Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.40 supl.1 Santa Marta Dec. 2011
ENDEMIC SHALLOW REEF FISHES FROM MALPELO ISLAND: ABUNDANCE AND DISTRIBUTION*
PECES ENDÉMICOS DE ARRECIFES SOMEROS DE LA ISLA MALPELO: DISTRIBUCIÓN Y ABUNDANCIA
Luis Chasqui Velasco, Diego L. Gil-Agudelo and Ramón Nieto
Instituto de Investigaciones Marinas y Costeras-INVEMAR. Cerro Punta de Betín, A.A. 1016, Santa Marta, Colombia. luis_chasqui@invemar.org.co (L.C.V.); dl_gil@yahoo.com (D.L.G.A.); ramon_nieto@invemar.org.co (R.N.).
ABSTRACT
The fish species endemic to Malpelo Island have been scarcely studied, resulting in a lack of information on their densities and habitat preferences. The distribution and abundance of the endemic reef fish species of Malpelo were estimated using underwater visual census techniques. The most abundant species were Axoclinus rubinoffi (0.18 fish/m2) and Lepidonectes bimaculatus (0.08 fish/m2). The highest abundance was found in rocks covered by coralline algae in the Bajo de Junior site.
KEY WORDS: Endemic fishes, Malpelo, Axoclinus, Lepidonectes, Acanthemblemaria.
RESUMEN
Las especies de peces endémicos de la isla Malpelo han sido poco estudiadas, al punto que no se cuenta con información sobre sus densidades y preferencias de hábitat. Mediante censos visuales se estudió la distribución y abundancia de las especies de peces arrecifales endémicos de Malpelo. Las especies más abundantes fueron Axoclinus rubinoffi (0.18 peces/m2) y Lepidonectes bimaculatus (0.08 peces/m2). La mayor abundancia se encontró en el Bajo de Junior y en relación con sustrato rocoso cubierto con algas coralináceas incrustantes.
PALABRAS CLAVE: Peces endémicos, Malpelo, Axoclinus, Lepidonectes, Acanthemblemaria.
INTRODUCTION
Malpelo is an oceanic island situated in the Tropical East Pacific (TEP) and is the only emerged portion of the Malpelo Ridge. Due to its volcanic origin as part of an isolated ridge dating around 17 MY, Malpelo has never been connected to the mainland nor to other TEP islands (Hoernle et al., 2002). The distance between Malpelo and other landmasses such as the other TEP islands and mainland Colombia (about 360-500 km; López-Victoria and Rozo, 2006), as well as water depth inbetween these sites (over 3000 m) are considered important barriers for the regular flow of continental fauna and shallow water marine species (Graham, 1975). This isolation has driven the evolution of marine and terrestrial endemic species via allopatric speciation of founder populations.
In Malpelo's marine realm, at least five endemic fish species have evolved: Halichoeres malpelo, Axoclinus rubinoffi, Lepidonectes bimaculatus, Chriolepis lepidotus, and Acanthemblemaria stephensi (Robertson and Allen, 2008). Little is known about the ecology and biology of these species despite of their endemic status. Factors such as habitat availability for larvae settlement and competition for key resources are some of the drivers of reef fish population size. Understanding the physical and biological factors driving the success of the species is an essential tool for the management of geographically restricted species, such as Malpelo's endemic fishes; in that sense, this study provides the first insight into the distribution and abundance of these species and describes some characteristics of their habitat.
STUDY AREA
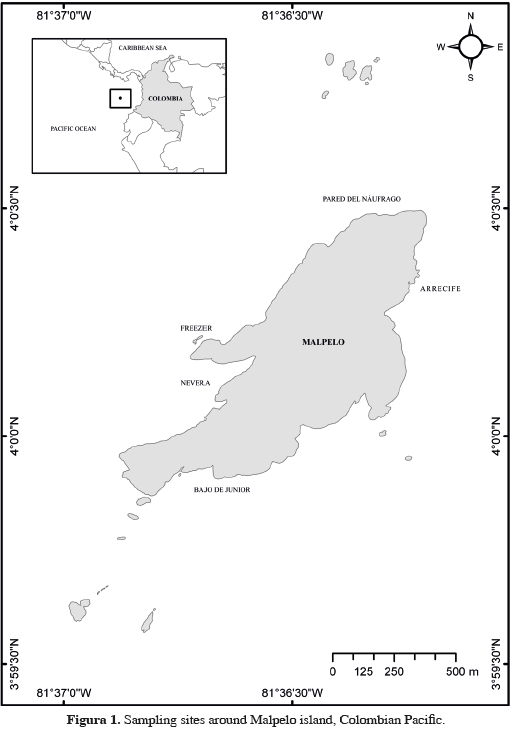
Malpelo (4°0' N, 81°36'30" W) is located 500 km from the Colombian port of Buenaventura and it is the only oceanic island in the Colombian Pacific. The island and its islets are part of the Malpelo Fauna and Flora Sanctuary (SFF Malpelo), one of 56 protected areas of the Colombian National Park System, and have belonged to the World Heritage List of UNESCO since 2006. The island of Malpelo is of volcanic origin and is subject to constant erosion of its coastal cliffs. The seabed around the island is dominated by steep walls and is mostly covered by loose boulders resulting from landslides, although there are some small terraces with sand at about 30 m depth.
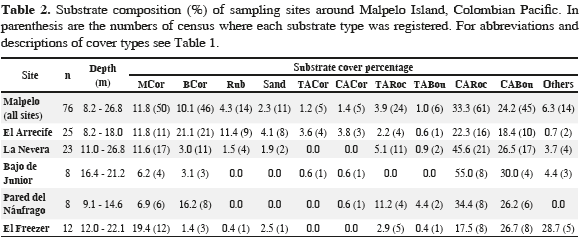
Visual censuses were used to assess the abundance of these fish species in five distinct areas around the island: El Arrecife, La Nevera, El Freezer, La Pared del Náufrago and El Bajo de Junior (Figure 1). El Arrecife is Malpelo's largest coral formation with an extension of 2.3 ha, located between 4 and 30 m depth (Chasqui and Zapata, 2007). La Nevera is a small bay located at the southwest face of the island harboring a small coral patch (0.46 ha) mainly composed of massive corals at depths between 20 and 30 m. El Freezer is a rocky wall with a small landslide zone characterized by rocks and boulders covered with coralline algae and lacking coral formations. La Pared del Náufrago is a rocky wall with a small coral patch between 8 and 15 m deep with some boulders covered with crustose coralline algae. Finally, El Bajo de Junior is a shoal in the southern part of the island at depths of 10 to 25 m; it has a gentle slope and a bottom covered by both bare rocks and others covered by coralline algae.
MATERIAL AND METHODS
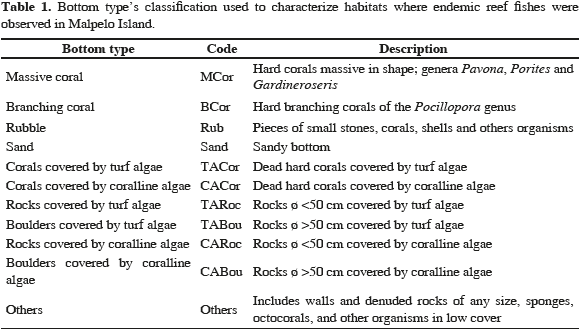
A total of 76 belt transects (20 x 2 m) were used to assess the presence and abundance of A. rubinoffi, L. bimaculatus and A. stephensi in the five study areas (total area of 3040 m2). Transects were performed at depths between 8 and 27 m where rocks and boulders covered with crustose coralline algae, and massive and branching corals are dominant. Along each transect, fish were counted and their habitats were noted, according to 11 bottom types and depth (Tables 1 and 2). For A. stephensi, abundance was estimated using 50 x 50 cm quadrants in areas with an abundance of barnacles, the habitat of this fish species.
The Kruskall-Wallis test was used to evaluate differences in species abundance in each site, differences among all evaluated sites, and differences in abundance of species between sites. Multiple comparisons using the Mann-Whitney test were performed among all possible pairs of species vs sites. Finally, contingency tables were used to explore associations between species abundance and substrate type, and the Spearman correlation analysis was used to assess the relation between species and each substrate type.
RESULTS
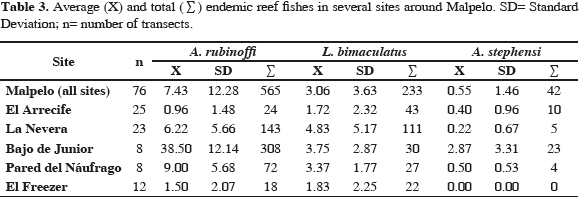
The most frequent species were A. rubinoffi and L. bimaculatus (Figure 2), while A. stephensi was less frequent (Figure 3, Table 3). Axoclinus rubinoffi was the most abundant species with 565 individuals in the 3040 m2 evaluated, and an average of 0.18 fish/m2, followed by L. bimaculatus with 233 fish (0.08 fish/m2). A. stephensi was scarce in tran sect counts (0.01 fish/m2). The Kruskall-Wallis test showed differences in abundance between species (H= 49.35; p <0.01), and the Mann-Whitney tests showed differences in abundance between A. rubinoffi and A. stephensi (U= 1340; p <0.01), L. bimaculatus and A. stephensi (U= 1142; p <0.01), but not between A. rubinoffi and L. bimaculatus (U= 2652; p >0.05).
Axoclinus rubinoffi abundance was significantly different among sites (Kruskall-Wallis, H= 41.87; p <0.01). This species was abundant in El Bajo de Junior (0.96 fish/m2) and scarce in El Arrecife (0.024 fish/m2; Table 3). Mann-Whitney paired tests showed differences between all site pairs but not in the case of El Freezer vs El Arrecife, and La Pared del Náufrago vs La Nevera (U= 128; p= 0.43 and U= 65; p= 0.22, respectively).
Differences in the abundance of L. bimaculatus were found between El Arrecife vs La Nevera (U= 145.5; p <0.01), El Arrecife vs La Pared del Náufrago (U= 42.5; p <0.05), El Arrecife vs El Bajo de Junior (U= 51; p <0.05), and El Freezer vs La Nevera (U= 79.6; p <0.05). This species was abundant in La Nevera, La Pared del Náufrago and El Bajo de Junior, and scarce in El Freezer and El Arrecife (Table 3).
Acanthemblemaria stephensi abundance was higher in El Bajo de Junior (0.07 fish/m2), being statistically different to El Arrecife (U= 41; p <0.01) and La Nevera (U= 31.5; p <0.01). The density of this species in El Bajo de Junior, estimated using 50 x 50 cm quadrants, was 3.3 fish/m2. A. stephensi was absent in El Freezer (Table 3).
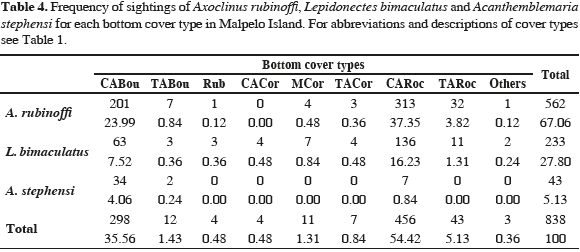
Sighting frequency analysis using contingency tables showed a relationship between species and substratum cover types (Cramer V= 0.206; Table 4), with an association between both variables (Cramer V= 0.206) and a low probability that observed frequencies would be expected if no relationship existed between species and substrate type (M-L X2= 70.03; p <0.01), suggesting that species are not randomly distributed in the substrate, but are associated to certain types of substratum.
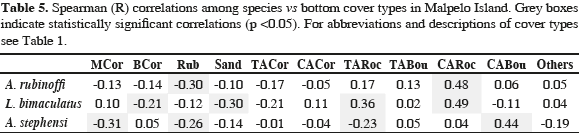
The Spearman test showed a positive correlation between the abundance of A. rubinoffi and the percentage of rocks covered by coralline algae (CARoc), as well as a negative correlation between L. bimaculatus and the percentage of branching coral (BCor), sand (Sand) and a positive correlation with the percentage of rocks covered with microalgae turf (TARoc) and rocks covered by coralline algae (CARoc). Regarding A. stephensi, abundance was negatively related to the percentage of massive coral cover (MCor), rubble (Rub), and rocks covered with algae turfs (TARoc), and positively related to the percentage of boulders covered by coralline algae (CABou) (Table 5).
DISCUSSION
This study provides the first attempt to understand the distribution, abundance and ecology of Malpelo's endemic reef fish species. Other than the short communication from Quimbayo et al. (2010), regarding the cleaning behavior of L. bimaculatus juveniles, there have been no studies involving A. rubinoffi and L. bimaculatus since their description in the 1990's (Allen and Robertson, 1992a).
Among the three studied species, A. rubinoffi was the most abundant followed by L. bimaculatus (Table 3). These species belong to the Tripterygiidae family (triplefin fishes), which includes some abundant and important species in the microcarnivore group (Kotrschal and Thomson, 1986). Gilligan (1991) found Axoclinus n.sp. and Axoclinus carminalis in abundance when collecting cryptobenthic species in shallow rocky habitats in the California Gulf; however, the taxonomic status of A. carminalis change to Enneanectes carminalis (Smith and Williams, 2002).
Abundance values found in this work are comparable to the values reported by Wellenreuther et al. (2007) when studying habitats used by the tripterigid community in New Zealand. These authors inspected 151 randomly located 4 x 4 m quadrants, finding 15488 organisms belonging to 17 species. Considering the total area sampled (2416 m2), a density of 0.38 fish/m2 was found. In Malpelo, when adding the abundance of both tripterigid species (A. rubinoffi and L. bimaculatus), a density of 0.26 fish/m2 is found. Other works in the Gulf of California also show an abundant tripterigid community (Kotrschal and Thomson, 1986; Gilligan, 1991), nonetheless, the sampling techniques used prevent further comparisons with the present study.
The low abundance values registered for A. stephensi could be the consequence of the sampling method used. This species, belonging to the family Chaenopsidae, lives within barnacle skeletons (Fischer et al., 1995), and as a result, their distribution is related to barnacle distribution. This species was found at depths of up to 21 m in El Bajo de Junior, however, the species is mainly located above 10 m depth, where very few censuses were made due to ocean conditions.
The abundance of A. rubinoffi and L. bimaculatus seems to be related to bottom cover, particularly large covers of coralline algae: A. rubinoffi was more abundant in El Bajo de Junior, where coralline algae cover was approximately 80 %, while this species was less abundant in El Arrecife, where coralline algae cover was less than 40 %. The triplefin fishes are often habitat specialists, being closely related to a particular type of bottom cover (Patzner, 1999), a common characteristic among blennioid fishes (Syms, 1995; Patzner, 1999; Wellenreuther and Clements, 2008).
Regarding the abundance of H. malpelo and C. lepidotus, census techniques used were not appropriate to obtain abundance estimates of these species, mainly because they have different habitats and behaviors to A. rubinoffi, L. bimaculatus and A. stephensi. Halichoeres malpelo is a wrasse species that swims close to the bottom, looking for invertebrates and small fish to prey on; it has been observed mainly in association with shallow coarse sand bottoms (Allen and Robertson, 1992b). On the other hand, the records of C. lepidotus are very scarce and always associated with rubble and sandy bottoms (Robertson and Allen, 2008). Therefore, it is necessary to carry out species-specific studies to determine the abundance and distribution of these two species. In conclusion, A. rubinoffi and L. bimaculatus are the most abundant endemic reef fishes in Malpelo, being primarily associated with rocky bottoms covered by coralline algae, a prevailing habitat in the shallower 30 m around the island.
ACKNOWLEDGEMENTS
The authors would like to thank the Marine and Coastal Research Institute "José Benito Vives de Andréis" INVEMAR, the Special Administrative Unit for the System of National Natural Parks of Colombia, Malpelo Fauna and Flora Sanctuary, the Walton Family Foundation, Asturias, and the Malpelo Foundation for their logistical support for the present investigation; also to the crew of the María Patricia and Nemo vessels.
LITERATURE CITED
1 Allen, G. and D. R. Robertson. 1992a. Three new species of Triplefins (Pisces: Tripterygiidae) from Malpelo and Socorro Islands, in the Tropical Eastern Pacific. Revue fr. Aquariol, 19: 53-56. [ Links ]
2 Allen, G. and D. R. Robertson. 1992b. Deux nouvelles espèces de Girelles (Labridae: Halichoeres) du Pacifique oriental tropical. Revue fr. Aquariol., 19: 47-52. [ Links ]
3 Chasqui, L. and F. A. Zapata. 2007. Tamaño y composición de dos formaciones coralinas del Santuario de Fauna y Flora Malpelo, Pacífico colombiano. 96-101. In: INVEMAR (Ed.). Informe del estado de los ambientes marinos y costeros en Colombia: Año 2006. Serie de Publicaciones Periódicas No. 8, INVEMAR, Santa Marta. 378 p. [ Links ]
4 Fischer, W., F. Krupp, W. Schneider, C. Sommer, K. E. Carpenter and V. H. Niem. 1995. Guía FAO para la identificación de especies para los fines de la pesca. Pacífico centro-oriental. Vol. II. Vertebrados - Parte 1. Roma, FAO. Vol. II: 647-1200. [ Links ]
5 Gilligan, M. R. 1991. Bergmann ecogeographic trends among triplefin blennies (Teleostei: Tripterygiidae) in the Gulf of California, Mexico. Environ. Biol. Fish., 31: 301-305. [ Links ]
6 Graham, J. B. 1975. The biological investigation of Malpelo Island, Colombia. Smithsonian Contr. Zool., 176: 1-8. [ Links ]
7 Hoernle, K., P. van den Bogaard, R. Werner, B. Lissinna, F. Hauff, G. Alvarado and D. Garbe-Schönberg. 2002. Missing history (16-71 Ma) of the Galápagos hotspot: Implications for the tectonic and biological evolution of the Americas. Geology, 30 (9): 795-798. [ Links ]
8 Kotrschal, K. and D. A. Thomson. 1986. Feeding patterns in eastern tropical Pacific blennioid fishes (Teleostei: Tripterygiidae, Labrisomidae, Chaenopsidae, Blenniidae). Oecología, 70: 367-378. [ Links ]
9 López-Victoria, M. and D. Rozo. 2006. Model-based geomorphology of Malpelo Island and spatial distribution of breeding seabirds. Bol. Invest. Mar. Cost., 35: 111-131. [ Links ]
10 Patzner, R. A. 1999. Habitat utilization and depth distribution of small cryptobenthic fishes (Blenniidae, Gobiesocidae, Gobiidae, Tripterygiidae) in Ibiza (western Mediterranean Sea). Environ. Biol. Fish., 55: 207-214. [ Links ]
11 Quimbayo, J. P., F. A. Zapata, S. R. Floeter, S. Bessudo and I. Sazima. 2010. First record of cleaning by a triplefin blenny in the Tropical Pacific. Coral Reefs, online. DOI 10.1007/s00338-010-0656-8. [ Links ]
12 Robertson, D. R. and G. R. Allen. 2008. Shorefishes of the tropical Eastern Pacific online information system. http://www.stri.org/sftep. 20/05/2011. [ Links ]
13 Smith, D. G. and J. T. Williams. 2002. History and status of the genera Enneanectes and Axoclinus (Teleostei: Blennioidei: Tripterygiidae). Zootaxa, 105: 1-10. [ Links ]
14 Syms, C. 1995. Multi-scale analysis of habitat association in a guild of blennioid fishes. Mar. Ecol. Prog. Ser., 125: 31-43. [ Links ]
15 Wellenreuther, M. and K. D. Clements. 2008. Determinants of habitat association in a sympatric clade of marine fishes. Mar. Biol., 154: 393-402. [ Links ]
16 Wellenreuther, M., P. T. Barrett and K. D. Clements. 2007. Ecological diversification in habitat use by subtidal triplefin fishes (Tripterygiidae). Mar. Ecol. Prog. Ser., 330: 235-246. [ Links ]
FECHA DE RECEPCIÓN: 26/08/2010 FECHA DE ACEPTACIÓN: 12/10/2011
*Contribución No. 1096 del Instituto de Investigaciones Marinas INVEMAR.