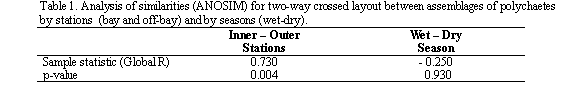Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.35 no.1 Santa Marta Jan./Dec. 2006
SPATIAL AND TEMPORAL CHARACTERIZATION OF SOFT BOTTOM POLYCHAETES IN A SHALLOW TROPICAL BAY (COLOMBIAN CARIBBEAN)
CARACTERIZACIÓN ESPACIAL Y TEMPORAL DE LOS POLIQUETOS DE FONDOS BLANDOS EN UNA BAHÍA TROPICAL(CARIBE COLOMBIANO)
Angela I. Guzmán-Alvis1 , Patricia Lattig2 and José A. Ruiz2
1Universidad Nacional de Colombia (sede Palmira), Carrera 32 vía La Candelaria, Palmira, Colombia. E-mail: aiguzmana@palmira.unal.edu.co
2Instituto de Investigaciones Marinas y Costeras (INVEMAR), Cerro Punta Betín, Santa Marta, Colombia. E-mail: plattigm@yahoo.com.mx (PLM); joseanibalruiz@hotmail.com (JAR)
ABSTRACT
The spatial and temporal variations of the polychaete assemblages were studied within and off a shallow (10-25 m) tropical bay (Bahía de Portete). The polychaete abundances at family level and their trophic mechanisms were used for this purpose. Sediment samples were collected at six stations in this bay during the wet and dry seasons. Multivariate analysis indicated that off- Bay polychaete assemblages were different from the bay ones; this spatial variation was related to sedimentary characteristics, depth and turbid-water conditions. On the other hand, these assemblages did not show significant differences between the dry and wet seasons. The differences between these two assemblages were given specially by the Syllidae, Gonidadidae, Nephtyidae, Dorvilleidae, Ampharetidae, Sabellidae, Glyceridae, Lumbrineridae, Opheliidae and Maldanidae families, being more abundant and frequent off the bay, while Magelonidae, Cirratulidae, Cossuridae and Eulephetidae were more abundant and frequent within the bay. The first ten families were related to a higher sand content, lower organic matter content and lower turbid-water conditions; while the last four were related to higher mud percentages, higher organic matter content and higher turbid-water conditions. Trophic guilds data showed similar assemblages as described above, which differ in their feeding mechanisms; the bay stations were dominated by surface and subsurface deposit feeders showing the importance of detritus; while off-bay stations the carnivores were the dominant organisms, reflecting the high predation. In these assemblages, the trophic and taxonomic structure is more affected by the spatial variation in the physical characteristics of the water column and sediment than by the seasonal variation.
KEY WORDS: Tropic, Polychaetes, Trophic groups, Taxonomic structure, Bahía de Portete.
RESUMEN
Las variaciones espaciales y temporales de las asociaciones de poliquetos fueron estudiadas dentro y fuera de una bahía somera (10-25m) tropical (Bahía de Portete). Para este propósito se usó la abundancia de poliquetos a nivel de familia y sus mecanismos tróficos. Las muestras de sedimentos fueron colectadas en seis estaciones de ésta Bahía durante la época húmeda y seca. Los análisis multivariados indicaron que las asociaciones de poliquetos fuera de la bahía fueron diferentes de las del interior; esta variación espacial estuvo relacionada con las características sedimentarias, la profundidad y las condiciones de turbidez del agua. Por otro lado, estas asociaciones no mostraron diferencias significativas entre la época seca y húmeda. Las diferencias entre estas dos asociaciones estuvieron dadas especialmente por las familias Syllidae, Gonidadidae, Nephtyidae, Dorvilleidae, Ampharetidae, Sabellidae, Glyceridae, Lumbrineridae, Opheliidae y Maldanidae, que fueron más abundantes y frecuentes fuera de la bahía; mientras que Magelonidae, Cirratulidae, Cossuridae y Eulephetidae fueron más abundantes y frecuentes dentro de la bahía. Las primeras diez familias estuvieron relacionadas con altos contenidos de arenas, bajos contenidos de materia orgánica en el sedimento y baja turbidez en la columna de agua; mientras que las cuatro últimas estuvieron relacionadas con altos porcentajes de cienos y materia orgánica en los sedimentos y alta turbidez. Los datos de los gremios tróficos mostraron asociaciones similares a los descritos anteriormente, los que se diferencian en sus mecanismos de alimentación; en las estaciones de la bahía dominaron los alimentadores de depósito de superficie y sub-superficie mostrando la importancia del detritus; mientras que en las estaciones afuera de la bahía dominaron los carnívoros, reflejando una alta depredación. En estas asociaciones, la estructura taxonómica y trófica está más afectada por la variación espacial de las características físicas de la columna de agua y sedimento que por la variación estacional.
PALABRAS CLAVE: Trópico, Poliquetos, Grupos tróficos, Estructura taxonómica, Bahía de Portete.
INTRODUCTION
There are many studies about the structure of sedimentary soft-bottom communities of coastal areas in temperate zones. Thus the theory related with assemblage structure is based mainly on these studies; by contrast, quantitative data from tropical areas are quite scarce (Alongi, 1990; Gray, 2002). The lack of information and the fast deterioration of tropical coastal zones make it difficult to understand and evaluate the natural - and anthropogenic- originated impact on the structure and function of these ecosystems.
The distribution of benthic fauna widely varies over time and space due to the heterogeneous distributions of benthic habitats (Mistri et al., 2000). Studies carried out in temperate zone at a small scale (1o latitude) have shown that the assemblage structure varies with small depth changes (Gray, 2002); and the distribution and abundance of benthic organisms are affected by spatial variations in salinity and sediment composition (Zajac and Whitlatch, 1982; Mannino and Montagna, 1997; Gray, 2002). At an annual scale, the climate in temperate regions is markedly seasonal and hydrodynamic conditions affect the patterns of organic matter sedimentation and benthic community.
There exists information favoring seasonal variations in the benthic communities in the tropics, as well as, evidence to the contrary. Parsons et al., (1995) show that the seasonal variations are small because of the relative homogeneous conditions in temperature and salinity, and that the changes occurring in benthic communities may be due to predation preventing space monopolization by one single species. On the other hand, Alongi (1989) shows that the climate and its effect on shallow benthos vary greatly within the tropics. The magnitude of seasonal fluctuations depends upon the distance from the equator; the habitats near equator show less seasonal variability than the tropical assemblages that are closer to the poles. For instance, in India the richness of tropical assemblages and benthic density is heavily influenced by seasonal changes in rainfall and run-off (Longhurst and Pauly, 1987; Alongi, 1990). In some Caribbean areas as Jamaica, Venezuela, Colombia, the richness and density do not change significantly with the season (Jackson, 1972; Bone and Klein, 2000; Guzmán-Alvis et al., 2001; Guzmán-Alvis and Carrasco, 2005b). However apparently there is no enough evidence describing the patterns in the tropics.
The benthic infaunal communities are organized structurally, numerically and functionally in relation to gradients of resource availability, modified by interactions with other environmental factors (Pearson and Rosenberg, 1987; Wieking and Kröncke, 2005). Since the primary food source for benthos originates, with a few localized exceptions, in euphotic surface waters, food availability decreases with depth and declining current speed. Water movement driven by currents, tides, b wind and other forces transports food particles in the water mass and causes resuspension of bottom sediments (Pearson and Rosenberg, 1987). This transport is of significant importance for the distribution of food to benthic animals. Species distribution may be seen as a response to the varying effects of these modified gradients. Such distributions are further affected, by other factors. These are, in general, physical factors contributing to the relative environmental harshness imposed on each species, e.g. sedimentary fluctuations in stability and in turbidity, salinity, oxygen, temperature and pressure. Other factors which exert effects independently of the primary gradients can be summarized as stochastic events and biotic interactions. These factors influence community distributions (Pearson and Rosenberg, 1987).
We hypothesized that the structural variability of these polychaete assemblages is more affected by the spatial pattern than by the seasonal one, because the spatial heterogeneity (the coastal shape determines the hydrodynamic conditions affecting the water column characteristics and the sediment) is ber than the seasonal variations.
The purpose of this study is to determine the spatial and temporal variations in the polychaete assemblages in relation to sediment (grain size and organic matter) and water column (salinity, transparency, dissolved oxygen content, temperature) variables, measured in both seasons (dry and wet) and two different places (within and off the bay). The structural changes were evaluated according to taxa composition and trophic groups.
STUDY AREA
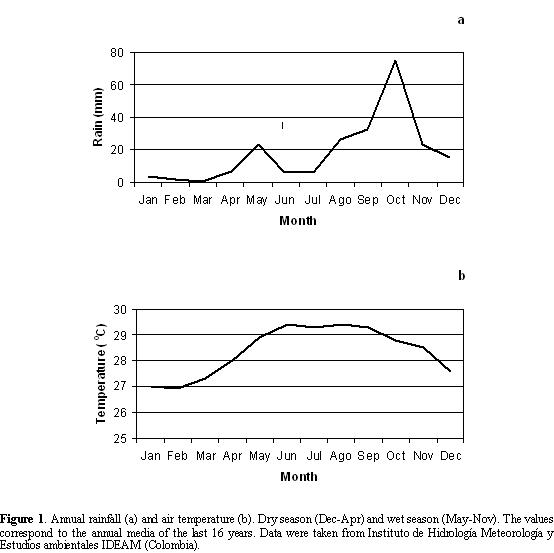
Bahía de Portete is located in the northeastern part of Colombia (12o07'N and 72o02'W). It has an approximate area of 125 km2 with an average depth of 9 m and a maximum of 20 m. The Bay's only connection with the Caribbean Sea is a 2 km inlet (Solano, 1994). The climate of the region is regulated by the NE trade winds; during the dry season the trade winds are b and continuous and the precipitation and temperatures are low (Dec-Apr) (Figure 1). During the wet season the winds are weak, the precipitation is high and the temperature increases (May-Nov) (Andrade, 2000).
Bahía de Portete shows high salinity values (34-37), high temperatures (25-30oC), and high turbidity in the water column (1-4 m). There is no oxygen deficiency (5-8 mg*1-1) (Solano, 1994); whereas water temperatures and salinities are the highest during the dry season.
A coastal upwelling occurs in this area especially during the dry season (Andrade, 2000); providing a high amount of nutrients to the bay ecosystems. The bay is a diverse
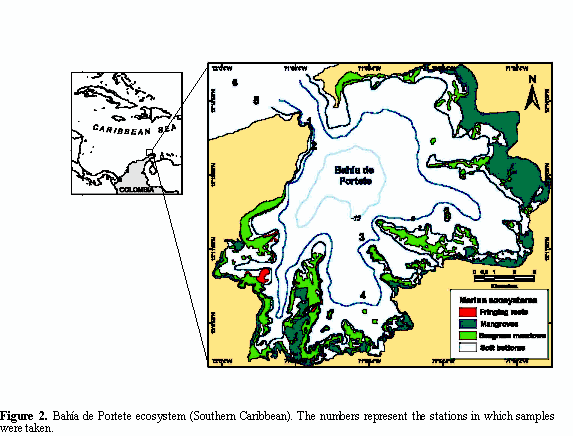
ecosystem comprised by a mangrove forest dominated by Rhizophora mangle and Avicennia germinans; seagrass meadows of Thalassia testudinum and Syringodium filiforme; and poorly developed fringing reefs at the east and south of the bay (Solano, 1994). The soft bottoms are basically mud covering a high proportion of the bay (Figure 2).
MATERIALS AND METHODS
Two replicate samples of sediment were taken from each of six stations using a van Veen grab (0.08 m2). Four stations were located within the bay and two off the bay. The samples were taken at the end of the wet season (December 2003) and during the dry season (January 2004) (Figure 2). The effect of rain and drought on the benthos is not considered immediate; this effect may lag at least one month (Zajac and Whitlatch, 1982; Kröncke et al., 1998; Guzmán-Alvis, 2004). In spite of the fact that the sampled months were consecutive, the lag effect allows to assess temporal differences. The sediment was sieved through a 0.5 mm mesh screen. The polychaetes were identified at family level because of the scarce taxonomic description to species level available for the area. According to different authors, the identification of organisms to species level is not always necessary to describe spatial patterns; indeed data at higher taxonomic levels assess much of community structure variations (Guzmán and García, 1996; Olsgard et al., 1998; Guzmán-Alvis and Carrasco, 2005a). Abundance was obtained as number of individuals per 0.08 m-2.
The two-way crossed layout ANOSIM (analysis of similarities) test was used to determine the differences of assemblages between stations (within and off the bay) and seasons (wet-dry) (using PRIMER 5: Clarke and Warwick, 2001). Family abundance data were square root transformed and similarity matrix between samples was generated using the Bray-Curtis similarity index (Clarke and Warwick, 2001). Two-way crossed layout ANOSIM tested the null hypothesis that there are no assemblage differences between treatments (stations and seasons). For the validity of the ANOSIM test it must be assumed that the groupings were decided 'a priori', or at least without reference to the assemblage data in any way (Clarke and Warwick, 2001). To determine the spatial and temporal distribution of the assemblages, the hierarchical clustering method with group average linking was used (Clarke and Warwick, 2001). The similarity matrix was performed in the same way as the ANOSIM.
Each family was assigned to eight trophic guilds: suspension feeders (sf), carnivores (ca), omnivores (o), selective and non selective surface deposit feeders (sdfs and sdfn), selective and non selective subsurface deposit feeders (ssdfs and ssdfn) and suspension feeders/surface deposit feeders (sf-sdf). These groups are based on information retrieved from literature sources (Fauchald and Jummars, 1979; Beesley et al., 2000; Rouse and Pleijel, 2001). Abundance values were summed for each trophic group at each station. This resulted in a station by trophic group table that was assessed in the same way as the taxonomic data, using cluster and redundancy analysis RDA.
The family abundance-composition patterns and trophic groups in relation to the environmental variables were analyzed with a redundancy analysis (RDA) (using CANOCO 4.5: ter Braak and Šmilauer, 2002). The environmental variables were the following: depth (D), transparency of the water column (Tr), dissolved oxygen (DO), temperature (T), salinity (S), organic matter (OM) and mud percentage (Mud). Abundance data were root square transformed, and the environmental data were standardized before statistical analyses to homogenize variances. For each variable value, subtract the mean and divide by the standard deviation over all variables for that station.
RESULTS
Significant assemblage polychaetes differences were found between stations within and off the bay (p < 0.05); whereas a temporal variation was not found (Table 1). For the latter one, the statistic Global R was no significant (p > 0.05); in this vein, the null hypothesis that there are no assemblage polychaetes differences between seasons were accepted. These results might be explained by the taxonomic composition between both seasons was similar.
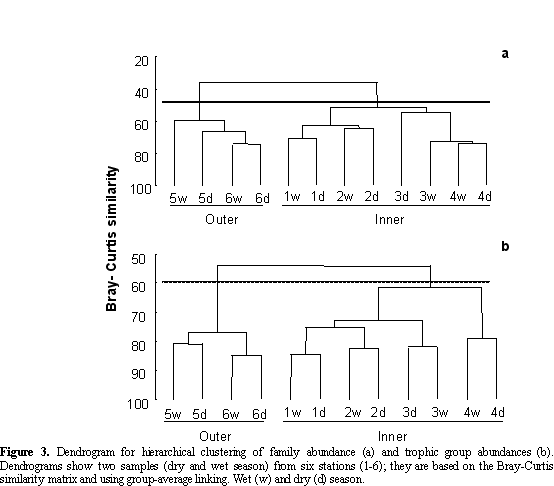
Two different assemblages of polychaetes in both taxonomic (Figure 3a) and trophic groups (Figure 3b) were formed; one cluster corresponded to bay stations (1-4)
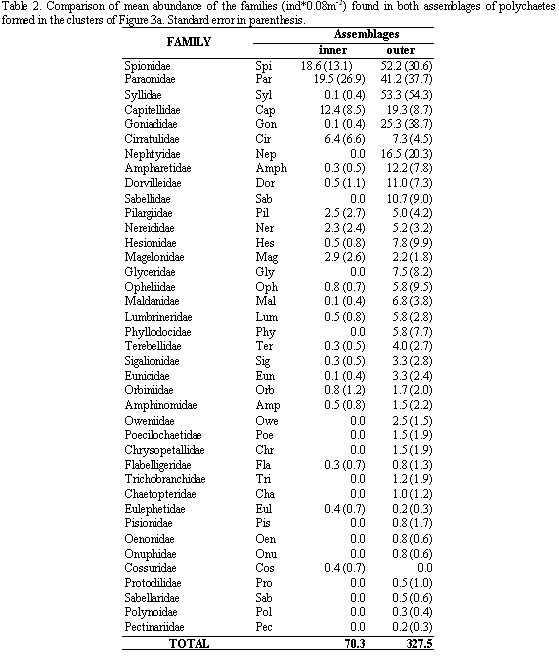
and the other the off-bay stations (5 and 6). The latter showed both a higher density (327.5 ind* 0.08m-2) and number of families (38) than the former (density: 70.3 ind* 0.08m-2 and number of families: 23) (Table 2).
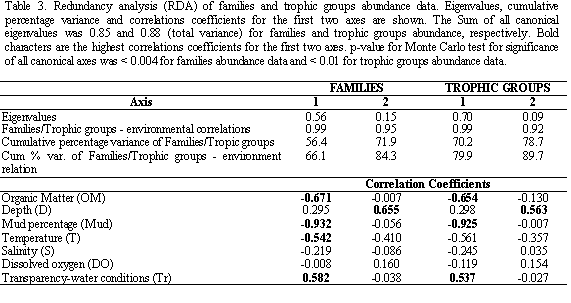
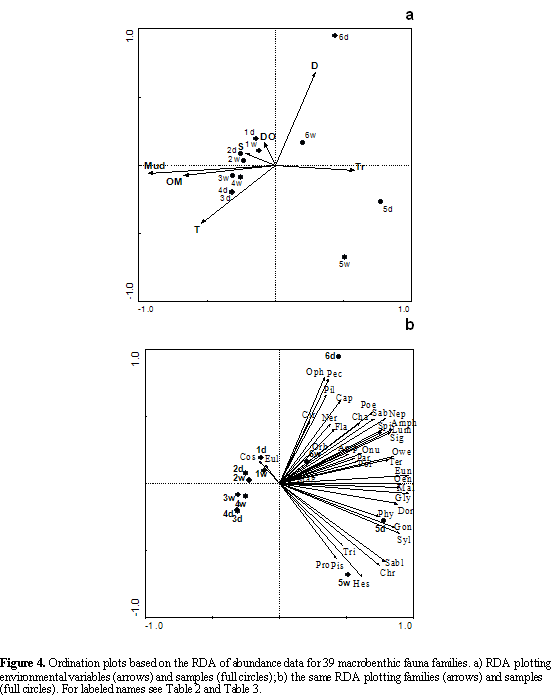
In the redundancy analyses (RDA) for family abundance data, the sum of all the canonical eigenvalues was 0.855 (total variance). The environmental variables that best explains the highest total variance quantity were the mud percentage (0.50), depth (0.12) and transparency of the water column (0.10); while other variables explained 0.13. The eigenvalues for the first two axes accounted for a high proportion of the total variance. The variation in the families composition had a high correlation with the environmental variables (Table 3). The first axis was defined by mud percentage, organic matter, transparency and temperature and the second axis by depth. The mud content had a high positive correlation with the organic matter in the sediment (arrows pointing in roughly with the same direction) (Figure 4a). The RDA also showed that the dry and wet season samples group together in each station (Figure 4ab).
In the RDA plots, the family abundances and the environmental variables indicated with long arrows were the most important for the analyses. In the off-bay stations, the families Opheliidae, Poecilochaetidae, Sabellidae, Nephtyidae, Ampharetidae and Lumbrineridae showed the highest abundances in deeper places with fine sand and low organic matter contents (Figures 4b and 5a), while Hesionidae, Syllidae, Goniadidae, Dorvilleidae, Glyceridae, Maldanidae and Eunicidae were found in deeper stations with coarse sand. In the bay stations Cossuridae and Eulepethidae were exclusive and presented low abundances; while Magelonidae, Cirratulidae showed high abundance and frequency in fine sediments (Figures 4b and 5a). The families with short arrows as Orbiniidae, Nereidae, Flabelligeridae, Amphinomidae and Paraonidae, and the families Capitellidae and Spionidae showed similar abundances and high frequency in both assemblages. Protodrilidae, Pisionidae, Sabellaridae, Oenonidae and Pectinaridae were rare families and were located only off-bay.
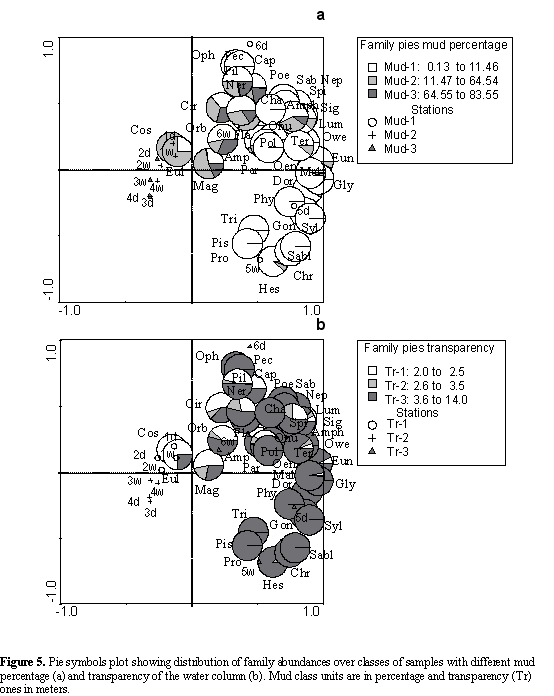
To summarize, the assessed physical and chemical variables that best explained the biological pattern were the mud percentages and water transparency. According to this, the families located to the right of Figure 5 preferred sediments with low mud contents and high transparency, while the families located in the center of the same figure preferred places with higher mud contents and a low transparency (Figure 5ab).
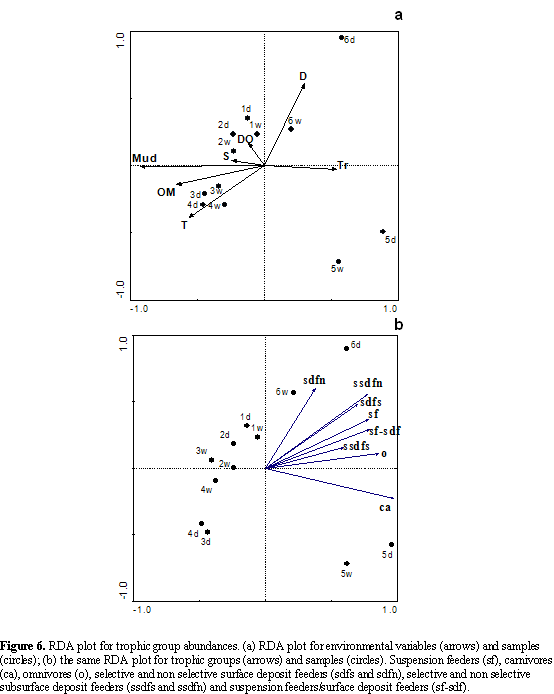
In the trophic structure RDA, the sum of all the canonical eigenvalues was 0.878 (total variance). The environmental variables that best explained the highest total variance quantity were the mud percentage (0.62), transparency (0.09) and depth (0.06); the other variables accounted for 0.11. The eigenvalues for the first two axes showed a high proportion of the total variance, and the variation on the trophic groups presented a high correlation with the environmental variables (Table 3). The best correlated variable on the first axis was the mud content, followed by organic matter and transparency; and
the second axis was defined by depth (Table 3). Figure 6a shows that the spatial and temporal distribution of the stations between the trophic groups and the environmental variables was similar to the distribution in Figure 4a.
The abundances of the different trophic groups increases from left to right in Figure 6b, showing a higher abundance in the assemblage of the off-bay. The carnivores (ca) presented the greatest abundances of this assemblage (the arrow is longer) and they were positively correlated with others trophic groups (arrows pointing in roughly with the same direction). The non selective subsurface deposit feeders (ssdfn), were highly
correlated with the selective surface deposit feeders (sdfs). The suspension feeders/surface deposit feeders (sf-sdf) were correlated with the suspension feeders (sf) and omnivores and selective subsurface deposit feeders (ssdfs). The trophic groups found in the bay, the subsurface and surface deposit feeders were more abundant than other trophic groups.
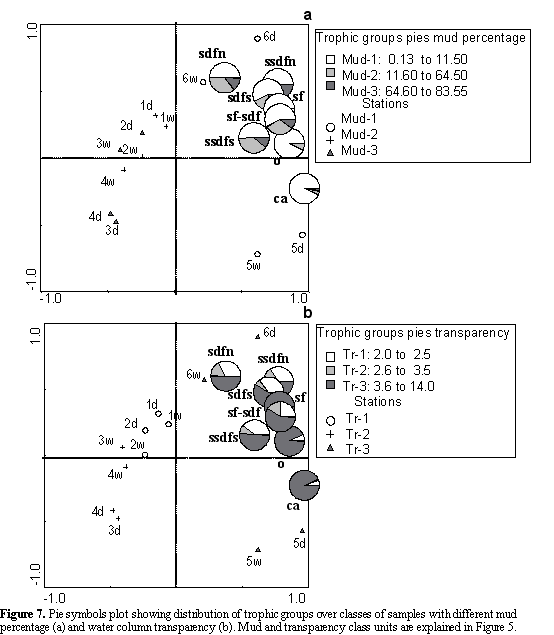
The selective subsurface deposit feeders (ssdfs), omnivores (o) and carnivores were positively correlated with transparency and negatively correlated with mud, organic matter and temperature (Figure 6ab). The suspension feeders, omnivores and carnivores were almost exclusively found in the sediments with low mud contents (sand) and high transparency of the water column (Figure 7ab); although the other trophic groups that preferred sediments with low mud contents and high transparency, were also distributed on sediments with intermediate and high mud contents and low and intermediate transparency.
DISCUSSION AND CONCLUSIONS
In ecological terms, it is important to identify the proportion of variance in biological data which may be attributed to environmental variability (Kröncke et al., 1998). The results of the relationship between spatial and temporal patterns of the organisms and environmental variables showed that, a higher percentage of the total variance was explained by variables measured in the water column and the sediment. The mud percentage and depth variables were those that best explained the variability in family and trophic abundances of soft bottom polychaetes in the bay and off-bay stations.
Most of the observed biological variability was spatial rather than temporal, and was determined by sediment heterogeneity. The mud content was the most important variable used to explain the variations in the trophic and taxonomic structures. The transparency and depth were also important in explaining the distribution of the families. These results can be compared to studies performed by Gray (2002) in temperate zones and small spatial scales, whereby species richness changes with sediment grain size and small depth changes.
Taxonomic and trophic structure of assemblages did not change seasonally. This result follows the temporal pattern seen in other soft bottom communities of the Colombian Caribbean. For example, the shallow water assemblages of Morrosquillo Gulf, Punta Canoas and Pozos Colorados were analyzed with a higher temporal frequency than the present study (3 and 4 times per year), showed no important changes between the dry and wet periods indicating no seasonal variability (and Carrasco, 2005b; Guzmán-Alvis, 2004; INVEMAR, 2004; Guzmán-Alvis and Solano, 2001). These results are in agreement with several studies in tropical soft-bottom and rocky shore communities, where neither number of taxa nor their abundances significantly changed during the year (Jackson, 1972; McCarthy et al., 2000). Alongi (1990) suggested that in dry tropics where rainfall is sporadic, there are high temperatures and desiccation (as in the study area), densities of most groups fluctuated over time without following seasons. The infaunal assemblages are characterized by displaying small, opportunistic and surface deposit feeder organisms.
The water movement and sediment structure affected the structure and function of polychaete assemblages. In exposed sandy areas (off-bay), suspension feeders were present. Their food mainly phytoplankton, may be produced in waters far away from their locations and transported to them by currents (Pearson and Rosenberg, 1987). The trophic group mutual exclusion hypothesis postulates that current speed controls community composition, through its effects on food supply and sedimentary composition; suspension feeders are abundant in areas of b water movement (off-bay) and deposit feeders in low flow areas (bay).
In this study, the deposit feeders (surface and subsurface) make up 39.0% of all the polychaetes; these trophic groups accounted for 65.6% of the bay assemblages showing the importance of detritus within the bay. Furthermore, obligate deposit feeders (surface and subsurface) which are generally semi-mobile or sessile, partition food and space resources through a variety of tube building and burrowing habitats. Such organisms are restricted to soft substrata and their predominance increase with decreasing particle size (Pearson and Rosenberg, 1987). Characteristic families inside the bay (defined as the families with abundance ≥ 60% and frequency ≥ 70% within the bay group), included burrowers such as Magelonidae, Cirratulidae and Cossuridae and predominated in the bay mud substrata.
In the off-bay assemblages the carnivores dominated with a 42%; characteristic families off-bay (defined as before), included active burrowers carnivores as Goniadidae, Nephtyidae and Glyceridae. The sands have greater spaces among the grains than the mud, making it easier for the carnivores in their search for and capture of potential preys. Furthermore, the distribution of the carnivores was more closely related to the abundance of their potential preys; the densities of these preys were higher in the off-bay. Predation can enhance coexistence between species of benthic organisms by preventing monopolization of space (Parsons et al., 1995).
The dominance of surface deposit feeders as consumers of newly sedimented food is related to the production in the water column (Gaston, 1987; Gaston et al., 1988; Josefson and Rasmussen, 2000). The present study does not present information on the biological productivity in the area. However, the region of the Guajira is characterized by a coastal upwelling that increases the productivity in the water column, and part of this production settles forming available food for the benthic community. Also, the benthic community receives food from the dissolved and particulate matter from the sea grass and mangroves in the bay. The deposit feeders (surface and subsurface) make up the 39% of the total assemblages, showing the importance of the detritus and benthic-pelagic coupling in these ecosystems.
The assemblage organization may be assessed by considering any convenient component unit, e.g. taxa and trophic groups; as assemblage structure change along any environment gradient so does its organization (Pearson and Rosenberg, 1978). Trophic relationships are particularly influenced by the gradient of organic input, and changes in trophic structure may, therefore, be considered as fundamental to any analysis of community change in relation to such inputs to the benthos.
Food supply is a key factor structuring marine benthic communities (Pearson and Rosenberg, 1978, 1987; Wieking and Kröncke, 2005). Dauwe et al., (1998) as with contrasting quantity and quality of organic matter and with different hydrodynamic environments. According to their results trophic structure reflects differences in the relative quality of organic matter.
The two assemblages found are associated with the area's hydrodinamics as follows: the off-bay area is exposed to b currents, characterized by low contents of organic matter and coarse sediments indicating dynamic environments. On the other hand, the area within the bay (a protected area) is characterized by high contents of organic matter and very fine sediments, typical of calm places.
ACKNOWLEDGEMENTS
The Instituto de Investigaciones Marinas y Costeras INVEMAR gave logistic support for the development of this study, which was carried within the project "Monitoring of the representative ecosystems in Bahia de Portete", requested and fully funded by the company Carbones del Cerrejón, LLC, as part of its environment protection programs.
LITERATURE CITED
1. Alongi, D. 1989. Ecology of tropical soft-bottom benthos: a review with emphasis on emerging concepts. Rev. Biol. Trop., 37: 85-100. [ Links ]
2. Alongi, D. 1990. The ecology of tropical soft-bottom benthic ecosystems. Oceanogr. Mar. Biol. Ann. Rev., 28: 381-496. [ Links ]
3. Andrade, C. 2000. The circulation and variability of the Colombian Basin in the Caribbean Sea. Thesis PhD School of Ocean Sciences. University of Wales. UK, 223 p. [ Links ]
4. Beesley, P. L., G. J. Ross and C. J. Glasby (eds). 2000. Polychaetes & Allies: The Southern synthesis. Fauna of Australia. Vol 4A Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing. Melbourne, 465 p. [ Links ]
5. Bone, D. and E. Klein. 2000. Temporal variations in a tropical Soft-Bottom community, Venezuela. J. Coast. Res., 16: 278-286. [ Links ]
6. Clarke, K. R. and R. M. Warwick. 2001. Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E: Plymouth, UK. [ Links ]
7. Dauwe, B., P. M. J. Herman, C. H. R. Heip. 1998. Community structure and bioturbation potential of macrofauna at four North Sea stations with contrasting food supply. Mar. Ecol. Prog. Ser., 173: 67-83. [ Links ]
8. Fauchald, K. and P. Jumars. 1979. The diet of worms: a study of polychaete feeding guilds. Oceanogr. Mar. Biol. Ann. Rev., 17, 193-284. [ Links ]
9. Gaston, G. R. 1987. Benthic polychaete of the middle Atlantic Bight: feeding and distribution. Mar. Ecol. Progr. Ser., 36: 251-262. [ Links ]
10. Gaston, G. R., D. A. Lee and J. C. Nasci. 1988. Estuarine macrobenthos in Calcasieu Lake, Louisiana: community and trophic structure. Estuaries, 11: 192-200. [ Links ]
11. Gray, J. S. 2002. Species richness of marine soft sediments. Mar. Ecol. Prog. Ser., 244: 285-297. [ Links ]
12. Guzmán-Alvis, A. 2004. Variaciones temporales de la macroinfauna sublitoral en la plataforma colombiana aledaña al río Magdalena asociadas con cambios climáticos. Tesis doctoral de Oceanografía. Universidad de Concepción. Chile, 103 p. [ Links ]
13. Guzmán-Alvis, A. and C. B. García. 1996. Taxonomic aggregation and the detection of patterns in a tropical marine benthos data set. Rev. Biol. Trop., 44: 907-910. [ Links ]
14. Guzmán-Alvis, A. y O. D. Solano. 2001. Cambios temporales en la estructura comunitaria de la macroinfauna y su relación con la precipitación (Caribe colombiano). Resúmenes IX Congreso Latinoamericano sobre Ciencias del Mar. San Andrés Isla, p 493. [ Links ]
15. Guzmán-Alvis, A., O. D. Solano, M. Córdoba-Tejada y A. López-Rodríguez. 2001. Comunidad macroinfaunal de fondos blandos someros tropicales (Caribe colombiano). Bol. Invest. Mar. Cost., 30: 39-65. [ Links ]
16. Guzmán-Alvis, A. and F. Carrasco. 2005a. Taxonomic aggregation and redundancy in a tropical macroinfaunal assemblage of the Southern Caribbean in the detection of temporal patterns. Sci. Mar., 69: 133-141. [ Links ]
17. Guzmán-Alvis, A. and F. Carrasco. 2005b. Influence of a tropical coastal lagoon discharge and depth on the structure of adjacent shelf macroinfauna (Southern Caribbean). Cahiers Biol. Mar., 46: 81-93. [ Links ]
18. INVEMAR. 2004. Establecimiento de la línea base primera fase del plan de monitoreo ambiental del proyecto para el tratamiento y disposición final de las aguas residuales de Cartagena de Indias. Aguas de Cartagena S.A. E.S.P. Santa Marta, 366 p. [ Links ]
19. Jackson, J. B. 1972. The ecology of the molluscs of Thalassia communities, Jamaica, West Indies. II. Molluscan population variability along an environmental stress gradient. Mar. Biol., 14: 304-337. [ Links ]
20. Josefson, A. B. and B. Rasmussen. 2000. Nutrient retention by benthic macrofaunal biomass of Danish estuaries: importance of nutrient load and residence time. Estuar. Coast. Shelf Sci., 50: 205-216. [ Links ]
21. Kröncke, I., J. Dippner, J. Heyen and B. Zeiss. 1998. Long-term changes in macrofaunal communities off Norderney (East Frisia, Germany) in relation to climate variability. Mar. Ecol. Prog. Ser., 167: 25-36. [ Links ]
22. Longhurst, A. R. and D. Pauly. 1987. Ecology of tropical oceans. Academic Press: San Diego. 407 pp. [ Links ]
23. Mannino, A. and P. A. Montagna. 1997. Small-scale spatial variation of macrobenthic community structure. Estuaries, 20: 159-173. [ Links ]
24. McCarthy, S.A., E.A. Laws, W. A. Estabrooks, J. H. Bailey-Brock and E. A. Kay. 2000. Intra-annual variability in Hawaiian shallow-water, soft-bottom macrobenthic communities adjacent to a eutrophic estuary. Estuar. Coast. Shelf Sci., 50: 245-285. [ Links ]
25. Mistri, M., E. A. Fano, G. Rossi, K. Caselli and R. Ross. 2000. Variability in macrobenthos communities in the Velli di Comacchio, Northern Italy, a Hypereutrophized Lagoonal Ecosystem. Estuar. Coast. Shelf Sci., 51: 599-611. [ Links ]
26. Olsgard, F., P. J. Somerfield and M. R. Carr. 1998. Relationships between taxonomic resolution, macrobenthic community patterns and disturbance. Mar. Ecol. Prog. Ser., 172: 25-36. [ Links ]
27. Parsons, T. R., M. Takahashi and B. Hargrave. 1995. Biological Oceanographic processes. Butterworth Heinemann Ltd. Oxford. 330 p. [ Links ]
28. Pearson, T. H. and R. Rosenberg. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Ann. Rev., 16: 229-311. [ Links ]
29. Pearson, T. H. and R. Rosenberg. 1987. Feast and fanime: structuring factors in marine benthic communities: 373-395. In: Gee J. and P. Giller (eds). Organization of communities: past and present. Blackwell Scientific Publications. Oxford. The 27th Symposium of the British Ecological Society Aberystwyth. [ Links ]
30. Rouse, G. W. and F. Pleijel. 2001. Polychaetes. Oxford University Press. N. Y. 354 p. [ Links ]
31. Solano, O. D. 1994. Corales, formaciones arrecifales y blanqueamiento coralino de 1987 en bahía Portete. An. Inst. Invest. Mar. Punta de Betín, 23:149-163. [ Links ]
32. ter Braak, C. J. and P. Smilauer 2002. Canoco reference manual and CanocoDraw for Windows User's guide: Software for Canonical Community Ordination (version 4.5). Microcomputer Power (Ithaca, NY, USA), 500 pp. [ Links ]
33. Wieking, G. and I. Kröncke. 2005. Is benthic trophic structure affected by food quality? The Dogger Bank example. Mar. Biol., 146: 387-400. [ Links ]
34. Zajac, R. N. and R. B. Whitlatch. 1982. Responses of estuarine infauna to disturbance. I. Spatial and temporal variation of initial recolonization. Mar. Ecol. Prog. Ser., 10: 1-14. [ Links ]
DATE RECEIVED: 14/09/04 DATE ACCEPTED: 23/01/06