Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Boletín de Investigaciones Marinas y Costeras - INVEMAR
versión impresa ISSN 0122-9761
Bol. Invest. Mar. Cost. v.36 n.1 Santa Marta ene./dic. 2007
FISSION IN THE ZOANTHARIA PALYTHOA CARIBAEORUM(Duchassaing and Michelotii, 1860) POPULATIONS: A LATITUDINAL COMPARISON
FISIÓN EN POBLACIONES DE PALYTHOA CARIBAEORUM (DUCHASSAING AND MICHELOTII, 1860): UNA COMPARACIÓN LATITUDINAL
Alberto Acosta and Ana M. González Pontificia
Universidad Javeriana, Departamento de Biología, Unidad de Ecología y Sistemática (UNESIS), Edificio 53 Oficina 112, Bogotá, Colombia. E-mail: laacosta@javeriana.edu.co (AA)
ABSTRACT
There are few regional studies attempting to compare the asexual reproductive output of marine populations, particularly when they are exposed to different environmental conditions. In this study we compared Caribbean and Southwestern Atlantic Palythoa caribaeorum populations in terms of ramet production, the minimum colony size for fission, and the relationship between fission frequency and colony size. Fission process was quantified in Ponta Recife and Praia Portinho, Sao Paulo, Brazil, and in Punta de Betín, Colombia, during the summer (December-January) of 1997 and 1998, respectively. Fission started at small colony size in both populations studied (> 4 cm2). The number of ramets produced per colony increased with colony size in Brazil and Colombia. Colombian zoanthids produced more ramets by fission than Brazilian populations. The populations shared early reproduction characteristics, and production of large numbers of ramets, which increased with colony size, even though they differed in fission frequency. Fission seems to be a conservative trait in P. caribaeorum, although its expression could vary depending on habitat conditions related to biotic and/or abiotic factors.
KEY WORDS: Asexual reproduction, Colony size, Fission, Latitudinal comparison, Palythoa caribaeorum.
RESUMEN
Existen pocos estudios regionales que intentan comparar el esfuerzo de reproducción asexual de poblaciones marinas, particularmente cuando éstas están expuestas a diferentes condiciones ambientales. En este estudio comparamos poblaciones de Palythoa caribaeorum del Caribe y del Atlántico Sur occidental en términos de producción de clones, mínimo tamaño de la colonia para fisionarse, y la relación que existe entre la frecuencia de fisión y el tamaño de la colonia. El proceso de fisión fue cuantificado en Ponta Recife y Praia Portinho, Sao Paulo, Brasil y en Punta de Betín, Colombia, durante el verano (Diciembre-Enero) de 1997 y 1998 respectivamente. La fisión comenzó en colonias de tamaño pequeño en las dos poblaciones estudiadas (> 4 cm2). El número de ramets producidos por colonia incrementó con el tamaño colonial en Brasil y en Colombia. Los zoantídeos de Colombia produjeron más ramets por fisión que poblaciones brasileras. Las dos poblaciones presentan ciertas características como reproducción temprana y producción de un gran número de ramets, los cuales incrementan con el tamaño colonial, aunque estas difieren en la frecuencia de fisión. La fisión parece ser una característica conservativa en P. caribaeorum, aunque su expresión podría variar dependiendo de las condiciones del hábitat relacionadas con factores bióticos y/o abióticos.
PALABRAS CLAVE: Reproducción asexual, Tamaño colonial, Fisión, Comparación latitudinal, Palythoa caribaeorum.
INTRODUCTION
Asexual reproduction is the process by which an increase in colony numbers is achieved without the aid of genetic recombination. Clonality is a common feature of plants (Cook, 1985) and benthic marine organisms. In Cnidaria, new colonies (ramets) can be formed asexually through several mechanisms, including polyp bail-out (Sammarco, 1982), coral polyp expulsion (Kramarsky et al., 1997), fragmentation (see review in Highsmith, 1982), asexually produced planulae (Fautin, 2002), and colony fission (Pearse, 2002) among others. Fission is a primary mode of asexual reproduction in numerous anthozoans, such as zoanthids and scleractinian corals (Hughes and Jackson, 1980; Tanner, 1999), and play an important role in population size (Tanner, 1999), dynamics (Acosta et al., 2005), and structure in several species (Karlson, 1991; McFadden, 1991). Despite the fission importance for ecology (Tanner, 2002), biogeography (Skold et al., 2002), and evolution (contributions of fission expression to genetic structure and genet survival; Fautin, 2002; Geller et al., 2005), few spatial comparisons have been published regarding how many ramets are produced via fission (Miller and Ayre, 2004; Zilberberg et al., 2006) and how these reproductive strategies affect the population size (Tanner, 2000).
Spatial variation in fission expression (i.e. frequency) between populations has been explained by a number of intrinsic (genetic) and extrinsic factors (Hughes, 1989; Skold et al., 2002; Zilberberg et al., 2006). The extrinsic abiotic factors proposed to control fission are: wave action, desiccation, temperature (Crump and Barker, 1985), seasonal variation (Mladenov, 1996), marginal habitats and disturbance level (Morris et al., 2004; Foster et. al., 2007), and biotic factors such as food availability (Tsuchida and Potts, 1994), and population density (Karlson et al., 1996; Tanner, 1999; 2002; Baums, et al., 2006). The magnitude of this change at regional scale (thousands of km) in populations exposed to different habitats however has been poorly studied (Zilberberg et al., 2006). Latitudinal variation in the frequency of asexual reproduction has been reported in an aquatic annual plant (Potamogeton pectinatus; Santamaría and García, 2004), marine sponges (Zilberberg et al., 2006), and corals (Miller and Ayre, 2004), but no causes have been explored.
P. caribaeorum is a common sessile epibenthic shallow water species occurring in reef areas (reef crest or reef flats; Díaz et al., 2000) in the Caribbean (Gleibs, 1994; Mueller and Haywick, 1995) and rocky shores along the Southwestern Atlantic (Acosta, 1999; Oigman-Pszczol et al., 2004; Perez et al., 2005). This species is present in Colombia under nearly all oceanographic conditions in continental and oceanic islands (Acosta A. pers. obs.), including upwelling zones (i.e. Santa Marta; Gleibs, 1994). Along the Brazilian coast, P. caribaeorum is one of the dominant benthic species (Migotto, 1997; Oigman-Pszczol et al., 2004; Perez et al., 2005); however, in the southern latitudinal limit of geographical distribution (Sao Paulo coast) this zoanthid is exposed to frequent storms, constant turbid waters, and high sedimentation rates (Acosta et al., 2005).
Nevertheless, the Caribbean and the Brazilian shallow water faunas are geographically separated due to freshwater and sediment discharged by the Orinoco and Amazon rivers (11 myo; Robertson, et al., 2006) which act as physical barrier (Lessios et al., 2001; Masson and Delecluse, 2001; Rocha, 2003). Fission in P. caribaeorum is known to occur in a wide distributional range from Florida Keys to São Paulo, Brazil (Acosta, pers. obs.); consequently, it is an ideal organism to test if fission is a conservative trait on geographically separated populations, if isolated populations exhibit similar fission frequency, and the relationship of fission to habitat and colony size.
P. caribaeorum reproduces asexually by fission and fragmentation all year round (Acosta et al., 2001; 2005). Fission seems to be more important than fragmentation in terms of ramets added to population growth in P. caribaeorum (Acosta et al., 2005), as it occurs with P. caesia (Tanner, 1997; 2000). Four types of fission are known for P. caribaeorum (Acosta et al., 2005). The first evidence of fission in this species is the presence of crevices over the colony’s surface caused by progressive degradation of the coenenchyme tissue connecting the polyps (Acosta, 1999). When the crevices become widely distributed over the colony, they join forming polyp-clusters, which are connected to each other. When a polyp-cluster becomes physically isolated from the parent colony (lost of basal coenenchyme) the process of fission is completed, and new ramets are added to the population (Acosta et al., 2005). Ramets were defined as a portion of tissue physically isolated from the parent colony (Cook, 1985; Hughes, 1989).
Colony size has been directly related with sexual reproduction (i.e. reproductive effort; Richmond, 1987; Tsuchida and Potts, 1994; Hall and Hughes, 1996), and particularly with the asexual reproduction in several species of the Phylum Cnidaria (Walker and Bull, 1983; Hughes and Connell, 1987; Karlson, 1988; Lasker, 1990; Acosta et al., 2005). Nevertheless minimal colony size for asexual reproduction is unknown for most coral or zoanthid species. The expression of this trait during the life cycle (starting earlier or later in life) has been demonstrated to change between populations of the same species. This has been related to the degree of disturbances and selective pressures experimented by colonies in a particular habitat (or environment), and the rate of mortality experienced at different stage classes. The adaptive strategy employed by Palythoa under an unpredictable environment is unknown at this time.
In this study we compare minimum colony size for fission, the relationship between fission and colony size, and ramet production via fission between tropical Colombian (11° N) and temperate Brazilian (23° S) P. caribaeorum populations.
MATERIALS AND METHODS
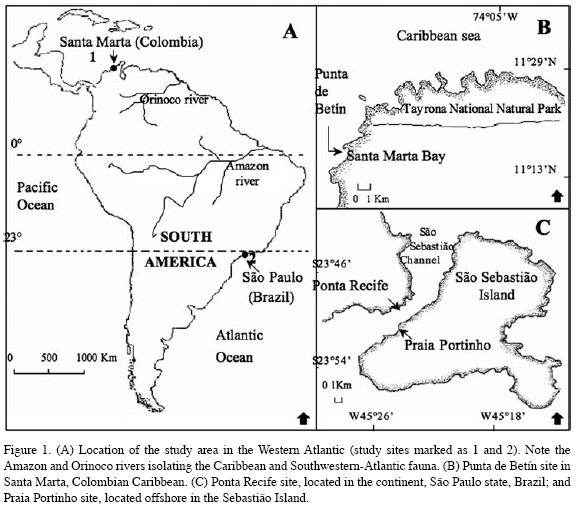
During the summer of 1997 (December-January; rainy season), signs of fission (presence of crevices and/or the number of polyp-clusters), and frequency of fission (number of ramets produced per colony) were recorded in 515 colonies of Palythoa caribaeorum on Praia Portinho and Ponta Recife, São Sebastião Channel, São Paulo, Brazil (23 °S - 45 °W; Figure 1). The presence of crevices (due to degradation of the coenenchyme tissue, according to Acosta et al., 2005), the number of polyp-clusters (differentiated polyp groups, although physically connected to each other), and the number of ramets produced per colony were quantified following Acosta et al. (2005). In Summer (December-January) of 1998, dry and windy season (upwelling zone), the same
variables were measured in 383 colonies in Punta de Betín, Santa Marta, Colombian Caribbean (11 °N - 74 °W; Figure 1). Colonies were sampled haphazardly between 0.5 and 4.0 m deep in Brazil, and 0.5 and 5.0 m deep in Colombia, and included a size range from 2 to 13440 cm 2, and 0.65 to 43400 cm2 area for Brazil and Colombia, respectively. Colonies exhibiting fission, those that showed crevices, connected polyp-clusters, and/or ramets were compared between sites using two a tail t-test (Sokal and Rohlf, 1995).
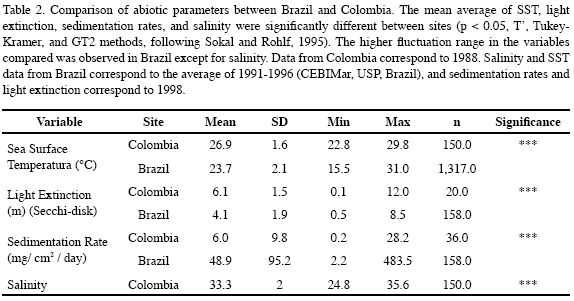
The percent cover of benthic organisms and substratum type was quantified by a random-point technique along a chain transect, sampling 300 points in Colombia, and 400 in Brazil. Differences in Palythoa caribaeorum percent cover between the two sites were assessed using a t-test (Sokal and Rohlf, 1995). In addition, measurements were made to compare environmental factors between the study sites. Assessment of light extinction (Secchi disc; weekly measurements), and sedimentation rates were done as well. Sedimentation rates were measured using 12 cylindrical PVC sediment traps per location (35 cm length x 5 cm diameter). These were fixed to the bottom, and collected and replaced monthly. Sea surface temperature (SST), and salinity were measured daily in the Centro de Biologia Marinha (CEBIMAR-USP-Brazil; 1991 to 1996) and the Instituto de Investigaciones Marinas (INVEMAR-Colombia; 1998). T’, Tukey-Kramer, and GT2 methods were used to compare abiotic parameters between Brazil and Colombia (Sokal and Rohlf, 1995).
Colony size was measured, and expressed as an area (maximum colony length x maximum width). The relationship between ramet production and colony size was performed using regression analysis at each site. Slopes different from zero were tested for each regression line (Kendall’s robust line-fit method, Sokal and Rohlf, 1995). Among regression lines we examine: a) differences between regression coefficients (F-test), and b) the equality of the intercepts (ANCOVA).
RESULTS
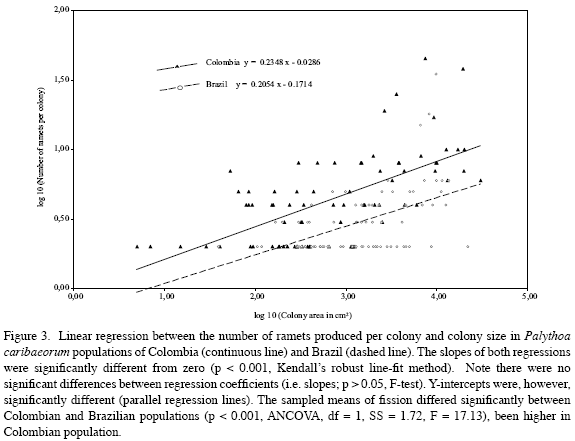
Palythoa caribaeorum was the benthic dominant organism in both sites (Figure 2). Colonies were relatively scarce and dispersed, and had much more hard substrate (rock) available to grow in Colombia than in Brazil. Both populations studied exhibited increased numbers of ramet production via fission (Table 1), although the relative frequency of the colonies exhibiting crevices, the number of polyp- clusters within the colonies, the number of colonies with ramets, and the total number of ramets produced by fission were higher in Colombian than in Brazilian populations (Table 1). A positive and highly significant linear relationship was found to occur between the number of ramets produced per colony and the colony size in both Brazilian and Colombian populations (Figure 3), though clonality was higher in Colombia than in Brazil when comparing colonies of similar size (Figure 3). Fission occurred at similar small colony size in both study sites (Table 1).
Significant differences in SST, salinity, light extinction and sedimentation rates were registered between the two sites. Colombia exhibited relatively better environmental conditions for SST, sedimentation rate and light extinction than Brazil, except for the high salinity variation (Table 2).
DISCUSSION
Several factors can explain the higher fission frequency found in Palythoa caribaeorum populations in Colombia vs. Brazil. a) Free space, small cover and low density of Colombian P. caribaeorum populations may stimulate colony fission, which may explain the differences observed in fission frequency with Brazil. This result agrees with Tanner (1999, 2000, 2002) who demonstrated that fission frequency increases at low density (i.e. cover) in P. caesia, and decreases when the population cover reaches > 90% of the substratum. Similarly, Acosta (1999) quantified low levels or no fission when P. caribaeorum covered 100% of the substrate in Brazil, indicating density-dependence effect. The density-dependent control of fission rates has also been demonstrated for corals and other marine organisms (Walker, 1975; Baums et al., 2006). P. caribaeorum has been considered a primary colonizer in shallow reef areas (Sebens, 1982), and it may become the dominant benthic species for long periods of time (Acosta, pers. obs.). It is possible that Colombian populations employed fission and less sexual recruits (Acosta, et al., 2005) as a mechanism to colonize the available substrate left by dead corals, as observed with other pioneer species after disturbance (Jackson et al., 1985; Coffroth and Lasker, 1998) or under stochastic environment (Lehmann et. al., 2006). In the last decade several authors have documented scleractinian coral decline (less coral cover and richness) at Santa Marta reefs (Colombia; Acosta and Martinez, 2005; Martinez and Acosta, 2005). In Brazilian populations the high density and cover may act in two opposite ways: 1. decreasing fission rate when population reach more than 84% cover, as demonstrated in P. caesia (Tanner, 1999), and 2. increasing fission rate, when there is no more substrate available for colony growth (Acosta et al., 2005); thus, fission can be used as an escape strategy to reach new habitats.
b) The positive relationship found in P. caribaeorum between fission frequency and colony size has also been reported in aquatic plants (Santamaría and García, 2004), scleractinians (Hughes and Connell, 1987), octocorals (Walker and Bull, 1983; McFadden, 1991), and the zoanthid P. caesia (Tanner, 1997). The higher colony size of Colombian than Brazilian populations could explain why the former may produce more ramets. Considering that asexual and sexual reproductive output in P. caribaeorum has been positively correlated to colony size (Acosta and Asbarh, 2000; Acosta A, unpublished data) we can infer that fitness may be enhanced by increasing colony size, as is known to occur in P. caesia (Tanner, 1997), and Alcyonium sp. (McFadden, 1991).
Although colony size may control the fission expression, the low regression coefficient observed in our populations suggests that it is not the only factor involved. Acosta et al. (2005) suggested that intrinsic (genetic) and environmental factors could act synergistically to modulate fission expression, but the relative importance of each will need future research.
c) Our results agree with Zilberberg et al. (2006) who demonstrated large differences in sponge clonality, where the two species from Bahamas (Caribbean) had a greater proportion of asexually produced individuals than those along the coast of Brazil. Larger proportion of ramets produced in Bahamas was explained by the more homogeneous and temporally stable environment, as may be the case for Palythoa Colombian population.
The results suggest that both populations studied are subject to environmental stress and disturbances (particularly in Brazil), thus colonies respond through high fission rates. Asexual reproduction has been observed to be more frequently associated with marginal isolated habitats (Miller and Ayre, 2004), and more severe with higher levels of disturbance (Morris et al., 2004). Palythoa populations in Brazil are not only at the geographic limit of distribution, but also exposed to harsh environment conditions (i.e. storms, sedimentation); as a consequence, partial mortality is exhibited by 57% of the population (i.e. disease; Acosta, 2001). These hostile conditions combined with rapid changing environment could explain, in part, the fission rate quantified in Brazil. According to Edmunds and Elahi (2007) and Foster et al. (2007) severe climate-induced disturbances have been recently revisited as important factors that explain the high fission rate in Montastraea annularis. Similar results were recently found in Colombian M. annularis populations exposed to Magdalena river disturbances (Alvarado E., unpublished data).
The relative low fission rate found in Brazil vs. Colombia could be explained by the negative effect that low light levels, high sedimentation rates, and high SST fluctuation produce in the colonies energy budget, at the expenses of fission (since fission implies energy expenditure). In such an unpredictable environment (i.e. higher SST and nutrients), it is expected, as proposed by Zhu et al. (2004), that Palythoa colonies decrease the energy input through photosynthesis, and increase the energy expenditure in cleaning processes (high sedimentation), fighting diseases (Acosta, 2001), recovering from bleaching (Migotto, 1997; Zhu et al., 2004), and consuming its own reserves during periods of low temperature, while remaining in diapause (Acosta, 2001).
d) Genetic differences among isolated populations as observed with corals (Miller and Ayre, 2004; Malagon et al., 2005) may also explain colony fission differences. The zoanthid P. caesia has shown significant genetic structure due to currents patterns in the Great Barrier Reef (Burnett et al., 1994). Genetic structure may appear by several reasons such as geographic barriers (Lessios et al., 2001, 2003; Rocha, 2003; Robertson et al., 2006), evolutionary partitions with habitat types (Rocha et al., 2005; Baums et al., 2006), isolated reef system (Miller and Ayre, 2004; Richards et al., 2007), divergent selective pressures (Rocha, 2003; Dorouszuk et al., 2006), or no genetic flow between populations due to oceanic or local currents direction (Whitaker, 2004), among others. There is also evidence indicating that reproductive isolation could exist between Brazilian and Colombian populations, since spawning occurs at different times, from April to beginning of May in Brazil (Acosta and Asbarh, 2000; Boscolo and Silveira, 2005) and from the end of May to the beginning of June in Colombia (Acosta A. unpublished data). Although Palythoa exhibits tele-planktonic planulae (Jackson, 1986) characterized by long duration in the water column (3 weeks to 7 months) and consequently long spatial dispersal (730 to 10000 Km; Ryland, 1997), the combination of potential reproductive isolation, restricted connectivity between populations due to oceanic currents, distance, or the effect of large rivers such as Amazon and Orinoco (low salinity decreasing gamets or planulae survival; Lessios et al., 2001, 2003; Robertson et al., 2006), plus selective pressure and genetic drift in ecologically distinct habitats, could promote not only genetically structured populations between Colombia and Brazil, but also different fission expression in Palythoa caribaeorum, and speciation in the Western Atlantic, as has been reported for tropical reef fish Halichoeres bivittatus (Rocha, 2003; Rocha et al., 2005; Robertson et al., 2006). All four hypotheses proposed here to explain differences in fission frequencies need, however to be demonstrated experimentally.
Despite what promotes fission frequency, the high number of P. caribaeorum ramets registered could potentially increase the population size in a short period of time (assuming ramets survival), this possible increment in size is more than three-fold the size reported for P. caesia (Tanner, 1997; 1999). The large numbers of potential ramets produced suggest that fission has a substantial influence on fitness, facilitating in this way the colonization of nearby habitats. Acosta et al. (2005) suggest that colonization and population dynamics in P. caribaeorum may be more heavily dependent upon asexual rather than sexual reproduction; while sexual reproduction can helps to maintain high genetic variability, which can enhance survival in this fluctuating environment characterized by frequent disturbances (i.e., cold fronts, rivers; Coffroth and Lasker, 1998; Skold et al., 2002).
P. caribaeorum populations reproduced by fission at small colony size in Colombia and Brazil. In other zoanthids, early asexual reproduction has also been reported in P. caesia (Ryland, 1997) and Zoanthus spp. (Karlson, 1988). Undergoing fission at small colony size may be selectively advantageous because it helps to: a) increase metabolic rate (Stoner, 1989), b) improve the efficiency of food capture (McFadden, 1986; Tanner, 2002), c) reproduce before death, d) decrease genet mortality risk, especially in unpredictable environments, through constant ramet supply (Cook, 1978; Coates and Jackson, 1985), e) increase fitness and the local dominance of well adapted genotypes (McFadden, 1991), f) escape from poor-quality microhabitats (Hunter, 1984), colonizing better environments during dispersal. But early reproduction at small colony size may have some disadvantages in P. caribaeorum: a) lesser and smaller number of ramets produced (Figure 3), b) higher ramet mortality rate (Jackson et al., 1985), which suggests that colonies must reach a minimum size to avoid mortality (Acosta et al., 2005), c) lower gamete output (Acosta and Asbarh, 2000), d) lower local genetic diversity in the short-term (Lehmann et al., 2006), e) less ability to adapt in a changing environment (Williams, 1975; Coffroth and Lasker, 1998), and f) lower dispersal capabilities vs. sexually reproduced larvae.
Similarly, early sexual reproduction has been observed in P. caribaeorum colonies in Brazil (Acosta and Asbarh, 2000) as well as at small colonies of P. tuberculosa (Yamazato et al., 1973). This could mean that Palythoa has evolved a strategy of maximizing a high energy investment in reproduction instead of growth in the earlier life stages. Karlson (1988) suggested that early reproduction may be an evolutionary response to harsh environmental conditions and high colony mortality rates in Zoanthus spp. If this is the case for P. caribaeorum, it is reasonable to think that its ancestor must have evolved this trait in a harsh environment (including high mortality rate). The conservativeness of the fission trait within the two different zoogeographical provinces in the Western Atlantic may support this idea.
The employment of both sexual and asexual reproductive strategies, even in small colony sizes, may help to explain the P. caribaeorum’s dominance of shallow benthic areas. Future studies will have to establish the real contribution of fission to population growth, particularly in Colombian populations, where we found the higher fission frequencies.
CONCLUSION
Although P. caribaeorum populations (Colombian Caribbean vs. Southwestern Atlantic-Sao Paulo, Brazil) exhibited similar fission characteristics, such as: a) reproduction by fission at small colony size, and b) production of large numbers of ramets directly related to colony size; they differed in fission frequency, which may be related to biotic and abiotic environmental conditions.
ACKNOWLEDGMENTS
We thank the Instituto de Investigaciones Marinas y Costeras INVEMAR (Punta de Betín; Santa Marta, Colombia) and CEBIMAR (Sao Sebastiao, Sao Paulo, Brazil) for supplying research facilities. The authors are grateful to Sandra Constantino for language editing and to two anonymous reviewers for their constructive suggestions on the manuscript.
LITERATURE CITED
1 Acosta, A. 1999. Ecologia da reprodução assexuada de Palythoa caribaeorum (Zoanthidea: Cnidaria). Tese de Doutorado. I.B. Universidade Estadual de Campinas, São Paulo, Brazil. 144 p. [ Links ]
2 Acosta, A. 2001. Disease in zoanthids: dynamics in space and time. Hydrobiologia, 460:113-130. [ Links ]
3 Acosta, A. and M. Asbarh. 2000. Reproductive effort in Palythoa caribaeorum. Proc. 9th Int. Coral Reef Symp. Bali, Indonesia. Abstracts. 295. [ Links ]
4 Acosta, A. and R.S. Martínez. 2005. Continental and oceanic coral reefs in the Colombian Caribbean after a decade of degradation. Proc. 10th Int. Coral Reef Symp, Okinawa, Japan. 2(5-6):1926-1930. [ Links ]
5 Acosta, A., P.W. Sammarco, and L.F. Duarte. 2001. Asexual reproduction in a zoanthid via fragmentation: the role of exogenous factors. Bull. Mar. Sci., 68:363-381. [ Links ]
6 Acosta, A, P.W. Sammarco, and L.F. Duarte. 2005. New fission processes in the zoanthid Palythoa caribaeorum: description, and quantitative aspects. Bull. Mar. Sci., 76:1-26. [ Links ]
7 Baums, I.B., M.W. Miller, and M.E. Hellberg. 2006. Geographyc variation in clonal structure in a reef-building Caribbean coral, Acropora palmata. Ecol. Monogr., 76(4):503-519. [ Links ]
8 Boscolo, H.K. and F.L. Silveira. 2005. Reproductive biology of Palythoa caribaeorum and Protopalythoa variabilis (Cnidaria, Anthozoa, Zoanthidea) from the Southeastern coast of Brazil. Braz. J. Biol., 65(1):29-41. [ Links ]
9 Coffroth, M.A. and H.R. Lasker. 1998. Population structure of a clonal gorgonian coral: the interplay between clonal reproduction and disturbance. Evolution, 52:379-393. [ Links ]
10 Cook, R.E. 1978. Asexual Reproduction: A Further consideration. Am. Nat., 769-772. [ Links ]
11 Cook, R.E. 1985. Growth and development in clonal plant populations. 259-296. In: Population Biology and Evolution of Clonal Organisms. Jackson, J.B.C., Buss, L.W. and Cook, R.E. (Eds.). New Haven, Yale University Press. [ Links ]
12 Crump, R.G. and M.F. Barker. 1985. Sexual and asexual reproduction in geographically separated populations of the fissioparus asteroid Coscinasterias calamaria (Gray). J. Exp. Mar. Biol. Ecol., 88:109-127. [ Links ]
13 Coates, A.G. and j.b. Jackson. 1985. Morphological themes in the evolution of clonal and aclonal marine invertebrates. In: Population biology and evolution of clonal organisms. J.B.C. Jackson, L.W. Buss, and R.E. Cook (eds.). Yale University Press, New Haven. 530 p. [ Links ]
14 Díaz, J.M., L.M. Barrios, M.H. Cendales, J. Garzón-Ferreira, J. Geister, M. López-Victoria, G.H. Ospina, F. Parra-Velandia, J. Pinzón, B. Vargas-Angel, F.A. Zapata, and S. Zea. 2000. Áreas Coralinas de Colombia. Instituto de Investigaciones Marinas y Costeras "José Benito Vives de Andreis" INVEMAR, Santa Marta, Serie de Publ. Esp. 5. 175 p. [ Links ]
15 Edmunds, P.J. and R. Elahi. 2007. The demographics of a 15-year decline in cover of the Caribbean reef coral Montastraea annularis. Ecol. Monogr., 77(1):3-18. [ Links ]
16 Fautin, D.G. 2002. Reproduction of Cnidaria. Can. J. Zool., 80(10):1735-1754. [ Links ]
17 Foster, N.L., I.B. Baums, and P.J. Mumby. 2007. Sexual vs. asexual reproduction in an ecosystem engineer: the massive coral Montastraea annularis. J. Anim. Ecol., 76(2):384-391. [ Links ]
18 Geller, J.B., L.J. Fitzgerald, and C.E. King. 2005. Fission in sea anemones: integrative studies of life cycle evolution. Integr. Comp. Biol., 45(4):615-622. [ Links ]
19 Gleibs, S. 1994. Role of a toxic secondary metabolite in the ecology of the genus Palythoa (Anthozoa) a zoanthid of the Caribbean. Diploma de thesis, Institut für Allgemeine und Spezielle Zoologie in the faculty of Biology, Justus-Liebig-Universität Giessen, Germany. 107 p. [ Links ]
20 Hall, V.R. and T.P. Hughes. 1996. Reproductive strategies of modular organisms: Comparative studies of reefbuilding corals. Ecology, 77:950-963. [ Links ]
21 Highsmith, R.C. 1982. Reproduction by fragmentation in corals. Mar. Ecol. Prog. Ser., 7:207-226. [ Links ]
22 Hughes, T.P. 1989. A functional biology of clonal animals. Chapman and Hall, New York. 331 p. [ Links ]
23 Hughes, T.P. and J.H. Connell. 1987. Population dynamics based on size or age? A reef-coral analysis. Am. Nat., 6:818-829. [ Links ]
24 Hughes, T.P. and J.B. Jackson. 1980. Do corals lie about their age? Some demographic consequences of partial mortality, fission and fusion. Science, 209:713-715. [ Links ]
25 Hunter, T. 1984. The energetics of asexual reproduction: Pedal laceration in the symbiotic sea anemone Aiptasia pulchella. J. Exp. Mar. Ecol., 83:127-147. [ Links ]
26 Jackson, J.B. 1986. Modes of dispersal of clonal benthic invertebrates: consequences for species distributions and genetic structure of local populations. Bull. Mar. Sci., 39:588-606. [ Links ]
27 Jackson, J.B, L.W. Buss, and R.E. Cook. 1985. Population biology and evolution of clonal organism. Yale University Press. pp. 296-337. [ Links ]
28 Karlson, R.H. 1988. Size dependent growth in two zoanthid species: a contrast in clonal strategies. Ecology, 69:1219-1232. [ Links ]
29 Karlson, R.H. 1991. Fission and the dynamics of genets and ramets in clonal cnidarian populations. Hydrobiologia, 216:235-240. [ Links ]
30 Karlson, R.H., H. Ronald, T.P. Hughes, and S. Karlson. 1996. Density-dependent dynamics of soft coral aggregations: The significance of clonal growth and form. Ecology, 77:1592-1599. [ Links ]
31 Kramarsky, E.W., m. Fine, and Y. Loya. 1997. Coral polyp expulsion. Nature, 387:137. [ Links ]
32 Lasker, H.R. 1990. Clonal propagation and population dynamics of a gorgonian coral. Ecology, 71:1578-1589. [ Links ]
33 Lehmann, L., N. Perrin, and F. Rousset. 2006. Population demography and the evolution of helping behaviours. Evolution, 60:1137-1151. [ Links ]
34 Lessios, H.A., J. Kane, and D.R. Robertson. 2003. Phylogeography of the pantropical sea urchin Tripneustes: contrasting patterns of population structure between oceans. Evolution, 57(9):2026-2036. [ Links ]
35 Lessios, H.A., B.D. Kessing, and J.S. Pearse. 2001. Population structure and speciation in tropical seas: global phylogeography of the sea urchin Diadema. Evolution, 55(5):955-975. [ Links ]
36 Magalon, H., M. Adjeroud, and M. Veuille. 2005. Patterns of genetic variation do not correlate with geographical distance in the reef-building coral Pocillopora meandrina in the South Pacific. Mol. Ecol., 14(7):1861-1868. [ Links ]
37 Martínez, R.S. and A. Acosta. 2005. Cambio temporal en la estructura de la comunidad coralina del área de Santa Marta-Parque Nacional Natural Tayrona (Caribe colombiano). Bol. Invest. Mar. Cost., 34:141-167. [ Links ]
38 Masson, S. and P. Delecluse. 2001. Influence of the Amazon river runoff on the tropical Atlantic. Phys, Chem. Earth Part B-Hydrol. Oceans Atmos., 26(2):137-142. [ Links ]
39 McFadden, C.S. 1986. Colony fission increases particle capture rates of a soft coral: Advantages of being a small colony. J. Exp. Mar. Biol. Ecol., 103:1-20. [ Links ]
40 McFadden, C.S. 1991. A comparative demographic analysis of clonal reproduction in a temperate soft coral. Ecology, 72:1849-1866. [ Links ]
41 Migotto, A.E. 1997. Anthozoan bleaching on the southwestern coast of Brazil in the summer of 1994. Proc. 6th Int. Conf. Coelenterate Biol. pp. 329-335. [ Links ]
42 Miller, K.J. and J. Ayre. 2004. The role of sexual and asexual reproduction in structuring high latitude populations of the reef coral Pocillopora damicornis. Heredity, 92(6):557-568. [ Links ]
43 Mladenov, P.V. 1996. Environmental factors influencing asexual reproductive processes in echinoderms. Oceanologica Acta, 19:227-235. [ Links ]
44 Morris A.B., R.L. Small, and M.B. Cruzan. 2004. Variation in frequency of clonal reproduction among populations of Fagus grandifolia in response to disturbance. Castanea, 69:38-51. [ Links ]
45 Mueller, E. and W. Haywick. 1995. Sediment Assimilation and Calcification by the Western Atlantic Reef Zoanthid, Palythoa caribaeorum. Bull. de l´Institut océanographique, Monaco, n° spécial 14, 2. [ Links ]
46 Oigman-Pszczol, S.S., M.A. Figueiredo, and J.C. Creed. 2004. Distribution of benthic communities on the tropical rocky subtidal of Armacao dos Buzios, southeastern Brazil. Mar. Ecol. Pubblicazioni della Stazione Zoologica di Napoli, 25:173-190. [ Links ]
47 Pearse, V.B. 2002. Prodigies of propagation: the many modes of clonal replication in boloceroidid sea anemones (Cnidaria, Anthozoa, Actiniaria). Invert. Reprod. Dev., 41:201-213. [ Links ]
48 Perez, C.D., D.A. Vila-Nova, and A.M. Santos. 2005. Associated community with the zoanthid Palythoa caribaeorum (Duchassaing and Michelotti, 1860) (Cnidaria, Anthozoa) from littoral of Pernambuco, Brazil. Hydrobiologia, 548:207-215. [ Links ]
49 Richards, V.P., J.D. Thomas, M.J. Stanhope, and M.S. Shivji. 2007. Genetic connectivity in the Florida reef system: comparative phylogeography of commensal invertebrates with contransting reproductive strategies. Mol. Ecol., 16:139-157. [ Links ]
50 Richmond, R.H. 1987. Energetic relationships and biogeographical differences among fecundity, growth and reproduction in the reef coral Pocillopora damicornis. Bull. Mar. Sci., 41:594-604. [ Links ]
51 Rocha, LA. 2003. Patterns of distribution and processes of speciation in Brazilian reef fishes. J. Biogeogr., 30(8): 1161-1171. [ Links ]
52 Rocha, LA, D.R. Robertson, J. Roman, and B.W. Bowen. 2005. Ecological speciation in tropical reef fishes. Proc. Royal Soc. Biol. Sci., 272:573-579. [ Links ]
53 Ryland, J.S. 1997. Reproduction in Zoanthidea (Anthozoa: Hexacorallia). Invert. Reprod. Dev., 31:177-188. [ Links ]
54 Santamaría, L. and A. García. 2004. Latitudinal variation in tuber production in an aquatic pseudo-annual plant, Potamogeton pectinatus. Aquat. Bot., 79:51-64. [ Links ]
55 Sammarco, P.W. 1982. Polyp bail-out: An escape response to environmental stress and a new means of reproduction in corals. Mar. Ecol. Prog. Ser., 10:57-65. [ Links ]
56 Sebens, K.P. 1982. The limits to indeterminate growth: An optimal size model applied to passive suspension feeders. Ecology, 63:209-222. [ Links ]
57 Skold, M., M.F. Barker, and P.V. Mladenov. 2002. Spatial variability in sexual and asexual reproduction of the fissiparous seastar Coscinasterias muricata: the role of food and fluctuating temperature. Mar. Ecol. Prog. Ser., 233:143-155. [ Links ]
58 Sokal, R.H. and F.J. Rohlf. 1995. Biometry. Third edition. WH Freeman, New York. 850 p. [ Links ]
59 Stoner, D.S. 1989. Fragmentation: a mechanism for the stimulation of genet growth rates in an encrusting colonial ascidian. Bull. Mar. Sci., 45:277-287. [ Links ]
60 Tanner, J.E. 1997. The effects of density on the zoanthid Palythoa caesia. J. Anim. Ecol., 66:793-810. [ Links ]
61 Tanner, J.E. 1999. Density-dependent population dynamics in clonal organisms: a modeling approach. J. An. Ecol., 68:390-399. [ Links ]
62 Tanner, J.E. 2000. Stochastic density dependence in population size of a benthic clonal invertebrate: the regulating role of fission. Oecologia, 122:514-520. [ Links ]
63 Tanner, J.E. 2002. Consequences of Density-dependent heterotrophic feeding for a partial autotroph. Mar. Ecol. Prog. Ser., 227:293-304. [ Links ]
64 Tsuchida, C.B. and D.C. Potts. 1994. The effects of illumination, food and symbionts on growth of the sea anemone A. elegantissima (Brandt, 1835) Ramet growth. J. Exp. Mar. Biol. Ecol., 183:227-242. [ Links ]
65 Walker, I. 1975. Density-dependent control of fission rates in marine ciliate Keronopsis-rubra (Hypotricha Oxytrichidae). J. Anim. Ecol., 44:707-717. [ Links ]
66 Walker, T.A. and G.D. Bull. 1983. A newly discovered method of reproduction in a gorgonian coral. Mar. Ecol. Prog. Ser., 12:137-143. [ Links ]
67 Williams, G.C. 1966. Adaptation and Natural Selection. Princeton Univ. Press., New York, 307 p. [ Links ]
68 Williams, G.C. 1975. Sex and evolution. Princeton University Press, Princeton. 210 p. [ Links ]
69 Whitaker, K. 2004. Non-random mating and population genetic subdivision of two broadcasting corals at Ningaloo Reef, Western Australia. Mar. Biol., 144: 593-603. [ Links ]
70 Yamazato, K., F. Yoshimoto, and N. Yoshihara. 1973. Reproductive cycle of a Zoanthid, Palythoa tuberculosa (Esper). Publ. Seto. Mar. Biol. Lab., 20:275-283. [ Links ]
71 Zilberberg, C., A.M. Solé-Cava, and M. Klautau. 2006. The extent of asexual reproduction in sponges of the genus Chondrilla (Desmospongiae: Chondrosida) from the Caribbean and the Brazilian coast. J. Exp. Mar. Biol. Ecol., 336 (2): 211-220. [ Links ]
72 Zhu, B.H., G.C. Wang, B. Huang, and C.K. Tseng. 2004. Effects of temperature, hypoxia, ammonia and nitrate on the bleaching among three coral species. Chin. Sci. Bull., 49(18):1923-1928. [ Links ]
FECHA DE RECEPCIÓN: 04/09/06 FECHA DE ACEPTACIÓN: 18/07/07