Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.36 no.1 Santa Marta Jan./Dec. 2007
ABUNDANCE OF THE EXCAVATING SPONGE CLIONA DELITRIX IN RELATION TO SEWAGE DISCHARGE AT SAN ANDRÉS ISLAND, SW CARIBBEAN, COLOMBIA
ABUNDANCIA DE LA ESPONJA EXCAVADORA CLIONA DELITRIX EN RELACIÓN CON DESCARGAS DE AGUAS SERVIDAS EN LA ISLA DE SAN ANDRÉS, CARIBE SUROCCIDENTAL, COLOMBIA
Andia Chaves-Fonnegra1, Sven Zea1 and Martha L. Gómez2
1Universidad Nacional de Colombia, Departamento de Biología y Centro de Estudios en Ciencias del Mar CECIMAR. INVEMAR, Cerro Punta Betín, A.A. 1016, Santa Marta, Colombia. E-mail: andia@invemar.org.co (AChF); szea@invemar.org.co (SZ)
2Instituto de Investigaciones Marinas y Costeras (INVEMAR), Laboratorio de Microbiología, Programa deCalidad Ambiental Marina, Cerro Punta de Betín, A.A. 10-16, Santa Marta, Colombia. E-mail: mlgomez@invemar.org.co
ABSTRACT
It is known that the encrusting and excavating Caribbean sponge Cliona delitrix may increase its abundance near sources of sewage. To ascertain whether its current conspicuousness in leeward reefs of San Andrés Island (SW Caribbean, Colombia) is related to organic pollution from local raw sewage discharges, quantitative data on density and cover of this sponge and other benthic components was obtained from belt and line transects at seven stations along the shallow (5-10 m deep) terrace. Coral mucus was sampled to quantify Escherichia coli bacteria, as an approximate indicator of sewage plume influence on benthic biota. A negative multiplicative regression between amount of E. coli in coral mucus and distance from the main raw sewage outlet demonstrated the domestic-wastes origin of the bacteria. Whereas significant E. coli counts occurred only up to 1-2 km from sewage sources, overall sewage influence may extend further as moderate C. delitrix abundances occurred throughout the West shallow terrace of San Andrés, apparently associated to the overall nutrient enrichment from sewage. C. delitrix abundances were lower in the Southwest, farthest from sewage influence, and generally increased towards sewage sources, but decreased near the main sewage outlet. Close to sewage sources, any positive effect on the sponge brought about by the increase in suspended organic matter is probably outweighed by the negative effect that excessive sedimentation has on the sponge itself, and on the quantity and quality of substratum available for settlement.
KEY WORDS: Sewage, Excavating sponges, Cliona delitrix, Corals, Caribbean, Colombia.
RESUMEN
Se conoce que la esponja incrustante y excavadora del Caribe Cliona delitrix puede aumentar su abundancia en las cercanías de fuentes de aguas servidas. Para determinar si su notable abundancia actual en los arrecifes de sotavento de la Isla de San Andrés (Caribe SO, Colombia) está relacionada con la contaminación orgánica generada por las descargas locales de aguas servidas, se obtuvieron datos cuantitativos de densidad y cobertura de esta esponja y otros componentes bentónicos, en transectos de banda y línea en siete estaciones a lo largo de la terraza somera (5- 10 m de profundidad). Se obtuvo mucus de coral para cuantificar la bacteria Escherichia coli, como indicador de la influencia de las plumas de aguas servidas en la biota bentónica. Una regresión potencial negativa entre la cantidad de E. coli en mucus de coral y la distancia del tubo principal de salida de aguas servidas, demostró que las bacterias provienen de los desechos domésticos. Aunque hubo conteos significativos de E. coli solamente hasta 1-2 km de las fuentes de aguas servidas, la influencia general de estas aguas puede extenderse mucho más lejos, ya que se encontraron abundancias moderadas de C. delitrix a lo largo de la terraza somera occidental de San Andrés, aparentemente asociadas con el enriquecimiento general de nutrientes de las aguas servidas. Las abundancias de C. delitrix fueron menores en el suroccidente, lejos de la influencia de aguas servidas, e incrementaron en general hacia las fuentes, pero disminuyeron cerca del tubo de salida principal. Muy cerca de las fuentes de aguas servidas, cualquier efecto positivo en la esponja producido por un aumento en la materia orgánica en suspensión, probablemente es neutralizado por el efecto negativo que el exceso de sedimentación tiene sobre la esponja misma y sobre la cantidad y calidad del sustrato disponible para la colonización.
PALABRAS CLAVE: Aguas servidas, Esponjas excavadoras, Cliona delitrix, Corales, Caribe, Colombia.
INTRODUCTION
Sponges are important components of coral reefs, especially in the Caribbean Sea (Rützler, 1975; 2002; Zea, 1993; Diaz and Rützler, 2001). Certain environmental conditions that favor sponges (Rose and Risk, 1985; Holmes, 1997) may have a negative impact on stony corals (Pastorok and Bilyard, 1985; Szmant, 2002). For example, low illumination, relatively high sedimentation rates, and high concentrations of suspended organic matter favor the presence and increase of sponges and other heterotrophs over predominantly phototrophic organisms such as algae and zooxanthellate stony corals (see Wilkinson and Trott, 1984; Parra-Velandia and Zea, 2003). It has been suggested that sponges, by being suspension feeders, respond positively to euthrophication, increasing in biomass and/or abundance near sources of runoff, sewage discharges, or other areas in which nutrients are concentrated (see Zea, 1994; Holmes, 2000, and references therein). However, it has also been observed that sponges located very close to a pollution source may have low cover and density (Muricy, 1989), probably owing to chronic clogging of their filtration system by sediments and/or lower quality of substrata on which to settle. Sponge diversity also usually reduces close to sources of contamination (Alcolado and Herrera, 1987; Muricy, 1989, 1991; Perez, 2000). Thus, it appears that the response of the sponge community to enrichment sources depends, in part, on the balance between the opposing effects of increased food availability and increased sedimentation, which vary with distance from the sources (Holmes, 1997).
The abundance of sponges that excavate limestone and coral skeletons has also been shown to increase towards sources of terrestrial runoff and sewage discharge (Rose and Risk, 1985; Holmes, 1997; Ward-Paige et al., 2005; Hutchings et al., 2005). In addition to the benefits of increased food availability brought about by nutrient enrichment, excavating sponges may find it easier to fill the spaces of the less dense and more porous skeletons that corals may secrete when exposed to high nutrients levels (Scoffin et al., 1989; Risk and Sammarco, 1991). Moreover, any elevated coral tissue mortality from chronic sedimentation, turbidity and diseases close to enrichment sources (Nugues and Roberts, 2003) may open new space for colonization by excavating sponges (López-Victoria and Zea, 2005). However, species-specific patterns of distribution result from the combination of species tolerance and the strength and spatial extent of the discharges (Muricy, 1991).
Faecal coliform bacteria include mainly bacteria of human intestinal origin, like Escherichia coli, plus other bacteria not always associated to human intestines such as Klebsiella pneumoniae and Enterobacter aerogenes (Madigan et al., 1998). These have been widely used as indicators of water pollution by humans and other warm-blooded organisms, and have been included in water quality standards throughout the world (see APHA, 1992; Bordalo, 1993; Bordalo et al., 2002). UV radiation, temperature, salinity, heavy metals, predation and competition, have a deleterious effect on the integrity of these bacteria (Bordalo et al., 2002); although they can survive and stay viable in sea water and marine sediments where they obtain nutrients and are protected from sunlight (Davies et al., 1995), they also survive in coral mucus and may constitute an indicator of potential risk for corals and overall reef health (Lipp et al., 2002). Albeit somewhat imprecise in the amount of mucus obtained in each sample, this methodology of quantifying enteric bacteria present in coral mucus offers a quick way of detecting if sewage pollution is reaching benthic organisms.
The Caribbean sponge Cliona delitrix is a bright scarlet sponge that simultaneously excavates and encrusts calcareous substratum, often able to colonize and then completely overpower massive live corals (Pang, 1973; Chaves-Fonnegra, 2006). It is currently one of the most conspicuous sponges in shallow, leeward reefs off the western margin of San Andrés Island (SW Caribbean, Colombia). As this sponge has been reported to increase its biomass and size in coral reefs exposed to sewage pollution (Rose and Risk, 1985; Ward-Paige et al., 2005), and untreated raw sewage is disposed directly at the shore in various points of W San Andrés (Díaz et al., 1995; INVEMAR, 2004), we suspected that its conspicuousness was related to organic pollution from raw sewage discharge. Thus, we undertook a study in several stations along the W margin of San Andrés to: (1) ascertain the linear extension of the influence of the sewage plume on the benthic biota, through quantification of coliform bacteria in coral mucus and; (2) determine how the abundance of the sponge and other benthic components is related to distance from raw sewage, including sites very close to the source.
MATERIALS AND METHODS
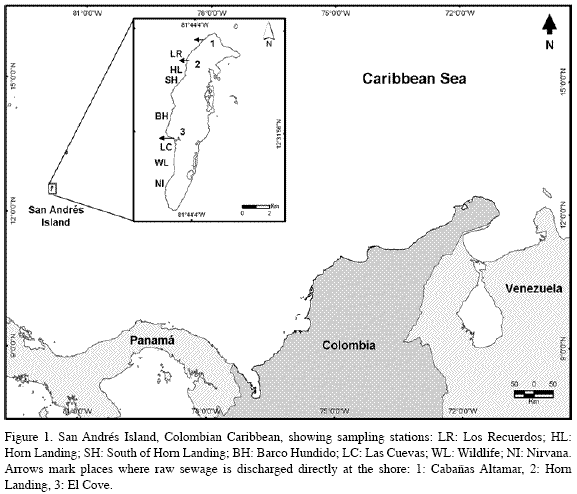
San Andrés (12°32’N, 81°43’W, Figure 1) is an oceanic island of coralline origin that is surrounded by a calcareous platform with a windward barrier reef, a lagoon with patch reefs, and leeward and windward fore reef terraces with coral carpets, all reefs being dominated by stony corals, gorgonians, sponges and algae (Díaz et al., 1995, 2000). The leeward, western margin is composed of two submerged terraces, parallel to the coast and slightly inclined, separated by a sandy trough: one terrace is shallow (4-10 m depth) along the shore; and the second is deeper (10-20 m depth), its outer edge dropping to the top of the insular slope below. The island’s main sewage outlet, located at Horn Landing (Figure 1), collects untreated wastes from the northern and most populated area of the island (Díaz et al., 1995). A smaller outlet further north, at Cabañas Altamar, disposes sewage somewhat continuously from its immediate neighborhood, and from a pumping station; to the south, at El Cove, a Colombian Navy infantry camp had an outlet pipe until 1999 (INVEMAR, 2003), and a fishing village disposes sewage directly from individual house outlets or indirectly via ground seepage from septic tanks (Contraloría General del Departamento Archipiélago de San Andrés, Providencia y Santa Catalina, 1997; personal observations, 2005) (Figure 1). Currents in the lee flow predominantly north to south owing to the influence of the NE trade winds (Geister, 1975; Andrade, 2001). To follow the main N to S influence of sewage plumes, seven stations were established at 6-10 m along the shallow terrace in sites with relatively similar physiognomy consisting of dispersed coral heads in variable density growing on a calcareous pavement that shallows gradually towards the shore, and is more steeply inclined, or even forms a cliffs at its seaward edge. Stations could not be placed in the shallow terrace off NW San Andrés, because excessive wave exposure produces a different physiognomy with very few corals. C. delitrix was already known to occur in appreciable numbers in the shallow terrace on the W and SW side of the island, whereas it is very sparsely distributed throughout the deep terrace, which is otherwise quite rich and abundant in other sponge species (S. Zea, pers. obs.).
Abundance of the enteric coliform bacteria in coral mucus was used as indicator of the approximate extension of the influence of the sewage plumes on the benthic biota. Following the methodology proposed by Lipp et al. (2002), samples of surface mucus (mixed with water) were obtained from 5 colonies of the coral Montastraea cavernosa selected haphazardly in each of the 7 stations. In September 2005, with a 60 mL sterilized, disposable plastic syringe, each sample was obtained by slowly sucking directly from the live coral surface over a square area of 6 x 6 cm, delimited by a frame of plasticized paper. Samples were kept in a cooler with ice and brought within 5 hrs to the laboratory of the San Andrés campus of Universidad
Nacional de Colombia. Fifty ml of each sample were then filtered through 0.45 μm membrane type HA filter (Millipore, MA, USA) under vacuum at 10 cm Hg pressure. Subsequently, the filter was placed over coliform selective Chromocult Merck medium (see Merck, 1996) prepared in 6 cm Petri dishes. Dishes were then incubated for 24 hrs at 35 °C, after which colony forming units (CFU) were counted. Due to logistical limitations, we could not incubate diluted aliquots from samples expected to yield high bacterial counts, but CFU could be distinguished and counted in all dishes.
As sewage plumes continuously vary in their intensity but the flow direction is predominantly to the south, bacteria associated with coral mucus probably integrate exposure to plume effluents over some time. To independently assess the likelihood that the coral-mucus associated coliform bacteria came predominantly from the sewage discharge pipes rather than from surface runoff or seepage, the amounts found in each station were related to their distance to the known outlets by linear regression with replicate samples. Distances were calculated after positioning the stations with a Garmin GPS map 168 sounder, and plotting their locations in georeferenced maps of known scale.
Quantitative data on sponge abundance and of cover of benthic components were obtained in 2-3, 40 m2 (20 m x 2 m) belt transects selected haphazardly at each station. A 20 m-long metric tape was stretched over the bottom. All individuals of C. delitrix, of all growth stages, found within 1 m of each side of the tape were counted; they were measured in their largest diameter to the nearest cm using a measuring tape attached to a 1 m-long PVC rod. The substratum on which the sponge was growing (species of living or dead coral, old unidentifiable dead coral, calcareous pavement) was recorded. This could not be determined when the sponge had completely overgrown a coral head, as it may either had settled originally on a coral that still had live tissue or an old dead coral head. When separate portions of sponge tissue were growing within about 10 cm of each other on the same coral head, we assumed that they were connected internally, thus belonging to a single physiological individual, although each portion was scored as a ramet. We had previously cut a few small coral heads to confirm that separate portions were indeed interconnected (Chaves-Fonnegra, 2006).
Percent cover of C. delitrix, stony corals (live and dead by species, plus old, unidentifiable dead), other macroorganisms (pooled as: macroalgae; other sponges; zoanthids; tunicates; gorgonians), calcareous pavement, and sand-rubble, were estimated as summed intercept lengths for each category underlying the 20-m tape measure. In a parallel study, it was found that at the studied stations C. delitrix biases its occupation of space towards elevated substratum (made by corals in the W terrace of San Andrés; cf. Chaves-Fonnegra, 2006; results to be published elsewhere). As stations varied in their amount of pavement vs. elevated substratum, we decided to normalize C. delitrix density by the amount of substratum available to its settlement, i.e., to total coral cover devoid of frondose algae and other macroinvertebrates (% live coral + % dead coral (old or current) + % C. delitrix; data obtained from cover transects). Per-station means (±1 standard deviation, SD) of C. delitrix density (individuals and ramets) and percent cover of benthic components were compared according to their position in relation to the different sewage discharges. Percent cover of live and dead coral and of C. delitrix were statistically correlated to each other using non-parametric Spearman, pair wise correlations (see Siegel and Castellan, 1988) to search for relationships.
The projected surface tissue area of each C. delitrix was calculated from its measured diameter, assuming a circular shape (summing all ramets for any individual); total abundance/transect was then estimated as the total area of all individuals, which was then also normalized to the total coral area devoid of algae and other invertebrates (see above). To assess the relationship of C. delitrix abundance to sewage discharges, per-station means of sponge tissue area and of coliform bacteria counts were related graphically.
RESULTS
Extension of sewage plume influence on the benthos
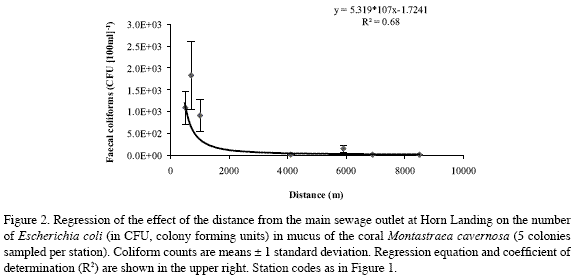
All coliform bacteria colony forming units (CFU) cultured from Montastraea cavernosa coral mucus were E. coli (see chromocult agar identification in Merck, 1996). There was a significant negative multiplicative regression between their mean number in each station and the distance to the main sewage outlet of Horn Landing (CFU=5.319*107*distance-1.72409; R2=0.68, F1,5=27.81, 0.001<p<0.005) (Figure 2). Replicate sample deviations from regression were also significant (R2=0.12, F5,28=3.56, 0.01<p<0.025), as variations were expected both from the method of mucus collection and from natural sources (among coral colonies). The three sites closest to the Horn Landing outlet (Horn Landing station: 500 m to the south; Los Recuerdos: 700 m to the north; south of Horn Landing: 1 km to the south), had the greatest mean number of E. coli, in about the same order of magnitude (1000 CFU[100 ml]-1, 1800 CFU[100 ml-1] and 900 CFU[100 ml]-1, respectively). Los Recuerdos showed the highest counts despite being up current and farther than Horn Landing from the main sewage outlet, possibly because it is also influenced by the northernmost outlet at Cabañas Altamar. From Barco Hundido and to the south (> 4 km), the number of E. coli was quite low (around 10 CFU[100 ml]-1), although in Las Cuevas (5.9 km) there was a slight increase (150 CFU[100 ml]-1), probably due to its nearness to El Cove sewage discharge (about 500 m) (Figure 2). Thus, it is likely that E. coli found in coral mucus originated in the sewage plumes. Also, it appears that E. coli from sewage plumes may settle on corals in significant numbers only up to a few hundred meters from El Cove and up to about 1-2 kilometers from Horn Landing and Cabañas Altamar combined, although general nutrient enrichment from sewage may extend much farther.
Cover and density of Cliona delitrix and other benthic components
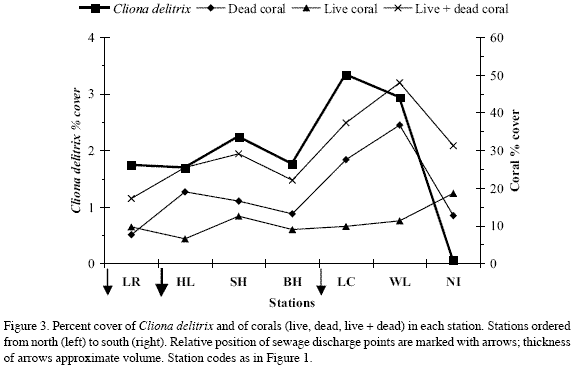
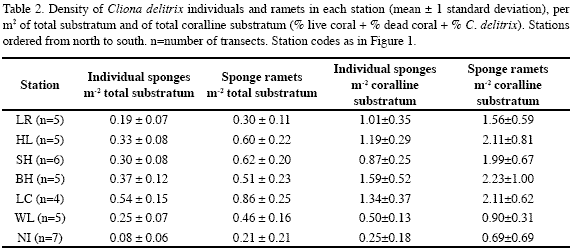
Nirvana, the southernmost station and the farthest from sewage sources, had the greatest live coral cover (19 %), and the lowest C. delitrix cover (0.1 %) and density (0.25 indiv. m-2 coralline substratum) (Figure 3, Tables 1-2). The remaining stations showed slightly higher C. delitrix cover (1.7-3.3 %) and density (0.5-1.6 indiv. m-2 coralline substratum). These stations showed live coral cover below 13 %, and dead coral cover (relative to live+dead coral) of 44 % or more (vs. 40 % in Nirvana) (data calculated from Table 1). Lowest live coral cover (6.5 %) occurred in the station nearest the main sewage outlet at Horn Landing. Thus, overall, between Los Recuerdos in the north and Wildlife in the south, the general nutrient enrichment brought about by sewage appears to have negatively affected reef corals and prompted moderate abundances of C. delitrix.
However, C. delitrix and live and dead coral cover were not correlated to each other (Spearman correlation, p>0.05); and between Los Recuerdos and Wildlife they did not show a linear pattern of increase or decrease towards sewage sources. In stations within the direct influence (500 m to 1 km distance) of the main sewage outlet at Horn Landing (Los Recuerdos, Horn Landing and south of Horn Landing) C. delitrix cover was 1.7-2.2 %, which overlaps that of Barco Hundido (1.8 %), 4.6 km off the main outlet. Its densities, in contrast, were lower near the main sewage outlet (0.9-1.2 indiv. m-2) and highest in Barco Hundido (1.6 indiv. m-2). Moreover, the highest C. delitrix cover was found in stations within 500 m (Las Cuevas, 3.3 %) to 1600 m (Wildlife, 2.9 %) of the smaller El Cove sewage source (with densities of 1.3 indiv. m-2 and 0.5 indiv. m-2, respectively). Hence, moderate exposure to sewage may have a greater positive influence on the cover and density of C. delitrix than higher exposure. On the other hand, C. delitrix cover was positively correlated with the amount of available coralline substratum (live+dead coral). Except for Nirvana, stations with the greatest amount of live+dead coral, i.e., south of Horn Landing (29 %), Las Cuevas (37 %), Wildlife (48 %), had the greatest sponge cover values (Table 1, Figure 3). Thus, availability of settling substratum may be interacting with sewage discharge to affect the current pattern of C. delitrix abundance.
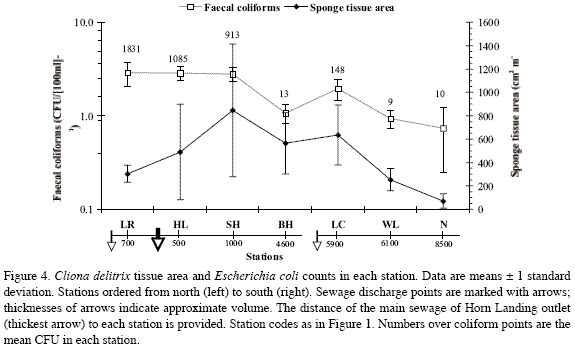
Cliona delitrix tissue area in relation to Escherichia coli in coral mucus
When plotted together from north to south, per-station C. delitrix tissue area and E. coli counts in coral mucus did not show a clear relationship (Figure 4). As with density and percent cover, relatively moderate to high C. delitrix mean tissue areas (ca. 250 cm2 to 850 cm2, per m2 of coralline substratum) occurred from Los Recuerdos in the north to Wildlife in the south. The smallest tissue areas (70±63 cm2m-2) occurred at Nirvana, the southernmost station, farthest from sewage influence (Figure 4). C. delitrix tissue area diminished in the vicinity of the main sewage outlet, but not in direct relation to E. coli abundance, which remained high where C. delitrix tissue area was the highest (south of Horn Landing, 846±566
cm2m-2). Thus, other factors operating close to the sewage source must be playing a role in influencing C. delitrix’s abundance. Farthest from the main sewage source, Barco Hundido, Wildlife and Nirvana had minimum E. coli counts but quite different C. delitrix tissue areas. However, there was a slight increase in C. delitrix’s tissue area (from 564±262 cm2m-2 at Barco Hundido to 635±257 cm2m-2 at Las Cuevas) near the minor sources of sewage at El Cove, associated with a ten-fold increase in E. coli counts, further indicating the positive influence of moderate sewage influence on C. delitrix abundance.
DISCUSSION
Our work confirms the findings of previous studies (Rose and Risk, 1985 at Grand Cayman; Ward-Paige et al., 2005 at the Florida Keys) that the excavating sponge C. delitrix may increase its abundance in areas exposed to organic pollution, being a potential bioindicator. However, we found that the positive response of C. delitrix to sewage pollution is greatest not closely, but at some distance from direct and continuous inputs, such as Horn Landing at San Andrés. When outputs have a lower or less steady flow, or when they are diffuse, through seepage or overflow, as in El Cove and in other studied areas (see Rose and Risk, 1985 and Ward-Paige et al., 2005), the sponge may have its greatest abundances closer to the sewage sources. Thus, the usefulness of this sponge as bioindicator must be judged against the nature and the intensity of sewage discharges. Moreover, our data also hint that other factors such as amount of available coralline substratum (live + dead coral, elevated over the flat pavement, see Chaves- Fonnegra, 2006) may be also important in controlling C. delitrix’s abundance.
Our finding of E. coli in coral mucus is further evidence that enteric waste bacteria may settle and dwell on the surface of corals (see Lipp et al., 2002). The negative multiplicative regression model found for abundance of E. coli in coral mucus with distance from sewage sources at W San Andrés also indicates that these bacteria originate in sewage and that their influence on benthic biota may extend up to 1-2 km from a major source such as the Horn Landing outlet, at least during the rainy season when our bacteria sampling took place. However, C. delitrix abundances were not closely related to E. coli counts, suggesting that the overall influence of nutrient enrichment brought about by sewage pollution extends farther than that of the E. coli present in coral mucus.
All coliform bacteria found in coral mucus at San Andrés were E. coli. The highest coliform bacteria values of our study (910-1800 CFU[100 ml]-1), occurring close to the main sewage outlet, were in fact higher than off the Florida Keys, where sewage pollution is more diffuse (74 CFU[100 ml]-1; Lipp et al., 2002). These latter values were similar to those we found in Las Cuevas (150 CFU[100 ml]-1), near the smaller El Cove sewage source. But the greatest abundances of C. delitrix were slightly higher at San Andrés than in other areas (San Andrés: 264 cm2 of tissue m-2 of total substratum; 0.54 indiv. m-2 total substratum; Grand Cayman: 0.23 indiv. m-2, Rose and Risk, 1985; Florida Keys: 800 cm2m-2; 0.32 indiv. m-2, combined for C. delitrix + Pione lampa, Ward-Paige et al., 2005.).
Although there were places at San Andrés with very low coliform bacteria counts and relatively high C. delitrix abundance, these were located within what we assume is the area generally influenced by sewage, covered by six of our seven stations, from Cabañas Altamar in the north to Wildlife in the south. Nirvana, our seventh site, farthest away from sewage sources, also had the minimum faecal coliform count (10 CFU[100 ml]-1) but had the lowest sponge abundance (22 cm2 of sponge tissue and 0.08 indiv. per m2 of total substratum). This site coincides with the lower bacterial counts (<5 CFU[100 ml]-1, Lipp et al., 2002) and lower C. delitrix abundances of the less contaminated sites at the Florida Keys (100 cm2 tissue m-2, 0.11 indiv. m-2, combined abundances for C. delitrix and P. lampa, Ward-Paige et al. 2005), and a control site at Grand Cayman (0.05 indiv. m-2, Rose and Risk, 1985). In lagoon and leeward terrace sites of three remote atolls located around San Andrés in the SW Caribbean, far from any sources of organic pollution, C. delitrix, when present, occurs in densities between 0.01 indiv. m-2 and 0.09 indiv. m-2 (Zea, 2001; unpublished data). Thus, abundances at Nirvana and the above-mentioned sites probably represent or are close to the natural “background” levels for this species, wherever it occurs.
The mechanisms by which growth, and perhaps reproductive and colonization abilities, of C. delitrix are favored in reefs exposed to sewage are not clear. Previously it has been hypothesized that C. delitrix may respond to an increase in suspended food brought about by sewage and other sources of nutrients. Greater bacterial cell counts (Rose and Risk, 1985), and greater ammonium and total nitrogen levels (Ward-Paige et al., 2005), have been associated with the greatest abundances of C. delitrix. In the latter study, 15N isotope concentrations in the tissues of this sponge were positively correlated with nitrogen concentrations in the water column, linking the amount of suspended food with sponge abundance (Ward-Paige et al., 2005). For sponges in general, being suspension feeders, the overall increase in their biomass and abundance with increasing suspended food, has been amply documented (Wilkinson and Cheshire, 1989; 1990; reviews in Holmes, 2000, and in Parra-Velandia and Zea, 2003). Sponges feed on bacteria, unicellular algae and possibly detritus (Goreau and Hartman, 1963; Reiswig, 1971a; Wilkinson et al., 1984; Pile, 1997), and filtering of E. coli from sewage has been demonstrated (Claus et al., 1967).
However, our results imply that any positive effects of sewage on C. delitrix seem to be eventually outweighed by the negative effects of excess sedimentation and the resulting unsuitability of the substratum for settlement, both of which are ber close to sewage sources. At San Andrés, abundance of C. delitrix decreased in stations located less than 1 km from the major sewage source. Excess sedimentation may inhibit sponge growth through clogging of ostia, which elicits body contraction and reduction of pumping rates during long periods (Reiswig, 1971a; 1971b). Through smothering, trapped sediments may in turn inhibit sponge larval settlement or prevent a young excavating sponge from penetrating the substratum. Like C. delitrix, Cliona carteri and Cliona viridis in the Mediterranean Sea show lowest abundances near pollution sites, higher abundances in intermediate sites, and again lower abundances farther from sources (Muricy, 1991). Tolerance to moderate rates of sedimentation have been invoked as the most likely cause of this pattern, with other species such as Cliona celata increasing exponentially in abundance towards the most polluted sites (Carballo et al., 1994; Muricy, 1991).
The greatest tissue area for C. delitrix at San Andrés was found just 1 km from the main sewage outlet, where E. coli counts in coral mucus were still high. Perhaps at this distance, the deleterious effects of turbidity and sedimentation on the substratum have diminished enough to permit settlement on the substratum; and currents have not yet dispersed all organic matter that serves as food for the sponge.
A previous finding of generally lower, or similarly low, abundance of the zooxanthellate encrusting and excavating sponges Cliona aprica and Cliona caribbaea off Horn Landing in the deep leeward terrace of San Andrés (10-20 m in depth) in comparison with places farther off to the south (López-Victoria and Zea, 2005) was, at the time, interpreted as contrary to findings elsewhere for non-zooxanthellate excavating sponges such as C. delitrix. Our study now confirms that C. delitrix also decreases in abundance off Horn Landing. Besides low substratum quality (turf algae heavily laden with fine sediments in the deep terrace off Horn Landing), turbidity was considered to be a negative factor from sewage affecting the sponges with photosynthetic symbionts. We do not know, however, if the assumed increased in nutrients some distance away from sewage sources would also favor these sponges as they appear to favor C. delitrix.
CONCLUSIONS
At San Andrés, faecal coliform bacteria are found in the mucus of the coral Montastraea cavernosa in amounts that are in general inversely proportional to the distance from sewage plumes. Mucus-associated bacteria are thus partly indicative of the extent of sewage influence on benthic biota.
Contamination by sewage and possibly other sources of mortality are affecting reef corals in the northern and central portions of the leeward shallow terrace of San Andrés. Associated with this phenomenon, currently the encrusting and excavating sponge C. delitrix is moderately abundant, in comparison to the southwestern portion of the island, farthest from sewage sources, where live coral cover is greatest and C. delitrix cover and density are the lowest.
Contrary to previous studies in other areas, C. delitrix increased in abundance towards sewage sources, but then decreased close to and directly off, the main sewage outlet. Thus, for this sponge to be used as bioindicator of domestic waste pollution, the volume of sewage discharge, the distance to the habitat affected, availability of substratum suitable for settlement, and doubtless many other variables, must be taken into account.
Possible mechanisms by which C. delitrix is positively affected by moderate influence from sewage runoff are: increased suspended food, greater availability of clean, recently dead coral for a settling substratum, and/or weaker, thinner and more porous coral skeletons. But when sewage influence increases, excess sedimentation and the resulting unsuitability of the substratum for settlement seem to outweigh these positive effects.
ACKNOWLEDGMENTS
Part of the thesis of A.Ch.-F., presented as partial fulfillment of the M.Sc. degree in Biology - Marine Biology, Facultad de Ciencias, Universidad Nacional de Colombia- INVEMAR. Our work was supported by the Colombian Science Fund - COLCIENCIAS (grant 110109-13544 to C. Duque and S. Zea), and by Universidad Nacional de Colombia and Instituto de Investigaciones Marinas y Costeras - INVEMAR, CAM, BEM and VAR Programs. A.Ch.-F. and S.Z. belong to the research groups “Productos Naturales Marinos y Aromas de Colombia” (Universidad Nacional de Colombia), and “Arrecifes coralinos” (INVEMAR). M.L.G. belongs to the research group “Calidad de Aguas” (INVEMAR). REDCAM and SIMAC gave access to baseline environmental data from San Andrés. Microbiological analyses were carried out at laboratories of San Andrés campus, Universidad Nacional de Colombia, with the help of Jairo Medina. CORALINA, San Andrés Archipelago’s environmental agency, allowed us to study their microbiological data and gave us official local support during the process of granting research permits by the Colombian Ministry of the Environment, Housing and Territorial Development. Special thanks to Carlos Andrés Orozco from CORALINA. We are grateful for help during field work to J. C. Márquez and Banda Dive Shop. Christine Ward-Paige and Erin Lipp helped with discussions and gave detailed information. The map was made by D. Rozo in INVEMAR’s GIS-Lab. Two anonymous reviewers greatly helped to improve the initial version.
LITERATURE CITED
1 Alcolado, P. and A. Herrera. 1987. Efectos de la contaminación sobre las comunidades de esponjas en el litoral de la Habana, Cuba. Rep. Invest. Inst. Oceanol., Havana, 68:1-17. [ Links ]
2 Andrade, C.A. 2001. Las corrientes superficiales en la cuenca de Colombia observadas con boyas de deriva. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 25(96):321-335. [ Links ]
3 APHA. 1992. Standard methods for the examination of water and wastewater, 15th edn. New York: Water Pollution Control Federation. [ Links ]
4 Bordalo, A.A. 1993. Effects of salinity on bacterioplankton: field and microcosm experiments. J. Appl. Bacteriol., 75: 393-398. [ Links ]
5 Bordalo, A.A., R. Onrassami and C. Dechsakulwatana. 2002. Survival of faecal indicator bacteria in tropical estuarine waters (Bangpakong River, Thailand). J. Appl. Bacteriol., 93:864-871. [ Links ]
6 Caraballo, J.L, J.E. Sánchez-Mollano and J.C. García-Gómez. 1994. Taxonomic and ecological remarks on boring sponges (Clionidae) from the Straits of Gibraltar (southern Spain): tentative bioindicators?. Zool. J. Linn. Soc., 112:407-424. [ Links ]
7 Chaves-Fonnegra, A. 2006. Mecanismos de agresión de esponjas excavadoras incrustantes y consecuencias sobre corales arrecifales en el Caribe colombiano. Tesis M.S.c. Biol. Mar. Univ. Nacional de Colombia - INVEMAR, Santa Marta, 116 p. [ Links ]
8 Claus, G., P. Madri and S. Kunen. 1967. Removal of microbial pollutants from waste effluents by the redbeard sponge. Nature, 216:712-714. [ Links ]
9 Contraloría General del Departamento Archipiélago de San Andrés, Providencia y Santa Catalina. 1997. Informe Ambiental. pp. 3-27. [ Links ]
10 Davies, C.M., J.A.H. Long, M. Donald and N.J. Ashbolt. 1995. Survival of fecal microorganisms in marine and freshwater sediments. Applied and Environmental Microbiology, 61(5):1888-1896. [ Links ]
11 Diaz, C.M. and K. Rützler. 2001. Sponges: an essential component of Caribbean coral reefs. Bull. Mar. Sci., 69(2): 535-546. [ Links ]
12 Díaz, J.M., L.M. Barrios, M. H. Cendales, J. Garzón-Ferreira, J. Geister, M. López-Victoria, G.H. Ospina, F. Parra-Velandia, J. Pinzón, B. Várgas-Angel, F. Zapata and S. Zea. 2000. Áreas coralinas de Colombia. Colombia: Invemar-Serie Publicaciones Especiales No. 5. 176 p. [ Links ]
13 Díaz, J.M., J. Garzón-Ferreira and S. Zea. 1995. Los arrecifes coralinos de la isla de San Andrés, Colombia: Estado actual y perspectivas para su conservación. Academia Colombiana de Ciencias Exactas, Físicas y Naturales Colección Jorge Álvarez Lleras N° 7. Editora Guadalupe LTDA. Colombia. 150 p. [ Links ]
14 Geister, J. 1975. Riffbau und geologische Entwicklungsgeschichte der Insel San Andrés (westliches Karibisches Meer, Kolumbien). Stuttgarter Beiträge Naturkunde Geol. Paläeont., 15:1-203. [ Links ]
15 Goreau, T.F. and W.D. Hartman. 1963. Boring sponges as controlling factors in the formation and maintenance of coral reefs. In: American Association for the Advancement of Science (Ed.) Mechanisms of hard tissue destruction. 75:25-54. [ Links ]
16 Holmes, K.E. 1997. Eutrophication and its effect on bioeroding sponge communitie. Proc. 8th Int. Coral Reef Sym., 2: 1411-1416. [ Links ]
17 Holmes, K.E. 2000. Effecs of eutrophicación on bioeroding sponge communities with the description of new West Indian sponges, Cliona spp. (Porifera: Hadromerida: Clionidae). Invert. Biol., 119(2):125-138. [ Links ]
18 Hutchings, P., M. Peyrot-Clausade and A. Osnorno. 2005. Influence of land runoff on rates and agents of bioerosión of coral substrates. Mar. Poll. Bull., 51:438-447. [ Links ]
19 INVEMAR. 2003. Red de Vigilancia para la conservación y protección de las aguas marinas y costeras de Colombia: Diagnóstico y evaluación de la calidad ambiental marina en el Caribe y Pacífico colombiano año 2002. Diagnóstico Nacional. Santa Marta, Colombia. 200 p. [ Links ]
20 INVEMAR. 2004. Red de Vigilancia para la conservación y protección de las aguas marinas y costeras de Colombia: Diagnóstico y evaluación de la calidad ambiental marina en el Caribe y Pacífico colombiano año 2003. Diagnóstico Nacional y Regional. Santa Marta, Colombia. 263 p. [ Links ]
21 Lipp, E.K., J.L. Jarrell, D.W. Griffin, J. Lukasik, J. Jacukiewicz and J.B. Rose. 2002. Preliminary evidence for human fecal contamination in corals of the Florida Keys, USA. Mar. Poll. Bull., 44:666-670. [ Links ]
22 López-Victoria, M. and S. Zea. 2005. Current trends of space occupation by encrusting excavating sponges on Colombian coral reefs, Marine Ecology, 26:33-41. [ Links ]
23 Madigan, M.T., J.M. Martinko and J. Parker. 1998. Brock: Biología de los Microorganismos. Prentice Hall. España. 986 p. [ Links ]
24 Merck. 1996. Microbiology Manual. Merck KGaA. Darmstadt, Germany. pp. 349-352. [ Links ]
25 Muricy, G. 1989. Sponges as pollution biomonitors at Arraial do Cabo, southeastern Brazil. Rev. Bras. Biol., 49:347-354. [ Links ]
26 Muricy, G. 1991. Structure des peuplements de spongiaires autour de légout de Cortiou (Marseille, France). Vie et Milieu, 41(4):205-221. [ Links ]
27 Nugues, M.N. and C.M. Roberts. 2003. Coral mortality and interaction with algae in relation to sedimentation. Coral Reefs, 22:507-516. [ Links ]
28 Pang, R.K. 1973. The systematics of some Jamaican excavating sponges. Postilla, 161, 75 p. [ Links ]
29 Parra-Velandia, F.J. and S. Zea. 2003. Comparación de la abundancia y distribución de algunas características de las esponjas del género Ircinia (Porifera: Demospongiae) en dos localidades contrastantes del área de Santa Marta, Caribe colombiano. Bol. Invest. Mar. Cost., 32:75-91. [ Links ]
30 Pastorok, R.A. and G.R. Bilyard. 1985. Effects of sewage pollution on coral-reef communities. Mar. Ecol. Prog. Ser., 21:175-189. [ Links ]
31 Perez, t., 2000. Evaluation of coastal areas quality by sponges: state of the art. Bull. Soc. Zool. Fr., Paris, 125(1):17-25. [ Links ]
32 Pile, A.J. 1997. Finding Reiswig´s missing carbon: quantification of sponge feeding using dual-beam flow cytometry. Proc. 8th. Coral Reef Sym., 2:1403-1410. [ Links ]
33 Reiswig, H.M. 1971a. Particle feeding in natural population of three marine Demospongiae. Biol. Bull., Woods Hole, 141(3):568-591. [ Links ]
34 Reiswig, H.M. 1971b. In situ pumping activities of tropical Demospongiae. Mar. Biol., 9:38-50. [ Links ]
35 Risk, M.J. and P.W. Sammarco. 1991. Cross-shelf trends in skeletal density of the massive coral Porites lobata from the Great Barrier Reef. Mar. Ecol. Prog. Ser., 69:195-200. [ Links ]
36 Rose, C. S. and M. J. Risk. 1985. Increase in Cliona delitrix infestation of Montastraea cavernosa heads on an organically polluted portion of the Grand Cayman. P.S.Z.N.I: Mar. Ecol., 6(1):345.363. [ Links ]
37 Rützler, K. 1975. The role of burrowing sponges in bioerosion. Oecologia, 19:203-216. [ Links ]
38 Rützler, K. 2002. Impact of crustose clionid sponges on Caribbean reef corals. Acta Geologica Hispánica., 37(1):61-72. [ Links ]
39 Scoffin, T.P., A.W. Tudhope and B.E. Brown. 1989. Fluorescent and skeletal density banding in Porites lutea from Papua New Guinea and Indonesia. Coral Reefs 7:169-178. [ Links ]
40 Siegel, S. and N.J. Castellan. 1988. Nonparametric statistics for the behavioral sciences. McGraw-Hill, Inc. Estados Unidos. 399 p. [ Links ]
41 Szmant, A.M. 2002. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries, 25(4b): 743-766. [ Links ]
42 Ward-Paige, C.A., M.J. Risk, O.A. Sherwood and W.C., Jaap. 2005. Clionid sponge surveys on the Florida Reef Tract suggest land-based nutrient inputs. Mar. Pollut. Bull., 51:570-570. [ Links ]
43 Wilkinson, C.R. and A. C. Cheshire. 1989. Patterns in the distribution of sponge populations across the central Great Barrier Reef. Coral Reefs, 8:127-134. [ Links ]
44 Wilkinson, C.R. and A. C. Cheshire. 1990. Comparisons of sponge populations across the barrier reef of Australia and Belize: evidence for higher productivity in the Caribbean. Mar. Ecol. Prog. Ser., 67:285-294. [ Links ]
45 Wilkinson, C.R. and L.A. Trott. 1984. Light as a factor determining the distribution of sponges across the central Great Barrier Reef. Proc. 5th. Int. Coral Reef Congr., 5:125-130. [ Links ]
46 Wilkinson, C.R., R. Garrone and J. Vacelet. 1984. Marine sponges discriminate food bacteria and bacterial symbionts: electron microscope radioautography and in situ evidence. Proc. R. Soc. Lond., 220:519- 528. [ Links ]
47 Zea, S. 1993. Cover of sponges and other sessile organisms in rocky and coral reef habitats of Santa Marta, Colombian Caribbean Sea. Caribb. J. Sci., 29:75-88. [ Links ]
48 Zea, S. 1994. Patterns of coral and sponge abundance in stressed coral reefs at Santa Marta, Colombian Caribbean. 257-264. In: R.W.M. van Soest., T.M.G van Kempen and J.C Braekman (Eds). Sponges in time and space, Balkema. Rotterdam. [ Links ]
49 Zea, S. 2001. Patterns of sponge (Porifera, Demospongiae) distribution in remote, oceanic reef complexes of the southwestern Caribbean. Rev. Acad. Colomb. Cienc., 25(97):579-592. [ Links ]
FECHA DE RECEPCIÓN: 27/02/06 FECHA DE ACEPTACIÓN: 08/06/07