Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.35 no.1 Santa Marta Jan./Dec. 2006
NOTE:
IS COMPETITION FOR SPACE BETWEEN THE ENCRUSTING EXCAVATING SPONGE CLIONA TENUIS AND CORALS INFLUENCED BY HIGHER-THAN-NORMAL TEMPERATURES?
¿ESTÁ LA COMPETENCIA POR ESPACIO ENTRE LA ESPONJA EXCAVADORA INCRUSTANTE CLIONA TENUIS Y LOS CORALES, INFLUENCIADA POR TEMPERATURAS MÁS ALTAS DE LO NORMAL?
Juan Carlos Márquez1, Sven Zea1 and Mateo López-Victoria2
1Universidad Nacional de Colombia, Departamento de Biología y Centro de Estudios en Ciencias del Mar - CECIMAR, c/o Instituto de Investigaciones Marinas y Costeras (INVEMAR), Cerro Punta Betín, Santa Marta, Colombia. E-mail: juancmarquezh@gmail.com (JCM); szea@invemar.org.co (SZ)
2Institut für Allgemeine und Spezielle Zoologie, Justus-Liebig Universität, Stephanstrasse 24, 35390 Giessen, Germany. E-mail: Mateo.Lopez-Victoria@bio.uni-giessen.de
RESUMEN
Temperatura del agua de mar por encima del promedio durante 2005 en el Archipiélago de las Islas del Rosario (Caribe Colombiano) causaron un blanqueamiento coralino masivo en aguas someras (hasta 2-3 m de profundidad). Para confirmar resultados previos en los que el estrés inducido por calentamiento del agua en los corales puede acelerar la velocidad a la que esponjas excavadoras incrustantes le ganan espacio a los corales vivos, se contrastaron las tasas de propagación lateral en individuos de la esponja Cliona tenuis previamente marcados, que estaban colonizando los corales Diploria strigosa y Siderastrea siderea a profundidades de 5 - 6 m. Se hicieron comparaciones pareadas del avance de cada individuo de esponja entre dos intervalos de medida aproximadamente semestrales para dos períodos: junio 2001-julio 2002, que no tuvo un calentamiento inusual, y agosto 2004-septiembre 2005, con el calentamiento significativo sobre el umbral de blanqueamiento de corales, iniciado en junio 2005. Contrario a lo esperado, los datos no mostraron una influencia consistente del incremento de temperatura en la interacción esponja-coral durante el episodio de calentamiento. Las tasas de avance de C. tenuis contra S. siderea permanecieron bastante constantes, y fueron algo variables contra D. strigosa. La ocurrencia o no de cambios en el color de las colonias de coral (palidez, blanqueamiento) tampoco estuvo asociada con tasas de avance de C. tenuis consistentemente mayores o menores. Aunque es posible que un efecto generalizado de este fuerte calentamiento se rezagara hasta después de nuestro último intervalo de medida, los datos sugieren además que existe una susceptibilidad diferencial al calentamiento excesivo entre las especies y las colonias de coral y quizás entre los individuos de esponja.
PALABRAS CLAVE: competencia por espacio, esponjas excavadoras, corales, blanqueamiento, calentamiento grado semana-1 , Islas del Rosario, Caribe.
Sponges of the genus Cliona (family Clionaidae, order Hadromerida) which simultaneously excavate and encrust calcareous substrata are able to spread laterally and displace live coral tissue at rates of several cm per year (Schönberg andWilkinson, 2001; Rützler, 2002; López-Victoria et al., 2003; 2006). The process is carried out through the extension of excavating tissue underneath live coral, undermining the polyp's support and resulting in coral tissue retraction and sloughing off (López-Victoria et al., 2003). The rates of lateral advance into live coral and the resulting bioerosion vary to a large degree, and depend on several factors, such as the species of coral and sponge involved and the angle of confrontation, i.e., the angle between the sponge and coral planes at the boundary (Schönberg and Wilkinson, 2001; Schönberg, 2002; 2003; López-Victoria et al., 2006). Also, of external factors such as pollution, sedimentary stress, physical damage, temperature, and mediation by other organisms (Rützler, 2002; López-Victoria et al., 2003; Márquez, 2005). Regarding temperature, Cortés et al. (1984) reported an increase in Cliona tenuis (as C. caribbaea) abundance on coral reefs of Costa Rica affected by warming-triggered bleaching. Rützler (2002) found greater rates of advance of C. tenuis in Belize during the season of higher temperatures (1.3-3.1 mm day-1 vs. 0.9-1.4 mm day-1 in the season of lower temperatures). Weil (2002) predicted that encrusting excavating sponges will prevail in competition for space with corals as corals will continue to be affected by global warming.
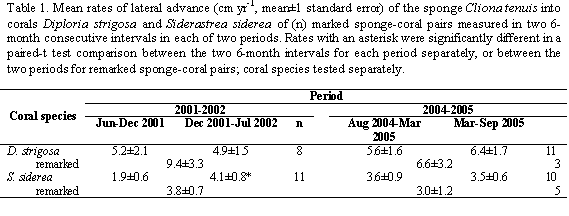
A remarkable and sudden increase in sea-surface water temperature in June 2005 triggered a massive bleaching event of reef cnidarians at Islas del Rosario Archipelago, Colombian Caribbean (10o 7' - 10o 14' N; 75o 37' - 75o 52' W), giving us the opportunity to test the hypothesis that the negative effect of higher-than-normal temperatures on corals would generate an increase in the rates of lateral advance of encrusting excavating sponges into live coral tissue. Since 2001, the advance of the sponge Cliona tenuis into the corals Siderastrea siderea and Diploria strigosa at 5-6 m depth on the windward fringing reef of Pajarales complex, NW Islas del Rosario was followed (see López-Victoria and Zea, 2004 for a description of the sites). We used data from a set of 19 C. tenuis-coral pairs, which had been marked and monitored from June 2001 to July 2002, a period without a significant increase in temperature, and a second set of 21 C. tenuis-coral pairs, which had been marked and monitored from August 2004 to September 2005, a period that included the phase of above-average seawater warming; several of the sponge-coral pairs marked for the first period were remarked for the second (Table 1). Steel nails had been driven at the sponge-coral boundary at the beginning of each period, and two measurements (to the nearest 0.5 mm) of sponge lateral advance (or retreat) were taken with plastic calipers at approximately 6-month intervals (6-7 and 13 months after initial marking for both periods). All marked sponge-coral boundaries had an approximately straight (≈180o) angle of confrontation between the sponge and coral planes, a situation in which the excavating sponge tissue continuously extends underneath the coral polyps
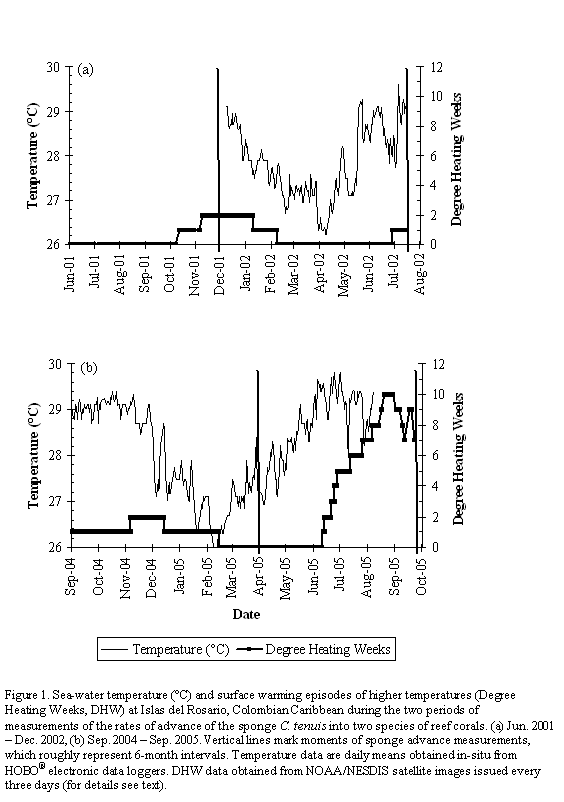
and is thus able to advance (López-Victoria et al., 2006). Measurements for all steel nails of each sponge-coral pair were averaged, generating a single datum per 6-monthly interval. Distances advanced were then standardized to rates of advance in cm yr-1. Two paired (i.e., non-independent) 6-month rates were thus obtained for each sponge (on its coral colony) within each period. The two 6-month intervals of each period could thus be compared in a typical "before-after" (first interval-second interval) contrast using a Student-t paired comparisons test (for each period and for each coral species separately). This procedure eliminates the problem of non-independence by calculating the difference between each of the two paired measurements, converting those two variables in a single one whose observations are now independent, and tests if the mean difference of all pairs is zero (Sokal and Rohlf, 1981; Underwood, 1997). Differences between intervals only for the second period when prolonged warming occurred were expected. The same test was used for sponges which were marked in both periods, comparing yearly advance rates between periods.As there were no shallow-water temperature data available for the studied periods, we used Degree Heating Week (DHW or Celsius degree week-1) data obtained from processed satellite images (50 km resolution) generated every 3-4 days by the U.S. National Oceanic and Atmospheric Administration/National Environmental Satellite, Data and Information Service (NOAA/NESDIS) (see http://www.coralreefwatch.noaa.gov/satellite/index.html). DHW images show "Hot Spots" where sea surface temperatures have remained a number of weeks above the maximum of the monthly summer values (about 29.5 oC for ground truth values in the Caribbean). One DHW is equivalent to one week, of the last 12, with temperatures staying at 1oC above 29.5 oC, or half a week, of the last 12, with temperatures staying 2oC above 29.5 oC (Liu et al., 2003). Of the single pixel that includes Islas del Rosario, we obtained a single DHW datum from every available image throughout the studied periods. For comparisons, we plotted in-situ daily mean temperature readings obtained from HOBO® electronic data loggers (readings every 5 hours), placed at a depth of 12 m at the leeward side of the archipelago.
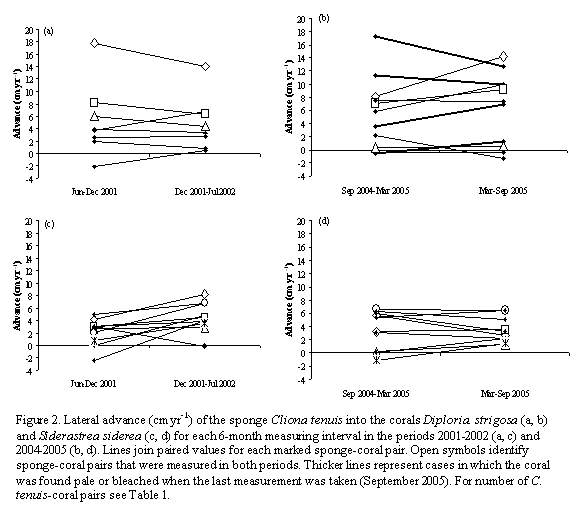
During the period 2001-2002, only a few months had values up to 1-2 DHW, occurring between October 2001 and February 2002, but not implying more than 2 weeks of continuous above-average temperatures (Figure 1a). For the period 2004-2005, a similar slight heating of 1-2 DHW was observed from September 2004 to January 2005 (most of the first 6-month interval of sponge measurements), but during the second 6-month interval temperatures remained above-average for several continuous weeks, starting in mid-June 2005, reaching a peak value of 10 DHW by the end of August 2005. By October 2005, when we did our last sponge measurements, values still reached 7-8 DHW (Figure 1b). The bleaching that ensued was massive at depths above 3-4 m, but less severe at greater depths (pers. obs. by SZ in early July 2005). In-situ temperature data at 12 m deep in general paralleled DHW trends, especially during the bleaching episode (Figure 1); daily mean temperatures were almost always above 29 oC since early June 2005, with a maximum value of 29.8 on July 1st. For the period 2001-2002, the mean advance of Cliona tenuis into Diploria strigosa was not significantly different between the two 6-month measuring intervals (paired t-test, t=0.46, p=0.66; Table 1; Figure 2a). In contrast, C. tenuis advance into S. siderea was greater for the second interval (t=-2.67, p=0.02, =7.1, p=0.02; Table 1; Figure 2c), which was not particularly warmer than the first (Figure 1a).
For the period 2004-2005, despite persistent warming during approximately four months of the second 6-month measuring interval, the mean advances of C. tenuis into both corals were not significantly different between intervals (paired t-test: D. strigosa, t=-0.77, p=0.45; S. siderea, t=0.16, p=0.89; Table 1; Figures 2b, 2d). In contrast to 2001-2002, in 2004-2005 the advance rate of C. tenuis into D. strigosa was more variable for each sponge individual between 6-month intervals. Although 4 out of the 11 D. strigosa colonies were pale or bleached when the last measurement took place, C. tenuis speed changes were not consistently related to color status (Figure 2b). C. tenuis rates of advance into S. siderea remained constant throughout period 2004-2005, and none of the colonies experienced color changes during the bleaching episode. There was also no significant difference between periods in the mean yearly rates of advance of remarked C. tenuis individuals (paired t-test; D. strigosa: t=1.52, p=0.27; S. siderea: t=-1.03, p=0.36; Table 1); the behavior of each sponge-coral pair in terms of sponge advance tended to vary between periods, with no apparent relation to the more prolonged warming of 2005.
Excavating sponges have benefited from coral mortality events associated to bleaching and diseases. Such was the case for Cliona tenuis, which occupied space freed after the mass mortality of the Elkhorn coral Acropora palmata in many shallow Caribbean fore reefs (López-Victoria and Zea, 2004). But contrary to what occurred in Belize where C. tenuis speeded up its advance into live corals in periods of greater-than-normal seasonal warming (Rützler, 2002), the 2005 warming event in the study area did not generate a consistent increase in its rate of advance during the periods of our study.
Instead, the response of the sponge and the corals to persistent above-average warming was variable: C. tenuis advance into Siderastrea siderea, which did not change color, remained constant; C. tenuis advance into Diploria strigosa was more variable, but not related to the intensity of coral bleaching. Color changes in marked C. tenuis individuals were not apparent, but shallower individuals located within the massive coral bleaching depth range were consistently grayer and softer than normal; thus, perhaps there was a physiological effect of temperature also on the sponge, which contributed to the variability in advance rates, or lack thereof. Another possibility is that a more consistent effect of warming on increasing or decreasing sponge spreading rates lagged beyond our last measurements, when DHW were still high and had lasted a little less than 4 months. On
the other hand, the advance of this sponge into corals is also determined by other factors (Rützler, 1975; 2002; Schönberg, 2002; 2003) and by organisms that may mediate in the interaction, such as turf algae or corallivorous fish (López-Victoria et al., 2003; 2006; Márquez, 2005). Nonetheless, there is no data on whether high temperature is affecting indirectly the outcome of the coral-sponge interaction through effects in other factors or in the behavior of mediating organisms. Our data might also overlap regular seasons limiting any perception of normal seasonal effects on sponge advance. In summary, there was not a clear and consistent influence of the increased temperature in C. tenuis rates of advance into corals during the time of our observations. However, there is a hint in the data that the combined physiological stress of warming on the sponge and the coral generated an effect in rates of advance of the sponge that depended in this case on the variability in susceptibility between and within coral species and perhaps on individual sponges.
ACKNOWLEDGMENTS
This research was funded by the Colombian Science Fund - COLCIENCIAS (grants 1101-09-10387 and 1101-09-13544), the Universidad Nacional de Colombia (Research Directorship - DINAIN), the Fondo para la Promoción de la Ciencia y la Tecnología del Banco de la República (contract 2001515), and the Instituto de Investigaciones Marinas y Costeras - INVEMAR. We thank Andia Chaves for her help in the field, Rafael Vieira of the Centro de Educación, Recreación e Investigación - CEINER, for his hospitality and logistical support at Islas del Rosario Oceanarium, and Diving Planet and Dolphin Dive Center of Cartagena for support in diving activities. In-situ temperature data were provided by INVEMAR's Colombian coral reef monitoring system - SIMAC; Alberto Rodríguez helped with its pre-processing.
LITERATURE CITED
1. Cortés, J., M.M. Murillo, H.M. Guzman and J. Acuña. 1984. Pérdida de zooxantelas y muerte de corales y otros organismos arrecifales en el Caribe y Pacífico de Costa Rica. Rev. Biol. Trop., 32(2): 227-231. [ Links ]
2. Liu, G., A.E. b and W. Skirving. 2003. Remote sensing of sea surface temperatures during 2002 Barrier Reef coral bleaching. EOS 84(15): 137-144. [ Links ]
3. López-Victoria, M and S. Zea. 2004. Storm-mediated coral colonization by an excavating Caribbean sponge. Clim. Res., 26: 251-256. [ Links ]
4. López-Victoria, M., S. Zea and E. Weil. 2003. New aspects on the biology of the encrusting excavating sponges Cliona aprica, Cliona caribbaea and Cliona sp. Boll. Mus. Ist. Biol. Univ. Genova, 68: 425-432. [ Links ]
5. López-Victoria, M., S. Zea and E. Weil. 2006. Competition for space between encrusting excavating Caribbean sponges and other coral reef organisms. Mar. Ecol. Prog. Ser., 312: 113-121. [ Links ]
6. Márquez, J.C. 2005. Mediación de los peces en las interacciones entre esponjas excavadoras y corales. Tesis M.Sc., Biología Marina, Universidad Nacional de Colombia-Instituto de Investigaciones marinas y Costeras-INVEMAR. 49 p. [ Links ]
7. Rützler, K. 1975. The role of burrowing sponges in bioerosion. Oecologia (Berl.), 19: 203-216. [ Links ]
8. Rützler, K. 2002. Impact of crustose clionid sponges on Caribbean reef corals. Acta Geol. Hisp., 37(1): 61-72. [ Links ]
9. Schönberg, C.H.L. 2002. Substrate effects on the bioeroding Demosponge Cliona orientalis. 1. Bioerosion rates. PSZN I: Mar. Ecol., 23(4): 313-326. [ Links ]
10. Schönberg, C.H.L. 2003. Substrate effects on the bioeroding Demosponge Cliona orientalis. 2. Substrate colonization and tissue growth. PSZN I: Mar. Ecol., 24(1): 59-74. [ Links ]
11. Schönberg, C.H.L. and C.R. Wilkinson. 2001. Induced colonization of corals by a clionid bioeroding sponge. Coral Reefs, 20: 69-76. [ Links ]
12. Sokal, R.R. and F.J. Rohlf. 1981. Biometry. The principles and practice of statistics in biological research, 2nd edition. W.H. Freeman and Co., San Francisco, xviii + 859 pp. [ Links ]
13. Weil, E. 2002. Sponge-induce coral mortality in the Caribbean. A potential new threat to Caribbean coral reefs. Boll. Mus. Ist. Biol. Univ., Genova 66-67: 211-212. [ Links ]
14. Underwood, A.J. 1997. Experiments in ecology. Their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge, xviii + 504 págs. [ Links ]
DATE RECEIVED: 01/11/05 DATE ACCEPTED: 19/09/06