sea. The study was performed in the dry season (February/March). During these months the water discharge of the Río Magdalena usually reaches its lowest level.
Three areas within this region, the Ciénaga Grande de Santa Marta, the Complejo Pajarales and the Complejo Isla Salamanca Occidental differing with respect to riverine or marine influences, were chosen. Three stations were located in the Ciénaga Grande, the largest coastal lagoon of Colombia. The station "Centro" represents the central part of this brackish lagoon, the station "Barra" is somewhat influenced by intruding coastal water from the nearby connection to the Caribbean Sea and the station "Boca R. Sevilla" receives river water from the nearby mouth of the Río Sevilla.
The three stations of the Complejo Pajarales are situated in a transition zone from a brackish water body (Ciénaga Pajaral) to an almost limnic system (Ciénaga La Redonda) with the Ciénaga La Luna between both extremes. Ciénaga La Redonda receives its fresh water from the Río Magdalena via the Caño Clarin.
Three further stations (Ciénaga La Piedra, Ciénaga EL Loro and Ciénaga El Torno) belong to the Complejo Isla Salamanca Occidental near the mouth of the Río Magdalena. Here only the Ciénaga La Piedra showed a slightly noticeable salinity. Apparently, small amounts of seawater leak through the strand which consists mainly of sand and shells. The two other lagoons contained pure river water.
The turbidity of the water in all lagoons was high as shown by Secchi depths between only 20 and 30 cm. Turbidity is caused mainly by plankton and organic detritus (Ciénaga Grande and C. Pajaral) or by suspended inorganic sediments introduced by the Río Magdalena.
For comparison a nearby-coastal marine region, Nenguange Bay, east of Santa Marta (11o20‘N, 74o05‘W) was also studied. Table 1 summarises the hydrological features of the locations studied. Additional information on climatological, hydrographical and biological properties of the study sites can be found in the comprehensive works of Botero and Mancera-Pineda (1996) and Polanía et al. (2001).
MATERIAL AND METHODS
Samples were taken with a Niskin sampler from approximately 0.5 m depth. They were kept on ice in an insulation box during transport to the laboratory. Processing of the samples started no later than 4-5 h after sampling.
Microscopic analysis. For microscopic analysis, subsamples were fixed with formalin. Identification and enumeration was done mostly with a standard microscope using phase contrast objectives. Since the phytoplankton densities (Ciénaga Grande and Ciénaga Pajaral) or the inorganic sediment loading (all other lagoons) were extremely high, Sedgewick Rafter counting cells (Sweetwater Products, GB) filled with 1 ml of the thoroughly mixed sample were used. The clear water sample from the Nenguange Bay was counted with an inverted microscope in a 100 ml sedimentation chamber. The study of Vidal (1995) was used for identification of the marine and brackish water algal species. Freshwater unicellular algae were identified according to Yacubson (1969; 1972; 1974).
Pigment analysis. 100 ml of the samples were filtered through glass fibre filters (Whatman GF/F) and stored at -20oC. HPLC-measurements were performed at the Institute of Marine Sciences at Kiel, Germany. For pigment extraction, filters were homogenised with glass beads and extracted with 5 ml of acetone (90%) in a cooled Vibrogen cell mill (Buehler, Germany). Afterwards the extracts were purified by centrifugation. This procedure corresponds with minor modifications to the method of Derenbach (1969). Chlorophylls and carotenoids were analysed by means of reverse-phase high performance liquid chromatography (rp-HPLC) following the method of Mantoura and Llewellyn (1983). Calibration of the system was carried out with commercially available standards. Chlorophyll a was purchased from SIGMA, all other pigments from the International Agency for 14C Determination, Denmark. Identification of pigments was carried out by comparing their retention times and absorption spectra obtained with the diode array spectrophotometer with those of the pigment standards. Calculation of the composition of the phytoplankton communities was executed using the CHEMTAX program from Mackey et al. (1996), converting the concentrations of HPLC analysed marker pigments to equivalents of chlorophyll a using in the initial pigment ratio matrix for tropical ocean species presented in the article of these authors.
Size fractionation of photosynthetic active algae. Water samples were filled into two clear and one black 30 ml polycarbonate flasks , amended with 4 µCi of NaH14CO3 and incubated in situ for 3 h roughly at the 50% light depth (incubation in Nenguange Bay lasted for 4 h). After incubation samples were transported on ice in dark boxes to the laboratory, where aliquots of 5 ml each were filtered through different pore sizes. Cellulose acetate membrane filters with 0.2 µm, polycarbonate filters with 2 and 5 µm and filter discs made from nylon gauze with 10 and 20 µm pore size were used. Radioactivity of the black flasks was subtracted from the mean of the clear ones. Photosynthesis of different size classes was calculated and expressed as percent of the photosynthetic activity of the whole phytoplankton assembly. The latter was obtained from the radioactivity retained on the 0.2 µm filters.
RESULTS
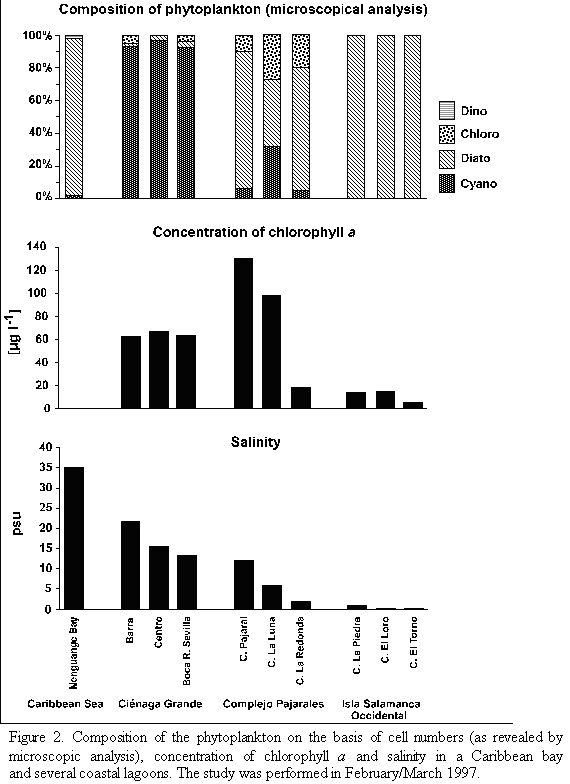
Hydrographic characterization. With respect to their salinity regimes and their amounts of phytoplankton the study sites can be arranged in three groups. As shown in table 1 and figure 2, Nenguange Bay represents the first group characterized by its clearly marked marine conditions and very low chlorophyll a concentrations (Table 1 and Figure 2).
The Ciénaga Grande with its three stations belongs to the second group. It forms a brackish water environment with very high chlorophyll a concentrations (Table 1 and Figure 2). The station Barra at about 2.5 km distance from the connection to the Caribbean Sea has a somewhat higher salinity than the large central part of the Ciénaga due to intrusion of sea water caused by tidal currents. In contrast, fresh water input from the Río Sevilla (albeit small during the dry season) slightly reduced the salinity at station Boca R. Sevilla. The influence of the marine or fresh water at both stations on the concentration of chl a, however, was almost negligible. Concerning salinity, Ciénaga Pajaral belongs to the same group as the adjacent Ciénaga Grande, its chl a concentration, however, was significantly higher (Table 1 and Figure 2).
The three lagoons of the Complejo Isla Salamanca Occidental represent the third distinct group. These lagoons were almost fresh water bodies with relatively low chlorophyll a concentrations (Table 1 and Figure 2).
The two remaining lagoons are situated within the Complejo Pajarales and form a transition between the adjacent brackish and fresh water systems, the Ciénaga La Luna being more influenced by the brackish and the Ciénaga La Redonda by the fresh water system.
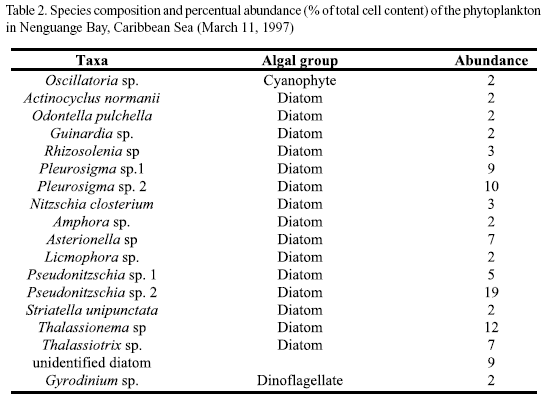
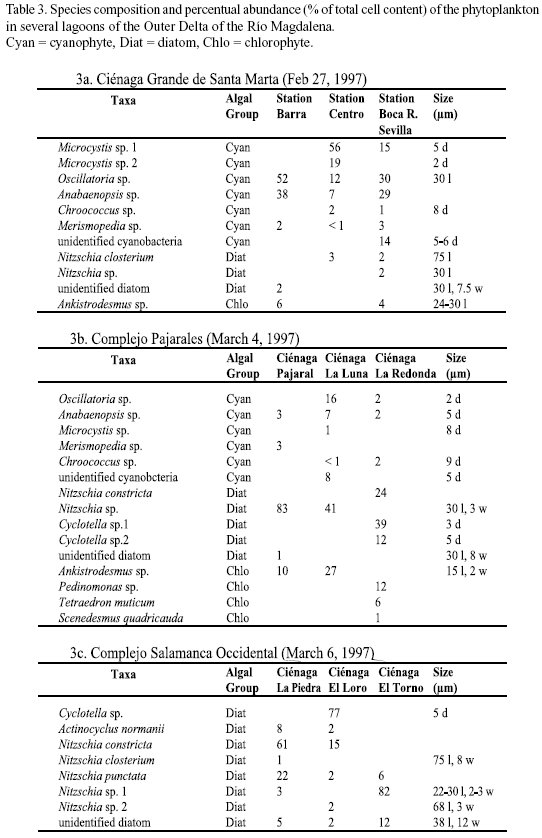
Microscopic analysis. The taxonomic composition of the phytoplankton (determined by microscopic analysis) at the different study sites is presented in tables 2 and 3a-c. The relative abundance of the species identified is given as percent of the total number of cells. In Nenguange Bay the pennate diatoms constituted by far the largest fraction of the phytoplankton assemblage (Table 2 and Figure 2). Cyanobacteria and dinoflagellates occurred only in smaller numbers.
At the three stations of Ciénaga Grande, cyanobacteria were the most abundant algal group accompanied by few diatoms and chlorophytes (Table 3a and Figure 2). Some other species were not included in the table since their fractions were much below 1%. Thus, at station Barra, several other forms, mostly marine diatoms but also some dinoflagellate species were detected and at station Boca R. Sevilla some freshwater algal species were present, e. g. Euglena sp. and Scenedesmus quuadricauda.
Table 3b and Figure 2 show the algal species which were encountered in the lagoons of the Complejo Pajarales. Here the microscopic analysis revealed that diatoms represented the most abundant algal group. Cyanobacteria and chlorophytes, however, were also present in significant numbers. Not mentioned in the table are the species occurring only rarely, e. g. Prorocentrum minimum (dinoflagellate) in Ciénaga Pajaral and Euglena sp. (euglenophyte) in Ciénaga La Redonda and Ciénaga La Luna.
In the lagoons of the Complejo Isla Salamanca Occidental only diatoms were detected by microscopic analysis (Table 3a and Figure 2 ).
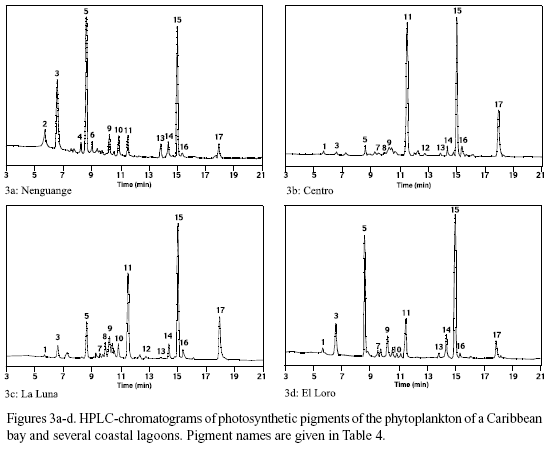
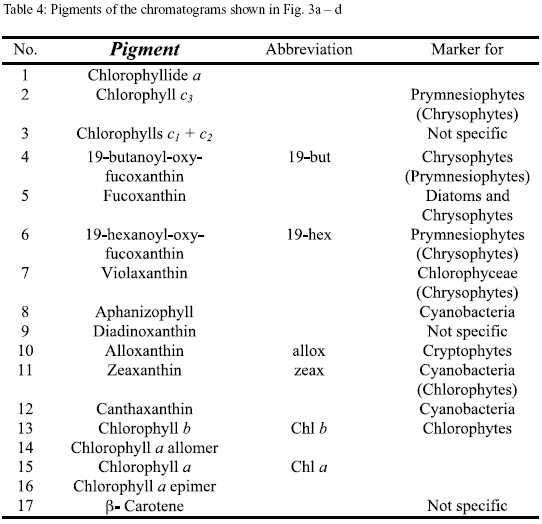
Pigment analysis. In figure 3a-d the pigment chromatograms of Nenguange Bay and of one representative of the different groups of lagoons are shown. The chromatograms are almost normalized to the chl a content (peak 15). This allows a direct comparison of the other components of the pigment composition of the phytoplankton. The names of the pigments and their distribution among the different phytoplankton groups are presented in table 4.
The pigment composition of Nenguange Bay phytoplankton (Figure 3a) was dominated by fucoxantin (in this case mainly from diatoms, because 19-butanoyl-oxy-fucoxanthin, the specific pigment of chrysophytes, which also contain fucoxanthin, occurred only in very small amounts) with small to negligible contributions from pigments of chrysophytes, prymnesiophytes, cryptophytes, chlorophytes and cyanobacteria. Pigments from Ciénaga Grande showed a very high contribution of zeaxanthin (cyanobacteria). Other marker pigments which are characteristic for diatoms, chlorophytes and cryptophytes comprised only an insignificant proportion of the pigment bulk (Figure 3b).
In Complejo Pajarales lagoons (Figure 3c) zeaxanthin (cyanobacteria) also represented the major pigment contribution. Its preponderance, however, at least in Ciénaga La Luna and Ciénaga La Redonda was much less marked than in Ciénaga Grande, since fucoxanthin (diatoms) and alloxanthin (cryptophytes) were also present in significant quantities.
In the freshwater lagoons of the Isla Salamanca Occidental (Figure 3d) fucoxanthin (diatoms) was the main marker pigment followed by zeaxanthin (cyanobacteria).
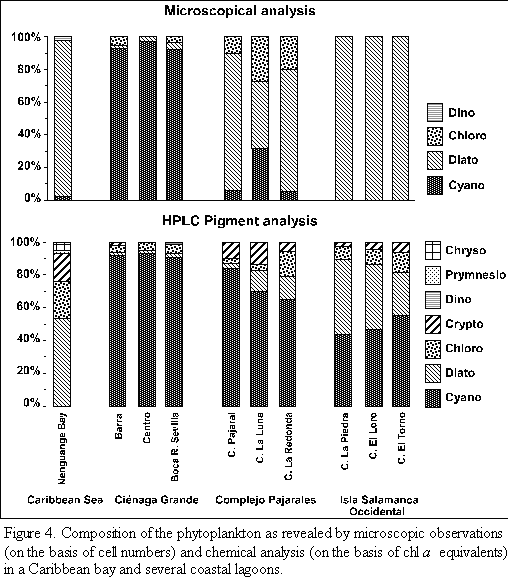
The composition of the phytoplankton communities calculated on the basis of the marker pigments is shown in figure 4. The phytoplankton of Nenguange Bay was dominated by diatoms. Fifty three per cent of total chl a belonged to this algal group followed by chlorophytes (23%) and cryptophytes (18%). Small amounts of chl a stemmed from chryso- and prymnesiophytes and insignificant ones from cyanobacteria.
The phytoplankton of the three stations in Ciénaga Grande consisted nearly exclusively of cyanobacteria (>90% of total chl a) with small contributions of chloro-, crypto-and dinophytes as well as diatoms.
Similarly the three lagoons of the Complejo Pajarales exhibited somewhat smaller, but still b contributions of cyanobacteria (65 to 84%). The remainder chl a stemmed from diatoms, chloro- and cryptophytes.
In the three lagoons of the Isla Salamanca Occidental the two most important phytoplankton groups were cyanobacteria (44 to 55%) and diatoms (26 to 45% of total chl a). Chloro- and cryptophytes contributed to the rest of chl a.
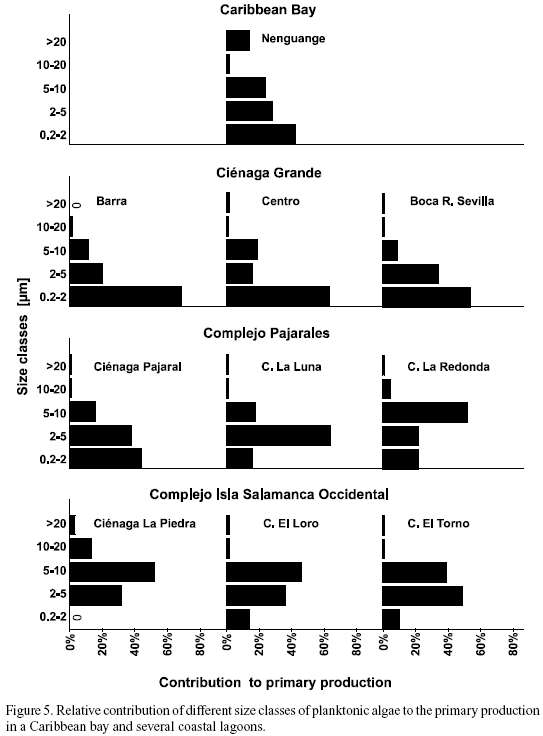
Size fractionation of photosynthetic active algae. With respect to the contribution of different size classes of algae to total primary production, the study sites can be arranged in three groups (Figure 5). These categories coincide well with those mentioned already with respect to salinity (Figure 2). The size spectrum of the photosynthetic algae of the marine Nenguange Bay differed significantly from that of the coastal lagoons. As in the brackish lagoons, the size class of 0.2 - 2 µm was the most important fraction. Its preponderance, however, was less marked (42%). In contrast to the coastal lagoons, the largest size class of algae (›20 µm) contributed 14% to the overall primary production (Figure 5).
The three stations of Ciénaga Grande together with Ciénaga Pajaral show an overwhelming preponderance of the smallest size class of 0.2 - 2 µm. The highest percentage measured was 68% (Barra), the lowest 44% (Ciénaga Pajaral). If the sum of the two smallest size classes is considered, then between 79% (Centro) and 88% (Barra and Boca R. Sevilla) of the primary production were due to algae smaller than 5 µm.
In the third group, the lagoons of the Complejo Isla Salamanca Occidental, the smallest algae played only a minor role. Here the size class of 2 - 5 µm (Ciénaga El Torno) and of 5 - 10 µm (Ciénaga La Piedra and Ciénaga El Loro) were much more important.
In the two "transient" lagoons, Ciénaga La Luna and Ciénaga La Redonda, the importance of the smallest size class (0.2 - 2 µm) decreased, whereas the role of the 5 - 10 µm class increased.
In all coastal lagoons studied the algae larger than 20 µm contributed only a negligible part to total primary production, nowhere more than 3% was surpassed.
DISCUSSION
The microscopic and chemical analyses of the phytoplankton from the different study sites lead to great discrepancies with regard to the taxonomical composition of the algal assemblages (Figure 4). The presence of several algal classes which were not encountered by microscopic analysis, could only be detected by pigment measurements. The crypto-, chryso- and prymnesiophytes belonged to these classes. The opposite case, i.e. that the occurrence of algal classes was detected only by microscopic but not by pigment analysis, was not observed.
A relatively good agreement between the two methods was only encountered in the samples of Ciénaga Grande, where both methods rendered almost the same contribution of cyanobacteria which dominated by far the phytoplankton of this eutrophic brackish coastal lagoon. (This agreement is a mere coincidence, since cell numbers were compared with chl a equivalents). The results from the other sampling sites, however, showed great discrepancies between the methods. Judging from microscopic counts, diatoms seemed to be the most important group in all sampling sites (up to 100% of cell number) except Ciénaga Grande. Only in Ciénaga La Luna did cyanobacteria play an important role. Contrary to these microscopic observations, the pigment composition revealed that not diatoms but cyanobacteria were the dominant component of phytoplankton biomass in all coastal lagoons. The only exception was found in the oligotrophic marine Nenguange Bay. Here the pigment analysis agreed with the microscopic observation showing that diatoms were the prevalent algal group. The important contributions of chloro- and cryptophytes to the phytoplankton community of the Nenguange Bay were only detected by pigment analysis. It should be stated ^^^^that our microscopic technique, especially the use of the Sedgewick Rafter counting cell, may have been partly responsible for the lack of agreement between the microscopic and and chemical analysis of the phytoplankton. A better technique (inverted microscope and a sedimentation chamber) would probably have resulted in a better agreement.
Several reasons might be responsible for the observed discrepancies between the results of the two methods. First, microscopic analysis renders cell numbers (which, of course, can be converted to biomass), whereas pigment analysis reveals equivalents of chlorophyll. Therefore, since cells of different algal classes vary in size and also in their content of chl a , the comparison between the microscopic and pigment analysis results will never lead to completely identical results even under most favourable circumstances. Second, many (if not most) picophytoplankton organisms cannot be identified by "normal" microscopic observations, whereas the detection of special marker pigments allows at least their assignment to a distinct algal class. Third, the number of individual cells of many life forms of phytoplankton (e.g. colonies or trichomes of cyanobaceria) are difficult to count. Their biomass (in terms of chl a equivalents) can be determined, however, quite easily using pigment analysis. (Gieskes and Kraay 1986; Murphy and Haugen 1985; Booth 1987).
Several investigators found poor agreements between the microscopic and chemical analyses of qualitative and quantitative composition of phytoplankton. Woitke et al. (1996) concluded that the determination of phytoplankton composition and algal class biovolumes on the basis of pigment chromatograms gave only semi-quantitative estimates. In a subtropical lake sometimes dramatic differences between both methods regarding relative biomass of different algal divisions of periphyton were observed by Havens et al. (1999), which may have been caused by variations in accessory pigment to chl a ratios. On the other hand, Roy et al. (1996) comparing HPLC and microscopic studies in the lower ST. Lawrence Estuary, concluded that both approaches gave similar results. Difficulties arose, however, when small algae were problematically to identify microscopicaly and/or the lack of specificity of some marker pigments reduced taxonomic precision. Also Yacobi et al. (1996) found good agreements between the microscopic study and the pigment composition of the phytoplankton in Lake Kinneret. Finally the study of Descy et al. (2000), who used the phytoplankton pigments as markers for the most important taxonomic algal groups in northern Wisconsin lakes, should be mentioned in this context. The authors stated that the CHEMTAX program lead to a reasonably good agreement of biomass estimates obtained from microscope counts and HPLC analysis.
Even though, as stated above, in some reports found in the literature the determination of the quantitative composition of phytoplankton was problematic, at least the measurement of the qualitative composition has been mostly satisfactory. The lack of agreement in our study even concerning the qualitative aspect may be due to the special characteristics of the coastal sites studied by us. Especially the high detritus and/or inorganic sediment loadings in the coastal lagoons made the microscopic counting extremely difficult. Assuming that the biomass of phototrophic organisms is at least fairly proportional to their photosynthetic rate, our size fractionation experiments (Figure 5) showed that from 0 to 68% of the phytoplankton was smaller than 2 µm and between 32 and 88% was smaller than 5 µm. However, these size classes played only an insignificant role in the microscopic analysis of the phytoplankton in the coastal lagoons (unfortunately the size was not determined for the Caribbean (Nenguange) phytoplankton). Instead, the results of the microscopic analysis showed a preponderance of large algae species (Tables 3a-c). These large forms, on the other hand, proved to be insignificant regarding primary production. This is a clear indication of the fact that very small organisms are underrepresented compared to larger forms, an observation which is even more valid in "difficult" samples.
We therefore conclude that our pigment analysis gave a more consistent picture of the taxonomical class composition and also of the relative share of these classes in the biomass of the phytoplankton of the Nenguange Bay and the lagoons of the Outer Delta of the Río Magdalena. In this context Schlüter et al. (2000) shall be cited who state that "the high sensitivity and reproducibility of the pigment method allow the detection of differences between phytoplankton populations which might not be easily detectable using standard microscopic techniques".
Nevertheless, only microscopic examination makes species identification possible, whereas pigment analysis renders information only as far as the class level is concerned. Furthermore, some phytoplankton species contain autotrophic symbionts of other classes and therefore show pigments not specific to their own group. Diatoms from oligotrophic regions, for example, may have cyanobacteria as symbionts (Werner, 1977), dinoflagellates may contain chrysophytes (Tomas and Cox, 1973; Withers and Haxo, 1975) and ciliates like Mesodinium rubrum a cryptophyte. Therefore the choice of methods depends on the scientific intent of the study.
ACKNOWLEDGEMENTS
The authors express their thanks to the director and to the scientific as well as to the technical board of the INVEMAR for constant encouragement during the study. For his most skilful aid as boat helmsman during the field trips we are especially indebted to Sr. Martin Montaño. The work was financed by COLCIENCIAS-INVEMAR (Colombia) and DAAD (German Academic Exchange Service).
LITERATURE
1 Booth, B. C. 1987. The use of autofluorescence for analyzing oceanic phytoplankton communities. Bot. Marina 30:101-108. [ Links ]
2 Botero, L. and J. E. Mancera-Pineda. 1996. Síntesis de los cambios de origen antrópico en los últimos años en la Cíenaga Grande de Santa Marta (Colombia). Rev. Acad. Colom. Cienc. 20: 465-474. [ Links ]
3 Derenbach, J. 1969. Zur Homogenisierung des Phytoplanktons für die Chlorophyllbestimmung. Kieler Meeresforsch. 25: 166-171. [ Links ]
4 Descy, J. P., H. W. Higgins, D. J. Mackey, J. P. Hurley and T. M. Frost. 2000. Pigment ratios and phytoplankton assessment in northern Wisconsin Lakes. J. Phycol. 36: 274-286. [ Links ]
5 Gieskes, W. W. C. and G. W. Kraay. 1986. Analysis of phytoplankton pigments by HPLC before, during and after mass occurrence of the microflagellate Corymbellus aureus during the spring bloom in the open northern North Sea in 1983. Mar. Biol. 92: 45-52. [ Links ]
6 Havens, K. E., A. D. Steinman, H. J. Carrick, J. W. Louda, N. M. Winfree and E. W. Baker. 1999. Comparative analysis of lake periphyton communities using high performance liquid chromatography (HPLC) and light microscope counts. Aquat. Sci. 61: 307-322. [ Links ]
7 Jeffrey, S. W. and G. F. Humphrey. 1975. New spectrophotometric equations for determining chlorophylls a, b, c1, c2 in higher plants and phytoplankton. Biochem. Physiol. Pfl. 167: 192-194. [ Links ]
8 Jeffrey, S. W., R. F. C. Mantoura and S. W. Wright. 1997. Phytoplankton pigments in oceanography. Monographs in oceanographic methodology. SCOR, UNESCO. Paris. p.661. [ Links ]
9 Letelier, R. M., R. R. Bidigare, D. V. Hebel, M. Ondrusek, C. D. Winn and D. K. Karl. 1993. Temporal variability of phytoplankton community structure based on pigment analysis. Limnol. Oceanogr. 38: 1420-1437. [ Links ]
10 Lorenzen, C. J. and S. W. Jeffrey. 1978. Determination of chlorophyll in seawater. UNESCO Technical Papers 34. [ Links ]
11 Mackey, M. D., D. J. Mackey, H. W. Higgins and S. W. Wright. 1996. CHEMTAX - a program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 144: 265-283. [ Links ]
12 Mantoura, R. F. C. and C. A. Llewellyn. 1983. The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reverse-phase high-performance liquid chromatography. Analyt. Chim. Acta 151: 297-314. [ Links ]
13 Millie, D. F., H. W. Paerl and J. P. Hurley. 1993: Microalgal pigment assessments using high-performance liquid chromatography: A synopsis of organismal and ecological applications. Can. J. Fish. Aquat. Sci. 50: 2513-2527. [ Links ]
14 Murphy, L. S. and E. M. Haugen. 1985. The distribution and abundance of phototrophic ultraplankton in the North Atlantic. Limnol. Oceanogr. 30: 47-58. [ Links ]
15 Polanía, J., A. Santos-Martínez, J. E. Mancera-Pineda and L. Botero Arboleda. 2001. The coastal lagoon Ciénaga Grande de Santa Marta, pp. 34 - 45. In: U. SEELIGER and B. KJERFVE (eds.): Coastal marine ecosystems of Latin Amerika. Springer Verlag Berlin Heidelberg. [ Links ]
16 Roy, S., J. P. Chanut, M. Gosselin and T. Sime-Ngando. 1996. Characterization of phytoplankton communities in the lower St. Lawrence Estuary using HPLC-detected pigments and cell microscopy. Mar. Ecol. Progr. Ser. 142: 55-73. [ Links ]
17 Schlüter, L., F. Møhlenberg, H. Havskum and S. Larsen. 2000. The use of phytoplankton pigments for identifying and quantifying phytoplankton groups in coastal areas: testing the influence of light and nutrients on pigment/ chlorophyll a ratios. Mar. Ecol. Prog. Ser. 192: 49-63. [ Links ]
18 Stockner, J. A. 1988. Phototrophic picoplankton: An overview from marine and freshwater ecosystems. Limnol. Oceanogr. 33: 765-775. [ Links ]
19 Tomas, R. N. and E. R. Cox. 1973. Observation on the symbiosis of Peridinium balticum and its intracellular algae. I. Ultrastructure. J. Phycol. 9: 304-323. [ Links ]
20 Vidal, L. A. 1995. Estudio de fitoplankton en el sistema lagunar estuarino tropical Ciénaga Grande de Santa Marta, Colombia, durante el año 1987. - Tesis M. Sc. Univ. Nacional de Colombia, 207 p., 62 plates. [ Links ]
21 Werner, D. (ed.). 1977. The diatoms. Botanical Monographs, University of California Press: 13. [ Links ]
22 Withers, N. and F. T. Haxo. 1975. Chlorophyll-c1 and c2 and extraplastidic carotenoids in the dinoflagellate Peridinium foliaceum Stein. Plant Science Letters 5: 7-15. [ Links ]
23 Woitke, P., T. Schiwietz, K. Teubner and J. G. Kohl. 1996. Annual profiles of photosynthetic lipophilic pigments in four freshwater lakes in relation to phytoplankton counts as well as to nutrient data. Arch. Hydrobiol. 137: 363-384. [ Links ]
24 Yacobi, Y. Z., U. Pollingher, Y. Gonen, V. Gerhardt and A. Sukenik. 1996. HPLC analysis of phytoplankton pigments from Lake Kinneret with special reference to the bloom-forming dinoflagellate Peridinium gatunense (Dinophyceae) and chlorophyll degradation products. J. Plankton Res. 18: 1781-1796. [ Links ]
25 Yacubson, S. 1969. Algas de ambientes acuáticos continentales nuevas para Venezuela (Cyanophyta, Chlorophyta). Bol. Cent. Invest. Biol., Univ. Zulia. Venezuela 3: 87 p., 18 plates. [ Links ]
26 Yacubson, S. 1972. Catálogo e iconografía de las Cyanofyta de Venezuela. Bol. Cent. Invest. Biol., Univ. Zulia. Venezuela 5: 78 p., 17 plates. [ Links ]
27 Yacubson, S. 1974. Catálogo e iconografía de las Cyanofyta de Venezuela. Bol. Cent. Invest. Biol., Univ. Zulia. Venezuela 11: 143 pp., 31 plates. [ Links ]
DATE RECEIVED: 30/10/02 DATE ACCEPTED: 27/08/03