Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.40 supl.1 Santa Marta Dec. 2011
NOTES ON THE ECOLOGY OF THE LIZARDS FROM MALPELO ISLAND, COLOMBIA*
NOTAS SOBRE LA ECOLOGÍA DE LOS LAGARTOS DE LA ISLA MALPELO, COLOMBIA
Mateo López-Victoria1,2, Pilar A. Herrón3 and Juan Carlos Botello4
1 Justus-Liebig-University, Department of Animal Ecology, Heinrich-Buff-Ring 29, 35392 Giessen, Germany. gf1617@uni-giessen.de (current address).
2 Instituto de Investigaciones Marinas y Costeras-INVEMAR, Cerro Punta Betín, Santa Marta, Colombia.
3 Fundación Malpelo y Otros Ecosistemas Marinos, Carrera 7 No 32-33 Piso 27, Bogotá, Colombia. herron_pilar@yahoo.com.
4 Universidad of Barcelona, Departamento de Biología Animal, Barcelona, España. jbotelca7@alumnes.ub.edu.
ABSTRACT
Observations of two of the endemic species of lizards of Malpelo Island provide new information on their natural history, ecology, and population size. Anolis agassizi, the most abundant and broadly distributed lizard, feeds mainly on insects and excrements of marine birds. It sleeps on large rocks, surfaces on hills or on man-made structures and, although it does not defend perch sites like most Anolis do, it does show preferences for high perches where, among other activities, it carries out copulation. Diploglossus millepunctatus, the largest and least abundant lizard, is an opportunistic-predator and scavenger that has a remarkable relationship with the land crab of the island (Johngarthia malpilensis) which it not only eats, but also competes with it for food. Behavior, higher density, larger body size and weight of individual D. millepunctatus living close to cabins suggest that these lizards accommodate to the presence of people by feeding on left-over food.
KEY WORDS: Anolis agassizi, Diploglossus millepunctatus, General behavior, Ecology, Population status.
RESUMEN
Observaciones de dos de las especies endémicas de lagartos de la isla Malpelo ofrecen características poco conocidas de su historia natural, ecología y estado poblacional. Anolis agassizi, el lagarto más abundante y ampliamente distribuido, se alimenta principalmente de insectos y excretas de aves marinas. Duerme sobre rocas grandes, en las paredes de los cerros o sobre estructuras construidas por el hombre y, aunque no defiende sitios de percha como la mayoría de Anolis, muestra predilección por sitios elevados en donde, entre otras actividades, realiza sus cópulas. Diploglossus millepunctatus, el lagarto de mayor tamaño corporal y menor abundancia, es un depredador-oportunista y carroñero que mantiene una relación inusual con el cangrejo terrestre de la Isla (Johngarthia malpilensis), al cual no solo depreda activamente, sino con el que también compite por alimentos. El comportamiento, mayor densidad, tamaño corporal y peso de los individuos de D. millepunctatus cercanos a las cabañas, sugieren que estos lagartos se han condicionado a la presencia de los humanos, al consumir periódicamente las sobras de sus comidas.
PALABRAS CLAVE: Anolis agassizi, Diploglossus millepunctatus, Comportamiento general, Ecología, Estado poblacional.
INTRODUCTION
The ecology of island-inhabiting reptiles has attracted much attention and interest amongst biologists because some of the more remarkable examples of evolution and adaptation have been studied on islands (i.e., Galapagos tortoise and iguanas; Darwin, 1859). Islands often contain relatively high numbers of endemic species. Among the best known reptile examples is the lizard genus Anolis, which has served as a model species for evolutionary studies (Losos, 1994; Nicholson et al., 2005). Almost all of the Caribbean and Pacific islands off the American continent have at least one endemic lizard. Malpelo Island harbors three unique species: Anolis agassizi Stejneger 1900, Diploglossus millepunctatus O'Shaughnessy 1874, and Phyllodactylus transversalis Huey 1975.
Twelve papers have been published on the three endemic lizard species inhabiting Malpelo Island. In addition to the original descriptions, studies have presented data on general aspects of the natural history of the lizards as well as minor details on their genetic variation and karyotypes. Most of these studies were based on observations and specimens obtained in 1972 during an expedition of the Smithsonian Institution (Huey, 1975; Kiester, 1975; Rand et al., 1975; Stamm and Gorman, 1975; Webster, 1975), while others are the result of sporadic observations made by scientists and collectors (Slevin, 1928; Dunn, 1939; Prahl, 1990; Brando et al., 1992; Álvarez-Rebolledo et al., 1999; López-Victoria, 2006).
We carried out density studies and we captured, marked, and followed specific individuals to fill in some gaps in our understanding of the ecology and behavior of these lizards. Herein, we present new data on the reproduction, feeding ecology, daily activity patterns, and current status of the populations of two of the three endemic lizard species of Malpelo.
STUDY AREA
Malpelo is an oceanic island surrounded by eleven islets, located 380 km from the nearest spot of the Colombian Pacific mainland coast (4°00'11" N, 81°36'30" W). It has a surface area of nearly 1.2 km2 and its maximum height is 300 m above sea level (López-Victoria and Rozo, 2006). Due to its volcanic origin, its topography is mainly rough, with steep slopes all around. The majority of the island surface lacks vegetation, apart from hosting a permanent cover of microalgae, lichens and mosses, scattered spots of grass, and a species of creeping fern not exceeding 200 m2. Scattered patches of grass, shrubs and ferns occur on some of the northern and southern islets (Wolda, 1975; Prahl, 1990; López-Victoria and Rozo, 2006).
MATERIALS AND METHODS
Colombia's biggest nesting colony of sea birds is found on Malpelo, which is particularly notable for the high number of Nazca Boobies (Sula granti) occuring there (around 40000 pairs; López-Victoria and Rozo, 2007). Apart from sea birds and lizards, the most conspicuous inhabitant of the island is a terrestrial crab, Johngarthia malpilensis, which is also an endemic species of Malpelo (Türkay, 1970).
In May-June of 2006, we carried out detailed and sporadic observations of Malpelo's lizards, from 0600 to 2400 hours, most of them on the eastern side of the island (surrounding the Colombian Navy facilities). We randomly captured 92 individuals of A. agassizi and 30 of D. millepunctatus. Each individual was measured for snout-vent length (SVL) and total length (snout to tail tip= TL) with a calibrator or metric tape, and then was weighed with a digital balance (averages are shown here with ± 1 standard error). After being measured all lizards were released.
We recorded the general distribution (presence/absence) of these two species on field maps of Malpelo and its surrounding islets. We estimated their density during daylight, based on seven band-transects of 100 x 4 m (400 m2). Estimated densities were extrapolated to the surface of Malpelo, excluding from the calculations those sectors where no individuals were registered or where their densities were very low. To facilitate tracking some individuals and to determine their distributional changes from day to night, we marked with color dots the backs of 30 A. agassizi individuals and 12 D. millepunctatus individuals. Unique forms of the tail tips of several individuals of D. millepunctatus living near the Naval residence huts allowed us to make repeated observations on these individuals. Observations on reproduction and ecological interactions are the results of tracking specific individuals combined with some events that were witnessed haphazardly. Some observations have been complemented with personal communications from colleagues and Colombian Navy staff.
RESULTS
Anolis agassizi
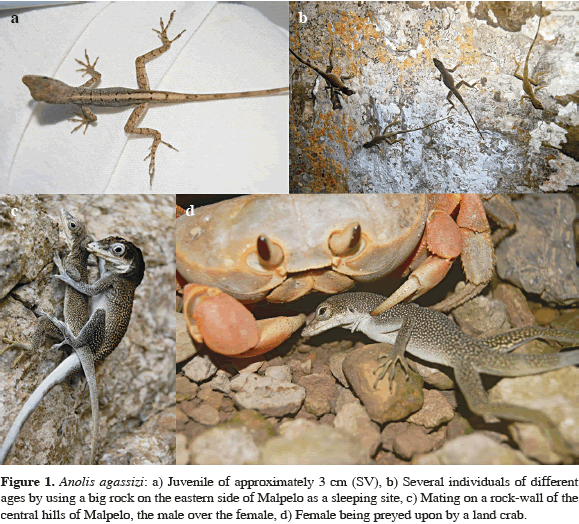
Females of this species are gray with clear spots on the back, and with greenish tones enhanced on the legs, tail and part of the abdomen. Maximum size of females was 8.3 cm SVL with a total length of 26 cm (average 21.5 cm TL ± 0.5). Females' maximum weight was 15 g. Males are of two morphotypes: one very similar to that of females, but with a more robust body and dark-gray color with green tones (max. size 8.4 cm SVL, 26.7 cm LT; average 23.5 cm LT ± 0.8; max. weight 17 g). The second morphotype, much more conspicuous, is dark gray in color on the back with clear spots, a blue belly and blue bands on the tail, and a very dark, prominent crest. Maximum size of this blue morphotype was 11 cm SVL with a total length of 33 cm (average 27.7 cm TL ± 0.5 cm); tail length was about twice the body length. The maximum weight recorded was 37.2 g, with an average of 25.3 g (± 1.5) for adults. Juveniles have a brown olive color with a marked longitudinal line of light tan color on the back and a variable pattern of small dark spots (Figure 1a). The smallest juvenile recorded was 2.7 cm SVL (6.7 cm TL).
We observed this species active only during daylight hours. When exposed rock surfaces became hot due to sun exposure, lizards were active but remained in shaded areas, like the shadows projected by Nazca boobies. During nighttime, A. agassizi gathered in high places, with several individuals congregating in sleeping sites of three kinds: a) on big boulders (>1 m diameter and >1 m height), b) on vertical walls of the hill sides where, at sunset, up to 30 individuals per m2 of both sexes and all ages, aggregated, and c) on man-made structures, such as the walls of the huts, steel cables, or antennae. Based on tracking of the 30 marked individuals, we established that at least 15 to 18 of them (50 to 60 %) returned to the same sleeping sites during seven consecutive nights, suggesting some fidelity toward those sites (Figure 1b). Individuals that lived within and on the huts remained active after dark, capturing insects attracted by the light of the lamps.
During daytime, individuals of A. agassizi were distributed around their sleeping sites and we found some of them more than 50 m away from the places where they had been marked. Individuals not wandering around on rocks hunting insects were distributed around nests of Nazca boobies, frequently in groups of up to eight, feeding on excrements dropped by the birds and on flies drawn by the birds' droppings. We also saw them preying upon small terrestrial crabs. In the vicinity of the huts we saw some individuals eating leftover food, but usually losing the contest for this resource to surrounding crabs and D. millepunctatus. Anolis agassizi was particularly active during periods of rain, taking advantage of flooded areas where insects were escaping from drowning. On these occasions A. agassizi also captured earthworms, which were underground and thus unavailable except during flooding. The predators of A. agassizi that we observed included adult terrestrial crabs, D. millepunctatus and, occasionally, migratory birds (Figure 1d).
On three occasions we observed what appeared to be stereotypic copulatory behavior. The male mounted the female dorsally, grasping her neck skin with the snout tip while introducing his hemipenis into her cloaca (Figure 1c). In two of the three events the pairs were adhering to a vertical wall and, in the other event, the pair was on top of a large rock. The three copulations lasted between 15 and 20 min. Despite intensive searches for eggs, we only found one, white in color with a soft texture, hidden between the branches of a terrestrial fern in the eastern side of the island. The egg contained a 1.6 cm SVL embryo.
This species is distributed throughout the main island and islets almost on every surface, including areas very close to the sea (supralittoral zone). The average density of A. agassizi was 37.3 inds 400 m-2 (± 9.7), ranging from 8 to 71 individuals per transect. Based on the surfaces inhabited by this lizard, and assuming a uniform average density in those surfaces (0.09 individuals m-2), we estimated a total population of between 60000 and 102000 individuals inhabiting Malpelo and its surrounding islets.
Diploglossus millepunctatus
This is a robust, strong species and the largest of the three lizard species living on Malpelo. Maximum size of adults was 28 cm SVL (average 21.9 cm ± 1.2) with tails of up to 23.5 cm. Few attain that size, however, because tails are lost, partially, during fights or from pecks given by boobies when lizards come close to their nests. Maximum adult weight was 498 g, with an average of 315.1 g (± 19.2); the smallest juvenile measured was 6.5 cm SVL and weighed 4.6 g (12.8 cm TL). Based on their external appearance, males of D. millepunctatus may only be differentiated from females by their larger size and, when they reach maturity, by relative head size (males have relatively larger heads). All individuals were dark metallic brown with clear small spots. Spots were much more conspicuous in juveniles.
We found the largest D. millepunctatus individuals around the Naval huts, and almost all of them exploited the permanent supply of wastes (leftovers of raw and processed food) as a food source. At least 20 individuals (marked or with individually recognizable tails) spent most of the day near the huts, or arrived at meal times (Figure 2a). Adult D. millepunctatus were active mainly during the day, although we also observed some hunting during the night. Juveniles were usually hidden among rocks during the day, and emerged from crevices at night to actively search for food.
Diploglossus millepunctatus consumes everything it finds in its path and may be classified as a scavenger-opportunist predator. It has a particular predilection for terrestrial crabs, amphibious crabs (Grapsus grapsus), bird carcasses, eggs and chicks of Nazca boobies, and lizards (including its own species). On two occasions we observed two large males eating juveniles and on two other occasions we recorded two males eating females of A. agassizi.
While tracking large adults, we were able to document their strategy for capturing and consuming terrestrial crabs. When an individual captures a juvenile crab, they chew slowly and swallow them almost entire (Figure 2b). When large crabs are captured, after being chased and cornered against rocks, the lizard initiates a methodic routine to dismember and consume them. First, it pulls off, one by one, the crab's walking legs and pincers, then opens the belly of the crab by hyper-extending the abdomen backwards and upwards, until the crab shell yields, exposing the soft internal parts. Once the soft internal parts of the crabs are eaten, D. millepunctatus sometimes feeds on the legs. Remaining portions of depredated crabs are consumed by other crabs or by A. agassizi. On some occasions, crabs hid inside crevices with their pincers projected outwards, so that they were able to repel lizard attacks.
Nazca booby chicks expelled from the nest represented an important food source for lizards, which continually wander around active nests. Crabs also may attain a chick when they outnumber the lizards in the vicinity. The fate of a chick depends on numerous factors: which predator species has first access to the prey, the lizard's size, and the number of crabs involved in the dispute for the chick. Eggs abandoned by boobies are also food sources disputed by lizards and crabs.
Fishes regurgitated by adult boobies when feeding their chicks occasionally fell on the ground and were stolen by lizards. Apparently, lizards recognize the movements and calls made by chicks and their parents at feeding time and remain alert, staying near the nests, from where they are expelled violently by the boobies that occasionally injure lizards with their sharp, pointed beaks. Only when an adult regurgitates inside the chick's beak and both of them have their beaks united are lizards able to obtain prey, if any has fallen in the process (Figure 2c). Also, sometimes a chick full of food vomits a fish, and lizards and crabs will take advantage.
The sole predators of adult lizards are some migratory birds (herons and hawks, for example), whereas juveniles are hunted by conspecific adults as well as, on occasions, terrestrial crabs. The lizards freely gathering together for feeding, even when they are surrounded by crabs, producing an interesting simultaneous relationship of predator-prey and food competition. The direction of the predatory-prey relationship may even be reversed in situations when crabs exceed the lizards in number, which occurs frequently (Figure 2a). When crabs are too abundant, lizards abandon the contest.
On four occasions we observed the pre-copulatory behavior of this species, in which one or two males grasped a female by the head with their jaws, holding this position for more than an hour (Figure 2d). We did not observe copulation in any of these four cases and neither did we witness any form of pre-coital courting.
Diploglossus millepunctatus is not a good climber and its distribution on Malpelo and the surrounding islets is limited to sectors with slopes of moderated inclination. Based on estimations of the surfaces inhabited by this lizard, and assuming an average density on those surfaces of 0.017 individuals m-2, we estimated a total population of 12000 to 18000 individuals inhabiting Malpelo and its surrounding islets.
DISCUSSION
Anolis agassizi exhibits sleeping site fidelity, although this lizard wanders during the day, reaching distances of from a few meters to up to 150 m or more from its sleeping site (Rand et al., 1975; Pers. obs.). Most species of Anolis are arboreal, typically perching in trees and shrubs (Fläschendräger and Wijffels, 1996). However, it is known that some species of Anolis may also live predominantly on the ground, and although some of them do so facultatively or segregated by gender (i.e., A. bimaculatus), others occupy that habitat even in sites with abundant vegetation (i.e., A. humilis) or in rocky biotopes (i.e., A. gadovi), like those available in Malpelo (Fläschendräger and Wijffels, 1996). The use by A. agassizi of high perches during the night might be both a strategy for resting safe from the ambush of crabs and Diploglossus, as well as a remnant characteristic inherited from its arboreal ancestors.
Rand et al. (1975) carried out an experiment with colored candies and established the preference of A. agassizi for orange and yellow colors. They suggested that the preference of these lizards for those two colors could be the result of their great interest in the birds' egg-yolks, assuming that boobies' eggs are broken in significant numbers during the reproductive season. We offer an alternative hypothesis; that the most likely explanation for the particular predilection for such colors is more related to bird droppings than to their eggs, because a portion of the bird excrement has a color between orange and yellow, and this particular portion is avidly eaten by A. agassizi just after it is projected out of the nest by the birds. In contrast, eggs usually are broken due to the action of crabs or D. millepunctatus, two competitors against which A. agassizi has few chances of winning, so they only occasionally are able to obtain some remainders of yolk or albumen spilled on the rocks (see López-Victoria and Werding, 2008).
Rand et al. (1975) reproduced some A. agassizi in captivity, providing data on the size of the eggs laid and the laying frequency as well as the size of the neonate at birth. The couplings of A. agassizi we observed are the first documented in its natural habitat and the egg found is the first indication of how this Anolis species protects its brood from predators such as D. millepunctatus and the terrestrial crabs. Because the vegetation cover of higher plants in Malpelo is low, it is expected that Anolis' eggs are laid in holes burrowed in the scarce soil or in crevices of the vertical walls, out of reach of the potential predators (though we failed to find any despite searching intensively).
Kiester (1975) recorded maximum size and weight for D. millepunctatus of 25 to 26 cm (SVL) and 268 g, while Prahl (1990) recorded sizes up to 26.4 cm (supposedly SVL) and weights up to 271 g. The biggest individual we found exceeded by just 2 cm the records of maximum size of those authors, but regarding weight, the difference was more than 220g! The enormous difference in weight we recorded is most likely due to overfeeding, as many of the lizards we measured around the huts feed from the leftovers produced by the soldiers. A comparative study on size and weight among both sectors of the island (eastern side vs. western side) would help to confirm this explanation and to evaluate the impact of this additional source of food on the population. Also the biggest terrestrial crabs were found in areas surrounding huts (López-Victoria and Werding, 2008).
In his scheme of trophic relations of Malpelo's organisms, Wolda (1975) left open the question regarding hunting by D. millepunctatus of terrestrial crabs. It is clear that the lizard not only avidly preys upon crabs of every size and age, but it has an effective strategy for chasing and hunting them, after which it dissects and eats the crabs. Nonetheless, the relationship between crabs and D. millepunctatus is hard to define, since they are at times in direct competition for food resources and at others, involved more in a predator-prey relationship.
Kiester (1975) described the feeding habits of D. millepunctatus as "unusual", suggesting that insects would be its main source of food, in particular during its juvenile stage. Although it is true that juveniles feed on insects and small crabs during the night, adults are predators and scavengers of a wide spectrum of prey, displaying little interest in insects, which are the typical prey of A. agassizi and P. transversalis. Remainders of crabs and feathers found in the stomachs of D. millepunctatus by Slevin (1928) and by Dunn (1939), as well as fish regurgitated by the boobies, suggested by Kiester (1975) as food craving by adult lizards, give good accounts of its assorted diet. Even today, a few individuals may be fed exclusively on leftovers produced by humans.
Kiester (1975) did not find intermediate sizes of D. millepunctatus, suggesting that individuals less than 18 cm long either hide in crevices or this lizard has a seasonal or erratic reproduction, and he studied the population "just before the next reproductive event". Although it is true that reproduction seems to be associated to the period of greater activity of the boobies, between April and the end of the year (López-Victoria and Estela, 2007), it is also likely that Kiester did not have the opportunity to see individuals of small or medium size because he did not carry out nocturnal observations, the time when juveniles of D. millepunctatus seem to be more active (maybe as a strategy to prevent depredation during the day). In any case, the reproductive cycles of this lizard, as well as many other aspects of its general biology (for example, juveniles' diet) have not been studied in detail.
Previous estimations of Kiester (1975) and Rand et al. (1975) on population sizes of A. agassizi and D. millepunctatus suggested between 140000 and 206000 for the former and nearly 100000 individuals for the second species. López-Victoria (2006) suggested ca. 120000 individuals for Diploglossus. Only the estimations on population size of Anolis made by Rand et al. (1975) coincides with our results. In the case of Diploglossus, the population size seems to be much less than that estimated in previous studies. The higher values found in the study of López-Victoria might be biased due to methodology, as that study utilized the same transects to estimate lizard density as were used to evaluate nesting activity of boobies. Since these lizards (Anolis and Diploglossus) have a distribution that seems to be highly influenced by nesting of marine birds (Kiester, 1975; Rand et al., 1975; López-Victoria, 2006), it is likely that previous density values were overestimated, because lizards gather around active nests searching for food.
ACKNOWLEDGEMENTS
We thank the financial and logistics support provided by: the Department of Animal Ecology of the Justus-Liebig University; the German Academic Exchange Service; Instituto de Investigaciones Marinas y Costeras-INVEMAR; the Colombian Ministry of the Environment, Housing and Territorial Development; the Malpelo Foundation; the Navy of the Republic of Colombia and the Fundación Zoológico de Cali. We also thank the support in the field provided by the ships ARC Valle del Cauca and ARC Caldas. In particular, we acknowledge the field support provided by S. Bessudo, G. Soler, and the Navy Personnel located on Malpelo and comments made by B. Bock and by an anonymous reviewer.
LITERATURE CITED
1 Álvarez-Rebolledo, M., F. Gast and S. Krieger. 1999. La fauna terrestre de la isla Malpelo. Instituto Humboldt-Biosíntesis, 12: 1-4. [ Links ]
2 Brando, A., H. von Prahl and J. R. Cantera. 1992. Malpelo: Isla oceánica de Colombia. Banco de Occidente, Cali. 195 p. [ Links ]
3 Darwin, C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray, London. 502 p. [ Links ]
4 Dunn, E. R. 1939. Zoological results of the George Vanderbilt South Pacific Expedition of 1937. Part III. The lizards of Malpelo Island, Colombia. Notula Naturae Acad. Nat. Sci. Phila., 4: 1-3. [ Links ]
5 Fläschendräger, A. and L. Wijffels. 1996. Anolis. Terrarien Bibliothek. Natur und Tier-Verlag, Berlin. 208 p. [ Links ]
6 Huey, R. B. 1975. A new gecko from Malpelo Island (Sauria: Gekkonidae: Phyllodactylus). Smithsonian Contr. Zool., 176: 44-46. [ Links ]
7 Kiester, A. R. 1975. Notes on the natural history of Diploglossus millepunctatus (Sauria: Anguidae). Smithsonian Contr. Zool., 176: 39-43. [ Links ]
8 López-Victoria, M. 2006. Los lagartos de Malpelo (Colombia): aspectos sobre su ecología y amenazas. Caldasia, 28: 129-134. [ Links ]
9 López-Victoria, M. and F. A. Estela. 2007. Aspectos sobre la ecología del Piquero de Nazca Sula granti en la isla Malpelo. 132-142. In: DIMAR-CCCP and UAESPNN (Eds.). Santuario de Fauna y Flora Malpelo: descubrimiento en marcha, DIMAR, Bogotá. 142 p. [ Links ]
10 López-Victoria, M. and D. Rozo. 2006. Model-based geomorphology of Malpelo Island and spatial distribution of breeding seabirds. Bol. Invest. Mar. Cost., 35: 111-131. [ Links ]
11 López-Victoria, M. and D. Rozo. 2007. Wie viele Nazcatölpel Sula granti brüten auf der Insel Malpelo? Vogelwarte, 45: 365-366. [ Links ]
12 López-Victoria, M. and B. Werding. 2008. Ecology of the endemic land crab Johngarthia malpilensis (Decapoda: Brachyura: Gecarcinidae), a poorly known species from the Tropical Eastern Pacific. Pac. Sci., 62: 483-493. [ Links ]
13 Losos, J. B. 1994. Integrative approaches to evolutionary ecology: Anolis lizards as model systems. An. Rev. Ecol. System., 25: 467-493. [ Links ]
14 Nicholson, K. E., R. E. Glor, J. J. Kolbe, A. Larson, S. B. Hedges and J. B. Losos. 2005. Mainland colonization by an island species. J. Biogeogr., 32: 929-938. [ Links ]
15 O'Shaughnessy, A. W. E. 1874. Description of new species of Scincidae in the collection of the British Museum. An. Mag. Nat. Hist., 13: 298-301. [ Links ]
16 Prahl, H. von. 1990. Malpelo la roca viviente. FEN Colombia, Editorial Presencia, Bogotá. 57 p. [ Links ]
17 Rand, A. S., G. C. Gorman and W. M. Rand. 1975. Natural history, behavior, and ecology of Anolis agassizi. Smithsonian Contr. Zool., 176: 27-38. [ Links ]
18 Slevin, J. R. 1928. Description of a new species of lizard from Malpelo Island. Proc. Cal. Acad. Sci., 16: 681-684. [ Links ]
19 Stamm, B. and G. C. Gorman. 1975. Notes on the chromosomes of Anolis agassizi (Sauria: Iguanidae) and Diploglossus millepunctatus (Sauria: Anguidae). Smithsonian Contr. Zool., 176: 52-54. [ Links ]
20 Stejneger, L. 1900. Description of two new lizards of the genus Anolis from Cocos and Malpelo Islands. Bull. Mus. Comp. Zool. Harvard Coll., 36: 161-164. [ Links ]
21 Türkay, M. 1970. Die Gecarcinidae Amerikas. Mit einem Anhang über Ucides Rathbun (Crustacea: Decapoda). Senckenbergiana Biol., 51: 333-354. [ Links ]
22 Webster, T. P. 1975. Electrophoretic estimates of genic variation in, and the relationships of, Anolis agassizi. Smithsonian Contr. Zool., 176: 47-51. [ Links ]
23 Wolda, H. 1975. The ecosystem on Malpelo Island. Smithsonian Contr. Zool., 176: 21-26. [ Links ]
FECHA DE RECEPCIÓN: 20/12/2010 FECHA DE ACEPTACIÓN: 12/10/2011
*Contribución No. 1095 del Instituto de Investigaciones Marinas INVEMAR.