Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.35 no.1 Santa Marta Jan./Dec. 2006
SIZE AT SEXUAL MATURITY IN THE QUEEN CONCH STROMBUS GIGAS FROM COLOMBIA
TALLA EN MADUREZ SEXUAL DEL CARACOL PALA STROMBUS GIGAS DE COLOMBIA
Omar Hernando Avila-Poveda1 and Erick Raúl Baqueiro-Cárdenas2
1Centro de Investigación y de Estudios Avanzados del IPN, Mérida, Yucatán, México.Presen taddress: Unidad Multidisciplinaria de Docencia e Investigación, Facultad de Ciencias UNAM, Puerto de abrigo S/N. Sisal, Yucatán, México. Teléfonos: +52-988-9120147/49 Fax: (988) 9120020.E-mail: omarhap@yahoo.com
2Recursos Naturales Costeros, CICATA-IPN, Unidad Altamira, km 14.5 carretera Tampico-Puerto Industrial Altamira. C.P. 89600. Altamira-Tampico, Tamaulipas, México
ABSTRACT
Size at sexual maturity was investigated in 346 queen conch, Strombus gigas, collected from the Archipelago of San Andres, Providencia and Santa Catalina (SAI), Colombia. Size at sexual maturity is defined as the size (based on total shell length and lip thickness) at which 50% of the population of queen conch sampled had mature and emission gonad developmental stages, based on microscopic examination (samples of gonads were processed by standardized histological methods). Only 12.14% of whole samples had mature and emission gonad developmental stages. The size at sexual maturity based on total shell length (STmat) was estimated to be STmat=249 mm for females (n=14), STmat=234 mm for males (n=28) and STmat=241 mm for both sexes (n=42). Lip thickness (LTmat) at sexual maturity was estimated to be LTmat=17.5 mm for females, LTmat=13 mm for males and LTmat=13.5 mm for both sexes. STmat and LTmat for females were greater than those estimated for males, according to plot of the cumulative size frequencies. The maximum sizes that had mature gonad stage were 285 mm ST and 24 mm LT for males (n=2) and 280 mm ST and 30 mm LT for females (n=2). The present size restrictions adopted by some countries with queen conch fisheries is 5 mm LT and/or 180-250 mm ST, which is not adequate to protect conch stocks. A lip thickness of 13.5 mm or greater appears from this study as to be a better maturity criterion. Therefore, this criterion should be adopted by Caribbean countries as a fisheries management tool in order to protect the queen conch fishery and ensure sustainability of the fishery.
KEY WORDS: Fishery management, Lip thickness, Shell length, Sexual maturity, Strombus gigas.
RESUMEN
La talla en madurez sexual fue investigada en 346 caracoles pala Strombus gigas, colectados del archipiélago de San Andrés, Providencia y Santa Catalina (SAI), Colombia. La talla en madurez sexual es definida como la talla (basada en longitud total de la concha y grosor del labio) en la cual el 50 por ciento de la población de Strombus gigas muestreada tenía estados de desarrollo gonádico de madurez y emisión, basado en examen microscópico (Las muestras de gónadas fueron procesadas por métodos histológicos estándares). Sólo 12.14% de todas las muestras presentó estado de desarrollo gonádico en madurez y emisión. La talla en madurez sexual basada en longitud total de la concha (STmat) fue estimada en STmat=249 mm para hembras (n=14), STmat=234 mm para machos (n=28) y STmat=241 mm para ambos sexos (n=42). El grosor del labio (LTmat) en madurez sexual fue estimada en LTmat=17.5 mm para hembras, LTmat=13 mm para machos y LTmat=13.5 mm para ambos sexos. STmat y LTmat para las hembras fue mayor que el estimado para machos, según el gráfico de las frecuencias acumuladas. Las tallas máximas que presentaron estado gonádico de madurez fueron de 285 mm en ST y 24 mm en LT para machos (n=2) y de 280 mm en ST y 30 mm en LT para hembras (n=2). La actual restricción de talla adoptada por algunos países con pesquerías de caracol pala es de 5 mm de LT y/o 180-250 mm de ST; la cual no es adecuada para proteger el stock. Un grosor del labio de 13.5 mm o mayor surge de éste estudio como un mejor criterio de madurez. Por lo tanto, este criterio debería ser adoptado por los países del Caribe como una herramienta de manejo pesquero para proteger el Caracol Pala y garantizar la sustentabilidad de la pesquería.
PALABRAS CLAVE: Manejo pesquero, Grosor del labio, Longitud de la concha, Madurez sexual, Strombus gigas.
INTRODUCTION
The queen conch fishery has a long tradition in the Caribbean region; however the commercial fishery has only been expanding since the mid to late seventies, due to the relatively recent increase in demand for Strombus meat, both within the Caribbean and in foreign markets, and also by the growing tourism industry that increased the demand for shells and jewelry. Most stocks are heavily exploited (Catarci, 2004). The ultimate aim is to establish a regional conservation and management strategy (CITES, 2002).
CITES (2003) indicated that, since the 1980's, several countries started to impose species-specific regulations and management measures for their conch fisheries and most have now implemented some form of fisheries management. The most common management tools include minimum size restrictions (shell length or meat weight). However, the effectiveness of these measures is largely dependent on adequate knowledge of the stock status, other biological and morphometric criteria (shell growth and size at maturity) and country-specific characteristics of the fishery. For example, the imposition of a minimum shell length restriction for Strombus gigas does not prevent the harvest of immature individuals, unless it is implemented in combination with a lip thickness requirement.
According to Medley (2005), the minimum size for queen conch should be related to size at sexual maturity. In general, unless the shell is landed, minimum size is difficult to apply. The meat size composition may indicate violations of a flared lip rule (only mature conch allowed), but only gross violations may be detectable. Hence, minimum size may prove less useful for direct enforcement, but remains a useful indicator of the performance of other controls. A restriction requiring fishers to take only those conchs with a flared lip would require cooperation from the fishers by preventing immature conch from being landed.
The size at sexual maturity is an important biological characteristic for fisheries management (Wenner et al., 1974; Annala et al., 1980; Conand, 1981; 1990; Appeldoorn, 1988). Several studies have included observations on the size at the onset of maturity in queen conch (Randall, 1964; Alcolado, 1976; Weil and Laughlin, 1984; Wilkins et al., 1987; Wicklund et al., 1991; Berg et al., 1992; Stoner et al., 1992; Appeldoorn, 1994;). However, those studies commonly used only external macroscopic characteristics of the gonad, reproductive behavior, or growth models as criteria for assessing maturity. Consequently, sexual maturity in queen conch is considered to occur after the shell lip has started to flare and has reached a thickness of approximately 5 mm.
More precision is needed in determining sexual maturity. Knowledge of which animals are capable of reproduction is required. Quantitative numbers, such as the size at which 50% of the population is sexually mature, are desirable. The purpose of this study was to assess the size (based on total shell length and lip thickness) at sexual maturity for both sexes of Strombus gigas in Colombian waters using microscopic gonadal characteristics.
MATERIAL AND METHODS
Strombus gigas, equal to or greater than 20 cm of total shell length, with and without a flared lip) were collected from the artisanal fishing site in the south cays of the Archipelago of San Andres, Providencia and Santa Catalina (SAI), Colombia: San Andres island (12o32'N, 81o42'W), Bolivar "Courtown" cays (12o24'N, 81o28'W) and Alburquerque cays (12o10'N, 81o51'W). Sampling was conducted monthly using local fishermen between February 2003 and January 2004. Total shell length (ST, measured as the length from the tip of the siphonal canal to the apex of the spire) and lip thickness (LT, measured in the mid-lateral region in a spot unaffected by plaits, and at a distance of 35-45 mm from the edge of the lip) of whole conch were recorded, as well as the sex, according to the methodology of Appeldoorn (1988).
Samples of the visceral mass (a 1 cm3 cross-section taken along the mid-length of the gonad and digestive glands) were dissected out and pre-fixed for 12 to 15 days in 10% buffer formalin prepared in seawater with sodium borate. Because the samples would not be processed immediately, they were preserved in 70% commercial alcohol with 0.1% glycerine for air transport purposes. Histological processing was done according to the dehydration, clearing, and embedding technique used by Avila-Poveda et al. (2005, 2006).
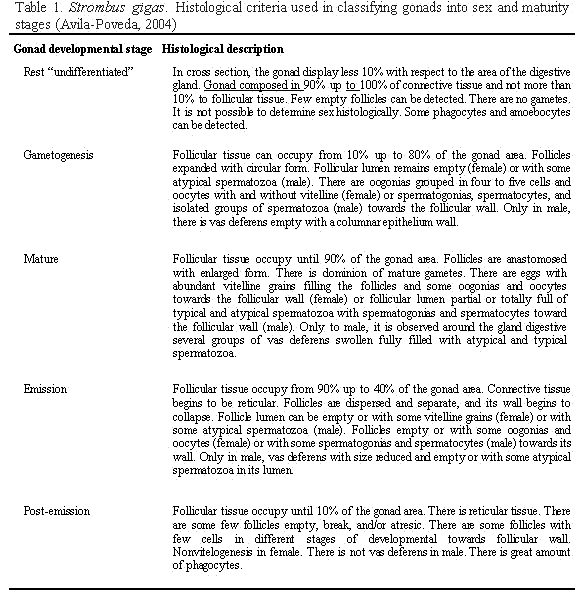
The maturity stage, as well as verification of sex, was assigned based on microscopic examination of the histological sections made from the gonads using the criteria in Table 1. Size at 50% sexual maturity (STmat and LTmat) was determined by plotting the cumulative size frequencies of individuals with mature and emission gonad
developmental stages (n=42) for both of total shell length (ST) and lip thickness (LT).
Conch with sexual aberrations (n=3, androgynous male) were excluded from this analyses (Table 2). In addition, the development of the secondary sexual characters (verge in male, egg groove in female) of these conch with mature and emission gonad developmental stages (n=42) was correlated with lip thickness and total shell length.
RESULTS
Of 346 Strombus gigas collected, only 12.14% had mature and emission gonad developmental stages. The secondary sexual characters of these conch in mature and
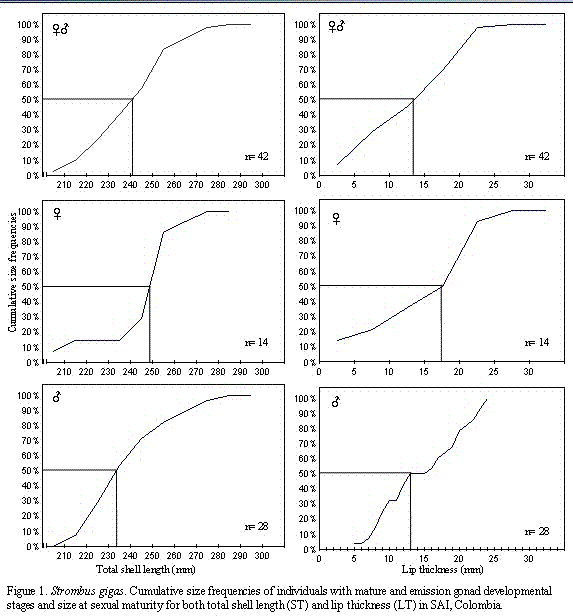
emission stages were clearly developing, i.e. males exhibited a genital groove up to the point at which the verge emerge and showing a small to large protuberance where the verge was forming; females exhibited an extended egg groove running down the side of the body to the foot. These observations allowed the size at which Strombus gigas initiates the sexual dimorphism to be assessed. At the macroscopic level, sexual polymorphism was not observed. However, upon microscopic examination of the gonads, three conch (lip thickness of 20-23 mm) were found to be feminized; they had a typical fully developed verge without an egg groove, but with gonads at mature stage completely female under microscopic examination (Table 2). Other studies (Reed, 1992, 1993, 1994, 1995) have found similar aberrations.Figure 1 shows that no queen conch were mature until the lip thickness reached a minimum of 2 mm in female, 5 mm in male, and 2 mm for both sexes and that 50% of the sample population reached sexual maturity at a lip thickness (LTmat) of 17.5 mm for females, 13 mm for males, and 13.5 mm for both sexes. In total shell length (STmat), 50% at sexual maturity was reached at 249 mm for females, 234 mm for males, and 241 mm for both sexes. STmat and LTmat is larger for females than those estimated for male. The 100% at sexual maturity recorded here were 24 mm LT and 285 mm ST for males (n=2) and 30 mm LT and 280 mm ST for females (n=2).
DISCUSSION
Size at sexual dimorphism
The development of the male secondary sexual characters, particularly with respect to the development of the verge, was found to occur at 214 mm ST with 8 mm LT on the smallest animal that had mature stage, when the conch exhibited a protuberance at the point of verge development. Appeldoorn (1988) observed this same characteristic around 222 mm ST and also indicated that verge development starts at 177 mm ST when a small protuberance is first visible. The development of secondary sexual characters (initiation of visible sexual dimorphism) in queen conch begins early in the juvenile stage, but the individual would not reach gonadal maturity until the lip thickness had reached at least 2 mm or when it had reached 205 mm in ST as found in the present study. The findings of this study agree with others that define the onset of maturation as when growth in length ceases and flared-lip formation begins (Randall, 1964; Alcolado, 1976; Wilkins et al., 1987; Appeldoorn, 1988; Wicklund et al., 1991; Berg et al., 1992; Stoner et al., 1992). Appeldoorn (1988) also indicated that the reproductive structures (verge in male, egg groove in female) are only fully developed in mature individuals, opposite to that observed by Avila-Poveda (2004), who observed queen conch of both sexes with completely formed secondary sexual characters but with gonads at undifferentiated or in gametogenesis stages.
Size at sexual maturity
The size at sexual maturity (LTmat and STmat) for Strombus gigas should be considered in the management of the fishery. A minimum legal size limit for this species in Colombian waters does not exist. Colombia has established fishery management measures for the queen conch (Resolution No. 000179 of 05 May, 1995), which is based on general knowledge of the species, a closed season, gear restrictions, closed areas, harvest quotas, and meat weight restriction that prohibits the capture of queen conch of less than 225 g without cleaning and 100 g clean by organism. Chiquillo-Espitia et al. (1997) recommended the implementation of other more effective morphometric measures, such as a minimum lip thickness, because there is no uniformity in the extraction and cleaning processes. Appeldoorn (1994) and Medley (2005) indicated that an alternative management strategy is to limit harvest to sexually mature conch.
In the literature, sexual maturity in queen conch only occurs when the shell lip has started to flare and has reached a thickness of approximately 5 mm, based on assessment of external macroscopic characteristics as criteria for maturity (Duque-Goodman, 1974; Brownell, 1977; Arango-López and Márquez-Pretel, 1993; Lagos-Bayona et al., 1996; Chiquillo-Espitia et al., 1997), reproductive behavior (Stoner et al., 1992), or growth models (Alcolado, 1976; CFMC and CFRAMP, 1999). Size at sexual maturity of Strombus gigas was examined with histological methods in only one study (Egan, 1985).
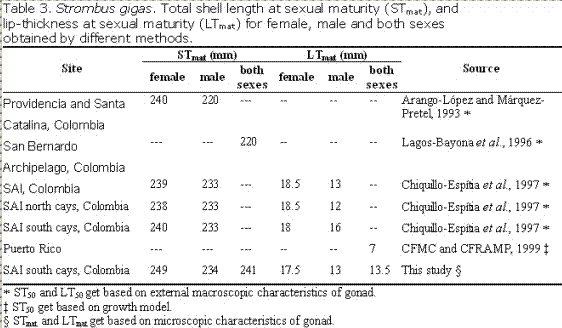
The STmat and LTmat found in this study are greater in females than in males. Arango-López and Márquez-Pretel (1993), and Chiquillo-Espitia et al. (1997) also reported that females were larger than males at ST50 and LT50 for the Colombian Caribbean; whereas, STmat and LTmat are similar to ST50 and LT50 reported by other authors (Table 3). Egan (1985) reported size frequency histograms for mature stages, but without details on STmat or LTmat; and only reported minimum and maximum lip thickness at which mature gonad tissue was present (minimum of 6 mm for females and 4 mm for males and a maximum of 29 mm for each sex. Similar values were observed in this study (Figure 1).
The present size restrictions or limits adopted by other Caribbean nations of 5 mm minimum LT and 180-250 mm in total shell length are not adequate to protect stocks should they be subject to heavy fishing pressure. The existence of a thin shell lip is not an efficient criterion of sexual maturity. A lip-thickness of 13.5 mm or greater appears
as to be a better maturity criterion. The LTmat and STmat obtained in this study are robust and allow implementation of a minimum size of capture for fisheries management in the Caribbean as recommend by CITES (2002) and Medley (2005).
ACKNOWLEDGMENTS
This paper is part of M.Sc. thesis of O.H. Avila-Poveda at CINVESTAV-IPN, Mérida, Mexico. Financial support from the CYTED II-7 and the Molluscan and Ichthyology Laboratories of CINVESTAV-IPN are acknowledged. The Environmental Ministry, "DAMA", Bogotá, Colombia, provided the certificate of exportation No. 15683 (25 August 2003) and No. CO/A/ 16470 (27 February 2004). We thank E.R. Castro-González, Clinton Pomare James, Katia Bent Escalona, Oscar Giovanni Romero and Elaisha Howard for logistics assistance at San Andres Islands. Estelman Puello Rambay made dedicated and active dissection, fixation and conservation of the samples. Our thanks also go to all the fishermen staff of cooperative "Fishingman Place Cove Sea Side": Granvill Nelson Henry, Virgilio Taylor Bowie, Alfonso Nelson Henry, Henry Mc'Nish Brackman, Kissinger Dawkins, Danny Downs, Ruben Hugdson and Camilo Hugdson who made their facilities and catches available. Liliane Frenkiel recommended tips to the histological process. Technical support in the laboratory from Teresa Cólas Marrufo and Victoria Patiño Suarez are recognized. Shawna E. Reed, Mario Rueda, and one anonymous review provided very useful comments and edited the English on the manuscript.
REFERENCES
1. Alcolado, P.M. 1976. Crecimiento, variaciones morfológicas de la concha y algunos datos biológicos del cobo Strombus gigas L. (Mollusca, Mesogastrópoda). Academia de Ciencias de Cuba, Serie Oceanología., 34. 36 p. [ Links ]
2. Annala, J.H., J. Mckoy, J.D. Booth and R.B. Pike. 1980. Size at the onset of sexual maturity in female Jasus edwardsii (Decapoda: Palinuridae) in New Zealand. N. Z. J. Mar. Freshw. Res., 14: 217-227. [ Links ]
3. Appeldoorn R.A., 1994. Queen conch management and research: status, needs and priorities. In: Appeldoorn R.A. and B. Rodríguez (Eds) Queen conch biology, fisheries and mariculture. Fundación Cientifica Los Roques, Caracas, Ven. [ Links ]
4. Appeldoorn, R.S. 1988. Age determination, growth, mortality and age of first reproduction in adult queen conch, Strombus gigas L., off Puerto Rico. Fish. Res., 6: 363-378. [ Links ]
5. Arango-López, L. and E. Márquez-Pretel. 1993. Evaluación de la población del Caracol pala Strombus gigas y la langosta espinosa Panulirus argus en las Islas de Providencia y Santa Catalina, Caribe colombiano. Fundación New-Reef/INPA, Informe Técnico. Santafé de Bogotá, Colombia. 92 p. [ Links ]
6. Avila-Poveda, O. H. 2004. Ciclo reproductivo del Caracol Pala Strombus gigas Linnaeus 1758 (Gastropoda: Caenogastropoda: Strombidae) del Archipiélago de San Andrés, Providencia y Santa Catalina, Caribe insular colombiano. M.Sc. Thesis, CINVESTAV-IPN, Unidad Mérida. Yucatán, México. 81 p. [ Links ]
7. Avila-Poveda, O.H., D. Aldana-Aranda, E.R. Baqueiro-Cárdenas and E.R. Castro-González. 2005. Preliminary data of the gametogenic cycle of Strombus gigas from Archipelago of San Andres, Providencia and Santa Catalina, Colombia (Seaflower Biosphere Reserve). Gulf and Caribbean Fisheries Institute, 56: 753-768. [ Links ]
8. Avila-Poveda, O.H., D. Aldana-Aranda and E.R. Baqueiro-Cárdenas. 2006. Histology of selected regions of the alimentary system of Strombus gigas Linnaeus, 1758 (Caenogastropoda: Strombidae). Am. Malacol. Bull., 21: 93-98. [ Links ]
9. Berg, C. J., Jr., F. Couper, K Nisbet and J. Ward. 1992. Stock assessment of queen conch, Strombus gigas, and harbor conch, S. costatus, in Bermuda. Gulf and Caribbean Fisheries Institute, 41: 433-438. [ Links ]
10. Brownell, W. N. 1977. Reproduction, laboratory culture, and growth of Strombus gigas, S. costatus and S. pugilis in Los Roques, Venezuela. Bull. Mar. Sci., 27: 668-680. [ Links ]
11. Catarci, C. 2004. World markets and industry of selected commercially-exploited aquatic species with an international conservation profile. FAO Fisheries Circular. No. 990. Rome, FAO. 186p. [ Links ]
12. CFMC and CFRAMP. 1999. Report on the Queen conch stock assessment and management workshop. 15-22 de marzo, 1999. Belice, Ciudad de Belice. 105 p. [ Links ]
13. Chiquillo-Espítia, E., J. Gallo-Nieto y J. F. Ospina-Arango. 1997. Aspectos biológicos del caracol pala, Strombus gigas Linnaeus, 1758 (Mollusca: Gastropoda: Strombidae), en el departamento Archipiélago de San Andrés, Providencia y Santa Catalina (Caribe colombiano). Boletín Científico INPA, 5: 159-179. [ Links ]
14. CITES. 2003. Review of Significant trade in Strombus gigas, AC19 Doc. 8. 71 pp. En: Review of Significant Trade in specimens of Appendix-II specie (Resolution Conf. 12.8 and Decision 12.75), Progress on the implementation of the Review of Significant Trade (Phases IV and V). Nineteenth meeting of the Animals Committee, Geneva, Switzerland, 18 -21 August 2003. [ Links ]
15. CITES. 2002. Interpretation and implementation of the Convention - Significant trade in specimens of Appendix II species - Strombus gigas, SC46 Doc.16.2. 2 pp. Forty-sixth meeting of the CITES Standing Committee, Geneva, Switzerland, 12-15 March 2002. [ Links ]
16. Conand C. (1981). Sexual cycle of three commercially important holothurian species (Echinodermata) from the lagoon of New Caledonia. Bull. Mar. Sci., 31: 523-544. [ Links ]
17. Conand C. (1990). The fishery resources of Pacific island countries. Part 2: Holothurians. F.A.O. Fisheries Technical Paper, Rome, No. 272 (2): 143 pp. [ Links ]
18. Duque-Goodman, F. 1974. Estudio biológico pesquero de Strombus (Tricornis) gigas L. mollusca, gastropoda en al Archipielago de San Bernardo (Bolivar). B.Sc. Thesis. Universidad Jorge Tadeo Lozano, Facultad de Biología marina. 75 p. [ Links ]
19. Egan, B.D. 1985. Aspect of the reproductive biology of Strombus gigas. M.Sc. Thesis. Department of Zoology, University of British Columbia. Vancouver, Canadá. 147 p. [ Links ]
20. Lagos-Bayona, A. L., S. Hernández-Barrero, H. Rodríguez-Gómez and P. Victoria-Daza. 1996. Algunos aspectos bioecológicos y reproductivos del caracol pala Strombus gigas Linnaeus, 1758 en el Archipiélago de San Bernardo, Caribe colombiano. Boletín CientíficoINPA, 4: 141-160. [ Links ]
21. Medley, P. 2005. Manual for the monitoring and management of queen conch. FAO Fisheries Circular. No. 1012. Rome, FAO. 58 p. [ Links ]
22. Randall, J.E. 1964. Contributions to the biology of the queen conch, Strombus gigas. Bul. Mar. Sci. of the Gulf and Caribbean 14: 246-295. [ Links ]
23. Reed, S.E. 1992. Histological comparison of masculinized females and androgynous males in the West Indian fighting conch, Strombus gigas. [Abstract]. J. Shellfish Res., 11: 205. [ Links ]
24. Reed, S. E. 1993. Gónadal comparison of masculinized females and androgynous males to normal males and females in Strombus (mesogastropoda: Strombidae). J. Shellfish Res., 12: 71-75. [ Links ]
25. Reed, S.E. 1994. Masculinized females in the genus Strombus: aspects of their biology and possible advantages for mariculture of conch. In: Appeldoorn, R.S. and B. Rodríguez Q. (Eds.), Queen conch biology, fisheries and mariculture, p. 213-221. Fundación Científica Los Roques, Caracas, Venezuela. [ Links ]
26. Reed, S.E. 1995. Sexual trimorphism in Strombus luhuanus Linné, 1758 (Mollusca: Gastropoda) at Shirahama, Japan. J. Shellfish Res., 14: 159-160. [ Links ]
27. Stoner, A.W., V.J. Sandt and I.F. Boidron-Metairon. 1992. Seasonality in reproductive activity and larval abundance of queen conch Strombus-gigas. U S National Marine Fisheries Service Fishery Bulletin. 90: 161-170. [ Links ]
28. Weil, E. y R. A. Laughlin. 1984. Biology, populations dynamic, and reproductions of the Queen conch Strombus gigas Linne in the Archipiélago de los Roques National Park. J. Shellfish Res., 4: 45-62. [ Links ]
29. Wenner, A.M, C. Fusaro and A. Oaten. 1974. Size at onset of sexual maturity and growth rate in crustacean populations. Can. J. Zool. 52 (109): 5-106. [ Links ]
30. Wicklund, R. I., L. J. Hepp and G. A. Wenz. 1991. Preliminary studies on the early life history of the queen conch, Strombus gigas, in the Exuma Cays, Bahamas. Gulf and Caribbean Fisheries Institute, 40: 283-298. [ Links ]
31. Wilkins, R.M., M.H. Goodwin and D.M. Reid. 1987. Research applied to conch resource management in St. Kitts/Nevis. Gulf and Caribbean Fisheries Institute, 38: 370-375. [ Links ]
DATE RECEIVED: 22/06/06 DATE ACCEPTED: 09/11/06