Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.44 no.1 Santa Marta Jan./June 2015
NOTES ON THE MARINE ALGAE OF THE INTERNATIONAL BIOSPHERE RESERVE SEAFLOWER, CARIBBEAN COLOMBIA IV: NEW RECORDS OF MACROALGAL EPIPHYTES ON THE SEAGRASS THALASSIA TESTUDINUM
NOTAS SOBRE LAS ALGAS MARINAS DE LA RESERVA INTERNACIONAL DE BIOSFERA SEAFLOWER, CARIBE COLOMBIANO IV: NUEVOS REGISTROS DE MACROALGAS EPÍFITAS SOBRE HOJAS DE THALASSIA TESTUDINUM
Margarita Rosa Albis-Salas1,2 and Brigitte Gavio1,2
1 Universidad Nacional de Colombia sede Caribe. Circunvalar San Luis Free Town No. 52-44, San Andrés, Colombia. mralbiss@unal.edu.co.
2 Universidad Nacional de Colombia sede Bogotá, Departamento de Biología, Ciudad Universitaria, Bogotá, Colombia. bgavio@unal.edu.co.
ABSTRACT
Nine species of macroalgae are newly reported for the Caribbean International Biosphere Reserve Seaflower. Of these taxa, Neosiphonia sphaerocarpa, Polysiphonia schneideri, Polysiphonia sertularioides, Cladosiphon occidentalis, and Phaeophila dendroides, have been previously reported from Colombian waters, whereas Ulothrix sp., Ulva flexuosa subsp. paradoxa, Chaetomorpha minima, and Cladophora liniformis represent new records for the country. All the algae were found growing epiphytically on Thalassia testudinum in shallow (<1 m) seagrass meadows around San Andrés Island. Their morphological features are discussed.
KEYWORDS: Colombia, Epiphyte, Marine algae, New records, Polysiphonia.
RESUMEN
Se registran por la primera vez para la Reserva Internacional de Biosfera Seaflower nueve especies de macroalgas. De estas especies, Neosiphonia sphaerocarpa, Polysiphonia schneiderii, Polysiphonia sertularioides, Cladosiphon occidentalis y Phaeophila dendroides, han sido registradas previamente para aguas colombianas, mientras Ulothrix sp., Ulva flexuosa subsp. paradoxa, Chaetomorpha minima y Cladophora liniformis son nuevos registros para el país. Todas las algas fueron encontradas epifitas sobre hojas de Thalassia testudinum, en praderas someras (<1 m) en la isla de San Andrés. Se discuten sus características morfológicas.
PALABRAS CLAVE: Colombia, Epifitos, Algas marinas, Nuevos registros, Polysiphonia.
INTRODUCTION
Seagrass meadows are very productive ecosystems of which a large proportion is often attributed to the epiphytes (Leliaert et al., 2001; Won et al., 2010). Epiphytes can represent up to 50% of the total above-sediment biomass of a seagrass meadow (Leliaert et al., 2001). Epiphytes can therefore play an important role in the functioning of seagrass ecosystems.
The most widely distributed seagrass in the Caribbean is Thalassia testudinum Banks ex König, which provides ample substrate for algal epiphytes (Cho et al., 2002; Barrios and Díaz, 2005; Corlett and Jones, 2007; Samper- Villarreal et al., 2008). However, few studies characterizing the epiphytic flora have been addressed in the Caribbean, there being only those in Florida (Dawes 1987, Won et al., 2010), in Costa Rica (Samper-Villareal et al., 2008), and in Venezuela (Barrios and Díaz, 2005). In Colombia, the studies on macroalgae epiphytes have been restricted to estimations of their biomass (Palacios et al., 1992). Our recent field surveys on Thalassia testudinum macroalgal epiphytes in San Andrés Island revealed some species previously unknown to the region (Albis-Salas and Gavio, 2011). We present nine new records for the Archipelago, three species of red algae, one brown alga and five green algae. Four taxa represent new records for Colombia. All these taxa are generally overlooked in the floristic treatments of the region, mainly due to their small size and difficult taxonomic treatment. We herein provide detailed morphological features of the specimens encountered and a comprehensive discussion on the taxonomic status of each species.
MATERIALS AND METHODS
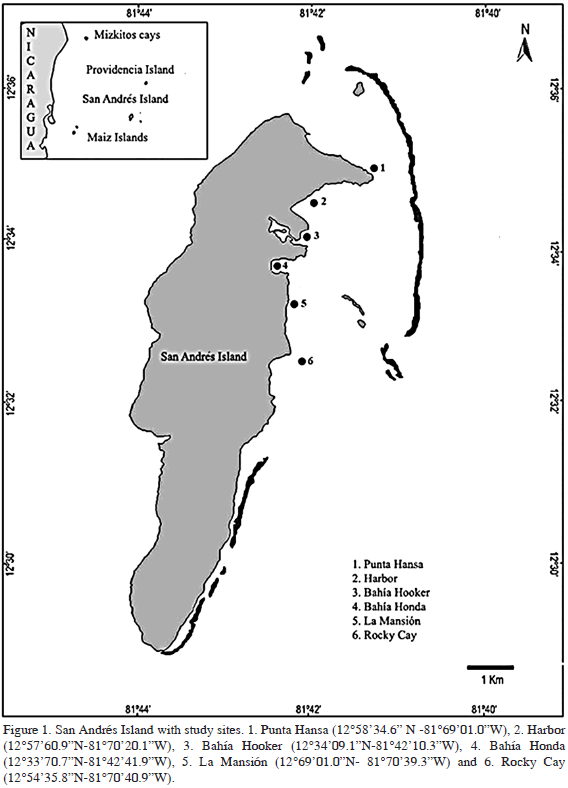
San Andrés (12°28'55"N; 81°40'49"W) is an oceanic island situated in the southwestern Caribbean, Colombia (Figure 1), being part of the San Andrés and Old Providence Archipelago, declared as International Biosphere Reserve Seaflower since 2000 (Coralina, 2007). For details on the study site, see Albis-Salas and Gavio (2011). During the wet (December 2007) and dry seasons (March 2008) we sampled in six sites, on the east coast of the island (Gavio et al., 2010; Albis-Salas and Gavio, 2011). All meadows are shallow (<1 m). The leaves of Thalassia were preserved in a 4% formalin/seawater solution. In the laboratory, algae were observed under an Olympus BX 51 microscope and identified with specialized bibliography for species identification (Littler and Littler, 2000; Dawes and Mathieson, 2008; Littler et al., 2008; Stuercke and Freshwater, 2010). Portions of the thalli were mounted on glass slides in 50% glycerin, after staining in aniline-blue solution. Information on the type localities of these taxa has been obtained from Silva (2013).
RESULTS AND DISCUSSION
We report a total of nine new records for San Andrés Island, four of which are new for Colombia. All species were found as epiphytes on leaves of Thalassia testudinum. We report three species of Rhodophyta, one Heterokontophyta and five Chlorophyta. Furthermore, this is the first report of the genera Cladosiphon, Neosiphonia, Phaeophila and Polysiphonia for the San Andrés and Old Providence Archipelago, and of the genus Ulothrix for Colombia.
The algae were mostly diminutive filamentous specimens growing as epiphytes or endophytes on the encrusting algae Hydrolithon farinosum and Pneophyllum fragile which were in turn overgrowing leaves of Thalassia testudinum. New records for Colombia are marked with an asterisk [*].
RHODOPHYTA
Order Ceramiales
Family Rhodomelaceae
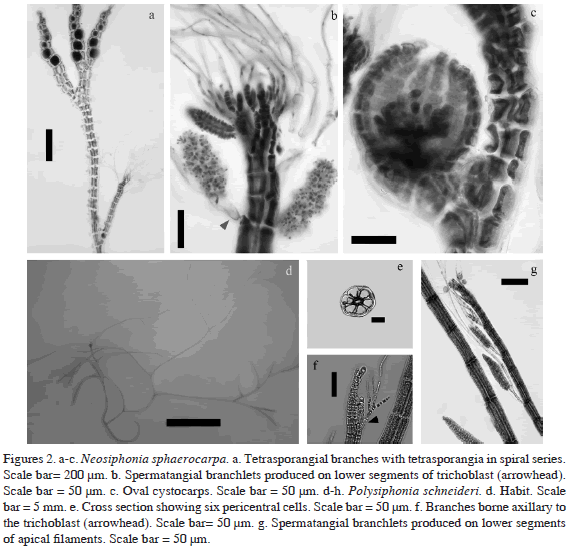
Neosiphonia sphaerocarpa (Børgesen) M.S. Kim and I.K. Lee (1999).
Type locality: St. Thomas, Virgin Islands.
Thallus filamentous, bushy, maroon in color, up to 1 cm tall. Branching alternate to pseudodichotomous. Thallus attached by discoid holdfast and secondarily by unicellular rhizoids that arise from distal ends of ventral pericentral cells with cross wall. Branches 60-90 µm diam, with four pericentral cells per segment, segments 80-140 µm long, 0.5-1.5 diameters long. Tetrasporangia 10-12 µm wide, 20-25 µm long, tetrahedrally divided, in spiral series (Figure 2a). Spermatangial branchlets cylindrical and lateral, produced on lower segments of apical filaments (Figure 2b). Cystocarps oval, 183 µm wide, 178 µm long (Figure 2c).
Site and season of collection: Dry season 07-10/12/2007, Bahía Honda, Punta Hansa.
Known western Atlantic distribution: Barbados, Belice, Cuba, Florida, Hispaniola, Lesser Antilles, Puerto Rico, Venezuela, Virgin Islands.
Polysiphonia schneideri B. Stuercke and D.W. Freshwater (2010) Reported by Díaz-Pulido and Díaz-Ruiz (2003) as P. denudata.
Type locality: Wrightsville Beach, North Carolina, USA.
Thallus filamentous, creeping, red-maroon to violet, without cortication (Figure 2d). The base was not observed in our specimens; however, there is a prostrate axis 130-180 µm diam, 0.8-1 diameters long, from which erect axes arise. Erect axes may reach 5 cm in length, frequently ramified, alternate proximally, unilateral distally. Lateral branches thinner, 70-100 µm diam; segments 1.5-2 diameters long; branches abruptly tapering distally to 50-55 µm diam, 1-1.5 diameters long; toward the apex the tapering is stronger, to 30-40 µm diam, 0.5 diameters long. Lateral adventive branches present. Pericentral cells 6-7 (Figure 2e). Apex conspicuous, 10-12.5 µm long, 10-12 µm diam. The branches are borne axillary to the trichome (Figure 2f). Trichoblasts abundant towards the apex, 2-3 times dicho- to subdichotomously ramified. Scar cells frequent and irregularly arranged. Rhizoids digitiform, cut-off from ventral pericentral cells, abundant, generally one per segment but we sometimes observed two rhizoids per segment, 20-25 µm diam, 500-800 µm long. Tetrasporangia in series, ellipsoidal, in the middle part of the thicker axes, sometimes dispersed in the whole thallus, 30-50 µm diam, 90-100 µm long. Spermatangial branchlets narrowly ovate and lateral, produced on lower segments of apical filaments, 18.2-32 µm diam 92-124 µm long (Figure 2g).
Known western Atlantic distribution: Bermuda, Colombia, Florida, Panamá, Puerto Rico, Texas, Venezuela.
Site and season of collection: Dry season 29-30/03/2008, Punta Hansa, Rocky Cay.
Remarks: Stuercke and Freshwater (2010) recently determined that the western Atlantic taxon known as Polysiphonia denudata is a previously unrecognized species, which they named P. schneideri. In their study, they included vouchers from continental Colombia, which belong to this new identity. The specimen that we found agrees with their description of P. schneideri, with the exception of the position of the tetrasporangia. Those authors described the tetrasporangia as being disposed in the distal portion of the branches, while our specimens had the tetrasporangia in the middle portion of the largest axes. Furthermore, tetrasporangia in our specimens were slightly smaller (30-50 um diam) than those reported by Stuercke and Freshwater (2010, 45-85 um diam), as well as the spermatangial branches (18-32 um diam x 92- 124 um long in our samples versus 35-60 um diam x 125-260 um long in the original description). Whether these variations represent normal variability in a population or reveal further cryptic species diversity should be assessed with molecular data.
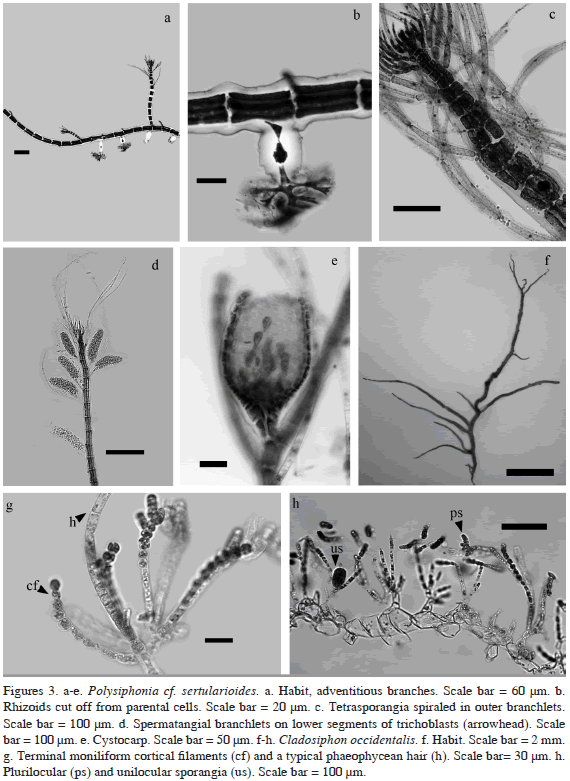
Polysiphonia cf. sertularioides (Grateloup) J. Agardh (1863) Type locality: Cette, Gulf of Lion, France.
Thallus filamentous, creeping, red to maroon, 0.5-5 mm tall. Erect branches alternate proximally, dichotomous distally. Prostrate axis (33) 75-100 µm diam, with four pericentral cells, segments 1-2 diameters long (Figure 3a). Erect axes (30) 40-55 µm diam, segments 0.5-3 diameters long. In some specimens secondary branching is rather sparse: the first branch may appear after 10-25 segments, and there may be 10-12 segments between branches; however, in adventitious short branches we observed an interval of only 2-4 segments between branches. Branchlets constricted at the base and gradually tapering toward the apex (Figure 3a), although we occasionally found specimens that were abruptly tapering toward the apex. Lateral branches forming in axils of trichoblasts. Trichoblasts deciduous, 1-3 times dichotomous to subdichotomous branched, with obvious scar cells spirally arranged. In some plants, we observed that scar cells appear after 5-9 segments. Scar cells give rise to adventitious branches. Rhizoids unicellular or multicellular, finger-like, cut off from parental cells (Figure 3b). Tetrasporangia spherical 70-90 µm, strongly spiraled in outer branchlets (Figure 3c). Spermatangial branchlets cylindrical on lower segments of trichoblasts (Figure 3d). Cystocarps oval, 130-190 µm wide, 240- 270 µm long (Figure 3e).
Site and season of collection: Wet season 07-10/12/2007, Harbor, Punta Hansa; dry season 29-30/03/2008, Harbor, Punta Hansa.
Known western Atlantic distribution: Bahamas, Belize, Colombia, Cuba, Florida, Panamá, Texas, Venezuela.
Remarks: The specimens we observed presented great morphological variation in thallus size and branching pattern. We observed individuals with scattered branching and prostrate axis of 33-40 µm diameter, and others with frequent branching and prostrate axis of 75-100 µm in diameter. There was scar cells variation as well, with specimens showing spirally arranged scar cells every segment, while others with scar cells appearing after the first 5-9 segments of the branch. Furthermore, sometimes the filaments gradually tapered towards the apex, while in other plants the tapering was rather abrupt.
Womersley (1979) proposed that P. sertularioides, originally described from the Mediterranean Sea, and P. flaccidissima Hollenberg, described from the Pacific coast of North America and later reported for the tropical Pacific, Caribbean Sea, and South Africa (Rojas-González and Afonso-Carrillo, 2010), should be considered taxonomic synonyms because they share many diagnostic characters, such as prostrate habit, rhizoids with close connection, conspicuous trichoblasts, presence of adventitious branches, frequent scar cells, lateral branches forming in axils of trichoblasts and spirally arranged trichoblasts. Later, Kapraun et al. (1983), Abbott (1999) and Womersley (2003) again suggested synonymy, pending new research. Abbott (1999), however, pointed out that P. flaccidissima has a much more developed prostrate system than P. sertularioides. The description of P. sertularioides by Lauret (1967), in his extensive work on the Mediterranean Polysiphonia, is very similar to Hollenberg (1942) original description of P. flaccidissima. However, there are differences between the two taxa which have been later dismissed by other authors. According to Lauret (1967), there is a very clear pattern in scar cell distribution in specimens of genuine P. sertularioides: in the upright segments there is always a scar cell before a branch, and after it there is a segment without scar cell, with a pattern SBN (scar cell, branch, no scar cell). On the other hand, in the original plates of Hollenberg (1942, p. 775, fig. 8) for P. flaccidissima, the pattern is reversed, i.e. the branch is preceded by a segment without scar cell and followed by one with scar cell (NBS). The robustness of this pattern as a taxonomic feature has not been considered again by other authors. In our specimens we mostly found a SBS pattern (scar cell, branch, scar cell), but in some specimens we observed also a SBN pattern, as in Hollenberg's original description. As we already mentioned, the morphological variation that we observed among specimens fitting the description of P. sertularioides was rather high, and this character was polymorphic as well. Mamoozadeh and Freshwater (2011), in a recent molecular study on Caribbean Polysiphonia, found genetic variation among three specimens of P. cf. sertularioides from Panama, indicating that the taxon is possibly a species-complex of cryptic taxa. Since the most recent published works on Polysiphonia maintain the synonymy between P. flaccidissima and P. sertularioides, we decided to follow this trend. As many other authors suggested, a thorough revision of the Polysiphonia sertularioides/flaccidissima complex is needed. Díaz-Pulido and Díaz-Ruiz (2003) reported Polysiphonia flaccidissima for the continental coast of Colombia in the Caribbean.
HETEROKONTOPHYTA
Order Ectocarpales
Family Chordariaceae
Cladosiphon occidentalis Kylin (1940)
Type locality: Dry Tortugas, Florida, USA.
Thalli erect, light brown to olive brown in color, soft and mucous, up to 11.5 cm high, with a monostromatic discoid holdfast, 0.5-1 mm diam, from which a main axis arises. Axes cylindrical (Figure 3f), to 1.5 mm in diameter, branched with unilateral to irregular branches abundant at base and sparse toward the apex, with short second-order branches up to 1 mm diam (Figure 3f). Medulla multiaxial, consisting of longitudinal filaments, the axis becoming hollow a short distance behind its apex. Branches often ending in a hair laterally displacing the distal portion of the medullary filaments (Figure 3g). Medullary cells 110-225 µm long and 30-75 µm diam. Thin subcortex 1 cell thick formed perpendicularly to the medullary filaments. Subcortical cells hyaline, subcylindrical to broad at base, (5)10-15 µm diam (Figure 3h). Primary cortical filaments simple, 100-225 µm long, composed of 6-13 cells, with proximal cells cylindrical, 17.5-27.5 µm long, 5-7.5 µm diam. Distal cells moniliform, 7.5-12.5 µm long, 7.5-12.5 µm diam (Fig. 3g). Phaeophycean hairs abundant, arising from subcortical cells, with a short, basal sheath 11- 12.5 µm diam (Figure 3g).
Plurilocular sporangia in groups of 3-6, on distal cells of cortical filaments, 25-26 µm long and 10-12.5 µm diam. Unilocular sporangia ovoid, 25-50 µm long and 25-45 µm diam, sessile and solitary, borne on proximal cells of cortical filaments or on distal subcortical cells (Fig. 3h).
Site and season of collection: Wet season 07-10/12/2007, Harbor; dry season 29-30/03/2008, Harbor.
Known western Atlantic distribution: Bahamas, Belize, Cuba, Florida, Panamá, Texas, Virgin Islands.
CHLOROPHYTA
Order Ulotrichales
Family Ulotrichaceae
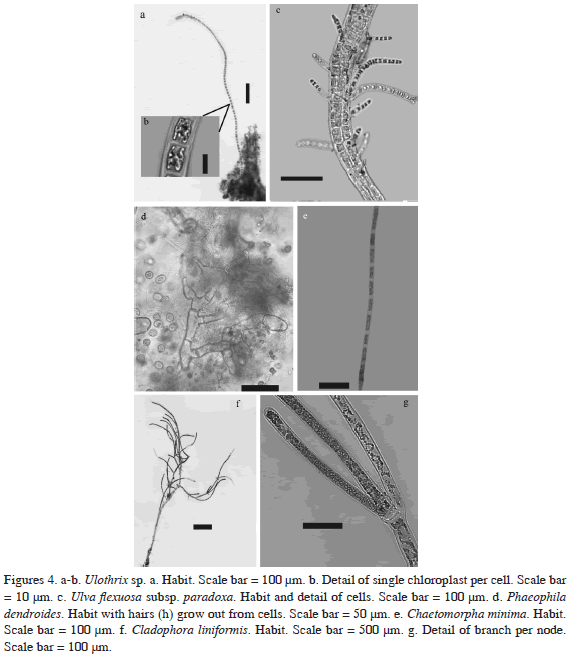
*Ulothrix sp.
Thallus minute, pale green, formed by simple uniseriate filaments to 1.1 cm high (Figure 4a). Filaments straight, neither upcurved nor bent. Cells cylindrical, 16 µm diam, 15-18 µm long with smooth cell walls to 3 µm thick. One chloroplast per cell, conspicuous, H-shaped, with one pyrenoid (Figure 4b). Apical cell rounded at tip, 10 µm, 12 µm long.
Site and season of collection: Dry season 29-30/03/2008, Harbor.
Remarks: Wynne (2011) included two species of Ulothrix in his checklist of benthic algae of the tropical and subtropical Western Atlantic, Ulothrix flacca and U. subflaccida. These two species are distinguished by the basal portion, attached by a basal cell and rhizoids formed as down-growing extensions from a few intercalary cells above in U. flacca and by tapering rhizoidal basal cells in U. subflaccida (John, 2007). In the single specimen we found, it was not possible to observe the basal portion of the plant, which is an important character to distinguish among the Ulothrix species present in the Caribbean flora. However, we consider this alga to be a member of the genus Ulothrix because of its diminutive habit, and the presence of just one chloroplast per cell (the genus Chaetomorpha has several, with the exception of C. philippinensis, Leliaert et al., 2011) (Figure 4b). The genus Uronema is composed of diminutive species, mostly restricted to freshwater habitat. The only marine species reported to date, Uronema marinum, is much smaller in size, the longest filament size to 600 µm, according to Kraft (2007), the chloroplast has a different appearance, and the apical cell is larger than the other cells (see Figure 6, p. 22 in Kraft, 2007). This is the first record of the genus for Colombia.
Order Ulvales
Family Ulvaceae
*Ulva flexuosa subsp. paradoxa (C. Agardh) M.J. Wynne (2005)
Type locality: Bangor, Wales.
Thallus flaccid, to 3 cm high, light green, branching abundant below, opposite to irregular, 30-325 µm diam (Figure 4c). Holdfast conspicuous. Main axis to 16 cells randomly arranged. Branchlets 1-4 cells thick. Cells rectangular to polygonal, 5-12.5 µm diam. and 5-15 µm long (Figure 4c).
Site and season of collection: Wet season 07-10/12/2007, Bahía Hooker, Harbor, Rocky Cay; dry season 29-30/03/2008, Bahía Hooker, Harbor.
Known western Atlantic distribution: Bahamas, Barbados, Brazil, Cuba, Curaçao, Florida, Hispaniola, Jamaica, Lesser Antilles, Panamá, Puerto Rico, Texas, Venezuela, Virgin Islands.
Family Phaeophilaceae
Phaeophila dendroides (P.L. Crouan and H.M. Crouan) Batters (1902)
Type locality: Brest, Finistère, France.
Thallus of uniseriate branched filaments, endophytic in Hydrolithon farinosum and Pneophyllum fragile. Cells cylindrical, 4.5-5 µm diam, 17.5-27.5 µm long with many irregular swellings (Figure 4d). Hairs grow out from vegetative cells, are undulate without cross-walls at base (Figure 4d).
Site and season of collection: Wet season 07-10/12/2007, Harbor, Punta Hansa, Rocky Cay; dry season 29-30/03/2008, Bahía Honda, Bahía Hooker, Harbor, La Mansión, Punta Hansa, Rocky Cay.
Known Caribbean distribution: Florida, Hispaniola, Panamá, Texas, Venezuela, Virgin Islands.
Order Cladophorales
Family Cladophoraceae
*Chaetomorpha minima Collins and Hervey (1917)
Type locality: Bermuda.
Thallus filamentous, inconspicuous, to 3 mm high, yellow-green. Filaments unbranched, uniseriate (Figure 4e), cells cylindrical, 7.5-20 µm diam and 37.5-100 µm long, 2-3 diameters long (Figure 4e), cells longer toward the base of the filaments. Apical cell blunt, 10-15 µm diam and 50 µm long. Attached by disc-like or finger like pad.
Site and season of collection: Wet season 07-10/12/2007, Bahía Honda, Bahía Hooker, Harbor, La Mansión, Punta Hansa, Rocky Cay; dry season 29-30/03/2008, Bahía Honda, Bahía Hooker, Harbor, La Mansión, Punta Hansa, Rocky Cay.
Known Caribbean distribution: Bermuda, Cuba, Florida and Venezuela.
Remarks: Although very common as individual filaments, it was not observed to form mats on the host plant as reported by Littler et al. (2008) in the Indian River Lagoon, Florida. It can attach either directly to Thalassia leaves or its epiphytic coralline algae (Hydrolithon farinosum and Pneophyllum fragile).
*Cladophora liniformis Kützing (1849)
Type locality: Lagoon of Venice (Chioggia), Italy.
Thallus bright yellow-green in older parts and dark green in younger cells. Thalli forming indefinite masses floating at the surface of protected waters among seagrass beds, loose-lying on protected sediments bottoms or like small specimens to 2.5 mm high above seagrass blades. Plants having an irregular organization alternate to pseudodichotomously below and unilateral above, with irregular scattered branch and branches with different lengths (Figure 4f). Growth by division of apical and intercalary cells followed by cell elongation. Branches predominately apically inserted, but subterminal insertion was also observed. One to three branches per node (Figure 4g). Ramification angle 40-80°. Chloroplasts rounded and may form a network. Apical cells mostly long and cylindrical, the end widened or slightly tapering, 15-20 µm diam, 180-250 µm long, 15-17.5 diameters long. Ultimate branches 20-35 µm diam., 200-375 µm long, 12-12.5 diameters long. Main axes 20-25 µm diam., 350 µm long, 14-17.5 diameters long. Basal cells 35 µm diam., 350 µm long. Filaments thicken slightly towards the base, which may reach 35 µm diam. Cell wall thickness in ultimate branches less than 5 µm.
Site and season of collection: Wet season 07-10/12/2007, Bahía Honda; dry season 29-30/03/2008, Bahía Honda.
Known Caribbean distribution: Bahamas, Cuba, Curaçao, Jamaica, Lesser Antilles.
ACKNOWLEDGEMENTS
The authors are grateful to Harley Bent, Samir Bent, family Jay-Padilla, Nacor Bolaños, Trisha Forbes, Sandra Pérez, Carlos Ballesteros, and Elizabeth Galeano, for helping in the field. We thank Omar Abril for assistance in managing Illustrator and Photoshop programs. Michael Wynne, Wilson Freshwater, Brian Wysor and Frederik Leliaert confirmed some of the species identifications. We thank Michael Wynne for kindly providing critical literature and improving the text. The project was developed with the research permit 01-08 for biological collecting issued by Coralina. This research was funded by the Universidad Nacional de Colombia, sede Bogotá, through the projects No. 20201009182 and No. 201010012700, and by the “Fundación para la Promoción de la Investigación y la Tecnología", Banco de la República, agreement Nr. 200921. This work is contribution No. 394 of CECIMAR, Universidad Nacional de Colombia and Programa de Posgrado en Biología - Línea Biología Marina.
LITERATURE CITED
1 Abbott, I. 1999. Marine red algae of the Hawaiian Islands. Bishop Museum Press. Honolulu. 465 p. [ Links ]
2 Albis-Salas, M. and B. Gavio. 2011. Notes on marine algae in the International Biosphere Reserve Seaflower, Caribbean Colombian I: new records of macroalgal epiphytes on the seagrass Thalassia testudinum. Bot. Mar., 54: 537-543. [ Links ]
3 Barrios, J. and O. Díaz. 2005. Algas epífitas de Thalassia testudinum en el Parque Nacional Mochima, Venezuela. Bol. Cent. Invest. Biol., 39: 1-14. [ Links ]
4 Cho, T. O., S. Fredericq and K. K. Yates. 2002. Characterization of macroalgal epiphytes on Thalassia testudinum in Tampa Bay, Florida. J. Phycol., 38: 4. [ Links ]
5 Coralina. 2007. Reserva de Biosfera Seaflower. (http://www.coralina.gov.co/). [ Links ]
6 Corlett, H. and B. Jones. 2007. Epiphyte communities on Thalassia testudinum from Grand Cayman, British West Indies: Their composition, structure, and contribution to lagoonal sediments. Sed. Geol., 194: 245-262. [ Links ]
7 Dawes, C.J. 1987. The dynamic seagrasses of the Gulf of Mexico and Florida coasts. Fla. Marine Research Publ. No. 42. p. 25-38. In: Durako, M.J., R.C. Phillips and R.R. III Lewis (Eds.). Proc. of Symp. on subtropical seagrasses of the S.E U.S. Florida Department of Natural Resources, Bureau of Marine Research, St. Petersburg, USA. 209 p. [ Links ]
8 Dawes, C. and A. Mathieson. 2008. The seaweeds of Florida. University Press of Florida. Gainesville, USA. 656 p. [ Links ]
9 Díaz-Pulido, G. and M. Díaz-Ruíz. 2003. Diversity of benthic marine algae of the Colombian Atlantic. Biota Colomb., 5: 203-246. [ Links ]
10 Gavio, B., P. Palmer-Cantillo and J.E. Mancera. 2010. Historical analysis (2000-2005) of the coastal water quality in San Andrés Island, Seaflower Biosphere Reserve, Caribbean Colombia. Mar. Poll. Bull., 60: 1018-1030. [ Links ]
11 Hollenberg, G.J. 1942. An account of the species of Polysiphonia on the Pacific coast of North America. I. Oligosiphonia. Am. J. Bot., 29: 772-785. [ Links ]
12 John, D.M. 2007. Ulothrix. In: Brodie, J., C.A. Maggs and D.M. John (Eds.). Green seaweeds of Britain and Ireland. 50-57. British Phycological Society. London. 242 p. [ Links ]
13 Kapraun, D.F., A.J. Lemus and G. Bula-Meyer. 1983. Genus Polysiphonia (Rhodophyta, Ceramiales) in the tropical Western Atlantic. 1. Colombia and Venezuela. Bull. Mar. Sci., 33: 881-898. [ Links ]
14 Kraft, G.T. 2007. Algae of Australia. Marine benthic algae of Lord Howe Island and the Southern Great Barrier Reef, 1. Green algae. ABRS, Canberra; CSIRO Publishing. Melbourne. 345 p. [ Links ]
15 Lauret, M. 1967. Morphologie, phénologie, répartition des Polysiphonia marins du littoral languedocien. I. Section Oligosiphonia. Nat. Monspel. sér. Bot., 18: 347-373. [ Links ]
16 Leliaert, F., W. Vanreusel, O. De Clerck and E. Coppejans. 2001. Epiphytes on the seagrasses of Zanzibar Island (Tanzania), floristic and ecological aspects. Belgian J. Bot., 134: 3-20. [ Links ]
17 Leliaert, F., D.A. Payo, H.P. Calumpong, and O. De Clerck. 2011. Chaetomorpha philippinensis (Cladophorales, Chlorophyta), a new marine microfilamentous green alga from tropical waters. Phycologia, 50: 384-391. [ Links ]
18 Littler, D.S. and M.M. Littler. 2000. Caribbean reef plants. OffShore Graphics. Washington D.C. 542 p. [ Links ]
19 Littler, D.S., Littler, M.M. and M.D. Hanisak. 2008. Submersed plants of the Indian River Lagoon: A floristic inventory and field guide. Offshore Graphics, Washington, D.C. 286 p. [ Links ]
20 Mamoozadeh, N.R. and D.W. Freshwater. 2011. Taxonomic notes on Caribbean Neosiphonia and Polysiphonia (Ceramiales, Florideophyceae): five species from Florida, USA and Mexico. Bot. Mar., 54: 269-292. [ Links ]
21 Palacios, D., G. Díaz and P. Rodríguez. 1992. Producción primaria de Thalassia testudinum y relación de su biomasa con el peso de epífitos, Isla Grande (Parque Nacional Natural Corales del Rosario), Caribe colombiano. Tomo II. 607-618. In: CCO (Ed.). Mem. VII Sem. Nal. Cienc. Mar. Congr. Centroam. Car. Cienc. Mar. Santa Marta. 1144 p. [ Links ]
22 Rojas-González, B. and J. Afonso-Carrillo. 2010. Morfología y distribución de las especies de Polysiphonia de las islas Canarias. 5. Polysiphonia sertularioides (Rhodophyta, Rhodomelaceae). Vieraea, 38: 99-108. [ Links ]
23 Samper-Villarreal, J., A. Bernecker and I.S. Wehrtmann. 2008. Inventory of macroalgal epiphytes on the seagrass Thalassia testudinum (Hydrocharitaceae) in Parque Nacional Cahuita, Caribbean coast of Costa Rica. Rev. Biol. Trop., 56: 163-174. [ Links ]
24 Silva, P. 2013. Index Nominum Algarum, University of California, Berkeley. online (http://ucjeps.berkeley.edu/CPD/). [ Links ]
25 Stuercke, B. and D.W. Freshwater. 2010. Two new species of Polysiphonia (Ceramiales, Florideophyceae) from the western Atlantic. Bot. Mar., 53: 301-311. [ Links ]
26 Womersley, H.B.S. 1979. Southern Australian species of Polysiphonia Greville (Rhodophyta). Aust. J. Bot., 27: 459-528. [ Links ]
27 Womersley, H.B.S. 2003. The marine benthic flora of southern Australia, Rhodophyta Part IIID: Ceramiales, Delesseriaceae, Sarcomeniaceae, Rhodomelaceae. Flora of Australia Biological Resources Series no. 18, Australian Biological Resources Study, Canberra. 533 p. [ Links ]
28 Won, B.Y., K.K. Yates, S. Fredericq and T.O. Cho. 2010. Characterization of macroalgal epiphytes on Thalassia testudinum and Syringodium filiforme seagrass in Tampa Bay, Florida. Algae, 25: 141-153. [ Links ]
29 Wynne, M.J. 2011. A checklist of benthic marine algae of the tropical and subtropical western Atlantic: third revision. Nova Hedwigia Beiheft, 140: 1-166. [ Links ]
FECHA DE RECEPCIÓN: 16/08/2013 FECHA DE ACEPTACIÓN: 09/06/2014