Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.44 no.2 Santa Marta July/Dec. 2015
CHECKLIST OF PLANKTONIC COPEPODA FROM A COLOMBIAN COASTAL LAGOON WITH RECORD OF HALICYCLOPS EXIGUUS KIEFER
LISTA DE COPÉPODOS PLANCTÓNICOS DE UN SISTEMA COSTERO DE COLOMBIA CON REGISTRO DE HALICYCLOPS EXIGUUS KIEFER
Juan M. Fuentes-Reinés1 and Eduardo Suárez-Morales2
1 Universidad del Magdalena, Grupo de Investigación en Biodiversidad y Ecología Aplicada, A. A. 731 Santa Marta, Colombia. juanmanuelfuentesreines@yahoo.com
2 El Colegio de la Frontera Sur, Unidad Chetumal, A.P. 424, 77014 Chetumal, Quintana Roo, México.
ABSTRACT
Sixteen species of planktonic copepods are reported from Laguna de Navío Quebrado, La Guajira, Colombia. Zooplankton samples were collected from the littoral zone with vegetation (macrophytes and mangroves) and from open water related to an oyster bank. Most of these species were recorded in the macrophytes-related area. One of them: Halicyclops exiguus Kiefer, 1934 is new to the Colombian copepod fauna; comparative morphological comments and illustrations of this species are also provided. The general composition of the local copepod community, including marine, estuarine and freshwater forms is representative of the wide salinity gradient (0-28) found in the surveyed area. This is the first report on the planktonic copepod fauna in this hydrological system. Comments on the morphology and photographs of selected species are provided.
KEY WORDS: Calanoida, Cyclopoida, Poecilostomatoida, Taxonomy, Distribution.
RESUMEN
Se registran dieciséis especies de copépodos planctónicos de la Laguna Navío Quebrado, en La Guajira, Colombia. Las muestras de zooplancton fueron recolectadas de la zona litoral con vegetación (macrófitas y mangles) y de aguas abiertas asociadas a un banco de ostras. La mayoría de las especies fueron registradas en el área relacionada con las macrófitas. Una de ellas, Halicyclops exiguus Kiefer, 1934, es nueva para la fauna de copépodos de Colombia; se proporciona un análisis morfológico comparativo e ilustraciones de esta especie. La composición general de la comunidad local de copépodos incluye formas marinas, estuarinas y de aguas dulces, que son representativas del amplio gradiente de salinidad (0-28) encontrado en el área de estudio. Este es el primer registro de la fauna de copépodos planctónicos en este sistema. Se proporcionan comentarios sobre la morfología y fotografías de especies seleccionadas.
PALABRAS CLAVES: Calanoida, Cyclopoida, Poecilostomatoida, Taxonomía, Distribución.
INTRODUCTION
Estuaries are highly productive hydrological systems harboring highly diverse and abundant fish and invertebrate communities (Beck et al., 2001). Among the estuarine invertebrates, copepods play a significant role in both the transportation of material and energy flux in these ecosystems (Dole-Olivier et al., 2000; Begon et al., 2006); they are the most dominant component of zooplankton considering their species diversity, abundance and range of distribution (Chih-Hao and Tai-Sheng, 1997). Estuaries are interesting because they can harbor copepods from different affinities, from marine to freshwater but also strictly estuarine forms (Suárez-Morales, 1994; Suárez-Morales and Gasca, 1996). The study of their copepod fauna is also important because invasive exotic species are most frequently introduced through these coastal environments (Bouley and Kimmerer, 2006) and they are likely to be detected by monitoring the local fauna. Among the Copepoda, members of the family Cyclopidae represent the most widespread group in freshwater and is highly diversified in all types of aquatic ecosystems (Dussart and Defaye, 2006). Species of the families Oithonidae and Cyclopidae have been known to occur also in brackish habitats (Dole-Oliver et al., 2000; Boxshall and Defaye, 2008). Members of the order Calanoida are primarily planktonic and are most abundant in the marine zooplankton (Campos-Hernández and Suárez-Morales, 1994) but some calanoid families (i.e. Pseudodiaptomidae, Acartiidae, Temoridae, Centropagidae, Aetideidae) have colonized estuarine and even freshwater and transitional habitats (Boxshall and Defaye, 2008). Within the order Poecilostomatoida, the genus Corycaeus is among the most widely distributed in the world seas; they inhabit coastal and oceanic environments in tropical, subtropical, and temperate latitudes (Tanaka, 1957; Suárez-Morales and León-Oropeza, 1999) Taxonomic studies on the Colombian planktonic copepod fauna of brackish or freshwater are scarce (Thiébaud, 1912; Pearse, 1915; Noodt, 1972; Reid, 1987, 1988; Gaviria, 1993, 1994; Fuentes-Reinés et al., 2012, 2013). There are no previous surveys about the planktonic copepod community in the large estuarine system of Laguna Navío Quebrado, Caribbean coast of Colombia. From the analysis of biological samples obtained in different areas of this coastal system, we provide the first checklist of free-living planktonic copepods in this coastal system. This survey contributes to increase the knowledge of these groups in Colombia and the Neotropical region. Descriptive notes and illustrations are provided for some of the species recorded.
STUDY AREA
The estuary Laguna Navío Quebrado is located at the cost of the Colombian La Guajira Department (11º25'N, 73º5'W) and has an area of 10.7 km2; it is characterized by the presence of an oyster bank in the limnetic area and vegetation (mangrove and beds of macrophytes) in the littoral zone. Water temperature ranged between 28 and 31 °C, salinity between 0-28, and pH values were 7.8-8.3.The climate conditions in the region can be characterized by two main periods: dry (January-April) and rainy (October-December).
MATERIALS AND METHODS
Plankton samples were taken from the Laguna Navío Quebrado, Colombia (11°24'15" N; 73°5'39" W) from April to December 2012, mainly in the littoral areas with vegetation (macrophytes and mangrove) but also from open water in areas close to oyster banks. Water salinity was measured with a WTW 3111 conductivity meter. Water samples were collected using a bucket of 25 L at both vegetated areas and open water. Samples were filtered with a zooplankton net (45 μm) and preserved in 70% ethanol.
Copepods were sorted from the original samples and then processed for taxonomic identification. Dissected specimens and appendages were mounted in glycerine and sealed with Canada balsam. The specimens were measured in lateral position, from the anterior end of the rostral area to the posterior margin of the caudal ramus. The taxonomic identification of the species recorded herein followed Bradford (1977), Reid (1985), Campos-Hernández and Suárez-Morales (1994) and Rocha et al. (1998). In order to provide a complementary set of information of these specimens, some of the appendages with taxonomic relevance for selected species were photographed using a Kodak Easy Share C140 digital camera adapted to a compound microscope. The morphological terminology follows Huys and Boxshall (1991). The following abbreviations are used in the descriptive section: P1-P6= first to sixth swimming legs, EXP= exopod, ENP= endopod. The specimens examined were deposited at the Museo de Colecciones Biológicas at the Universidad del Atlántico (UARC), Barranquilla, Colombia.
RESULTS
The taxonomic analysis of the copepods collected yielded the identification of 16 species belonging to six families and 11 genera (Table 1). These are all new records for the Laguna Navío Quebrado. The family Cyclopidae was represented by seven genera, followed by Acartiidae, Pseudodiaptomidae, Temoridae, Oithonidae, and Corycaeidae each with one genus. The cyclopoid genera Halicyclops and Microcyclops were the most speciose in this lagoonal system (three species each), followed by Acartia (two species). Most species were found in the macrophytesrelated habitats. A brief remarks and descriptions with illustrations about the relevant species confirming their presences to Colombia are given below.
Order Calanoida
Family Acartiidae Sars, 1903
Genus Acartia Dana, 1846
Acartia lilljeborgi Giesbrecht, 1889
Synonymy. Campos-Hernández and Suárez-Morales, 1994, 56.
Material examined. 4 adult females undissected. UARC149M.
Distribution. This species is found in the Gulf of Mexico, the Caribbean and also the Atlantic and Pacific coasts (0°-33°S) of South America (Campos- Hernández and Suárez-Morales, 1994; FADA, 2013).
Remarks. In some estuarine areas its distribution is indicative of transitional salinity conditions (Suárez-Morales, 1994; Escamilla et al., 2011). When it co-occurs with A. tonsa in estuarine zones it is more abundant at the areas with higher salinity values (Escamilla et al., 2001). Acartia lilljeborgi was common in the limnetic region where salinity was 28.
Acartia tonsa Dana, 1849
Synonymy. Campos-Hernández and Suárez-Morales, 1994, 58.
Material examined. 6 adult female undissected, 3 male undissected. UARC148M.
Distribution. It is considered a cosmopolitan species (FADA, 2013) with a wide distribution in coastal and estuarine systems of the Gulf of Mexico and the western Caribbean (Escamilla et al., 2011). In Colombia it has been recorded in San Andrés Island and Magdalena Department (Pearse, 1915; Martínez-Barragán, 2009; Fuentes-Reinés et al., 2013).
Remarks. Acartia tonsa was found in all the environments sampled, tolerating the entire salinity range observed (0-28), but it was more frequent in the limnetic region.
Family Pseudodiaptomidae G.O. Sars, 1902
Genus Pseudodiaptomus Herrick, 1884
Pseudodiaptomus marshi Wright, 1936
Synonymy. Walter, 1989, 604.
Material examined. 8 adult females undissected, 4 males undissected. UARC150M.
Distribution. It is a Neotropical species (FADA, 2013). Pseudodiaptomus marshi has been known to occur along the Atlantic coasts of the Neotropical region, including Costa Rica, Belize, and Mexico (Walter, 1989; Reid, 1990; Suárez- Morales et al., 2003). In Colombia it has been recorded in Magdalena Department (Medellín-Mora and Navas, 2009; Fuentes-Reinés et al., 2013).
Remarks. Pseudodiaptomus marshi was the most abundant calanoid in the surveyed area and it was found in both limnetic region and vegetation zone (0-28). It is considered a demersal copepod of shallow water (Walter, 1989) but it is also one of the few species of the genus known to dwell in freshwater conditions (Suárez-Morales, 2003).
Family Temoridae Giesbrecht, 1893
Genus Temora Baird, 1850
Temora turbinata (Dana, 1849)
Synonymy. Campos-Hernández and Suárez-Morales, 1994, 232.
Material examined. One adult female dissected. UARC163M-168M
Distribution. It is considered a widespread species (Bradford, 1977), which has been recorded from tropical, subtropical waters of the Atlantic and Indo-Pacific (Decker and Coetzee, 1979).
Remarks. Temora turbinata is a neritic epipelagic marine copepod (Decker and Coetzee, 1979) but in the surveyed area it was recorded at lower salinities (28). This species has been known to occur in estuarine waters (Ara, 2002) as a result of passive transportation by ballast water (Cordell and Morrinson, 1996) or by tidal currents.
Order Cyclopoida
Family Oithonidae Dana, 1853
Subfamily Oithoninae Dana, 1853
Genus Oithona Baird, 1843
Oithona oswaldocruzi Oliveira, 1945
Synonymy. Dussart and Defaye, 2006; 5.
Material examined. 8 adult females undissected, 2 male undissected. UARC183M
Distribution. Oithona oswaldocruzi has been recorded in South and Central America (Brazil, Ecuador, Venezuela, Honduras, El Salvador, Trinidad and Tobago, and Puerto Rico) (Ferrari and Bowman, 1980; Löffler, 1981; Rocha and Botelho, 1998; Dussart and Defaye, 2006). In Colombia, it has been reported in Magdalena Department (Fuentes-Reinés et al., 2013).
Remarks. Oithona oswaldocruzi is a planktonic copepod (Rocha and Botelho, 1998) and it is considered eurythermic and euryhaline (Björnberg, 1981). It has been reported from marine, brackish and freshwater environments with salinities ranging from 0 to 33.7 (Oliveira, 1947; Eskinazi-Sant'Anna and Tundisi, 1996; Fuentes et al., 2012). Our specimens were recorded at a salinity of 28.
Subfamily Cyclopinae Kiefer, 1927
Genus Apocyclops Kiefer, 1932
Apocyclops panamensis (Marsh, 1913)
Synonymy. Dussart and Defaye, 2006, 228-229.
Material examined. 3 adult females undissected, 3 male undissected. UARC184M.
Distribution. It has been record in the Neotropical, Nearctic, and Afrotropical regions (Reid, 1990; Dussart and Defaye, 2006). In Colombia this species has been recorded in Chocó and Magdalena Departments (Petkovski, 1988; Reid, 1988; Fuentes-Reinés et al., 2013).
Remarks. Apocyclops panamensis can be considered an oligohaline or freshwater species frequently recorded from coastal lagoons and estuaries. In the surveyed area it was found in both the limnetic and macrophytes zones with salinities ranging between 0 and 10, but it was most frequent in the macrophytes-related habitat.
Genus Microcyclops Claus, 1893
Microcyclops anceps anceps (Richard, 1897)
Synonymy. Dussart and Defaye, 2006, 100.
Material examined. 10 adult females undissected. UARC153M.
Distribution. It is considered a Neotropical, primarily freshwater species (Silva, 2008). In Colombia it has been recorded in Antioquia, Córdoba, Cundinamarca and Magdalena Departments (Gaviria, 1994; Gaviria and Aranguren, 2007; Fuentes-Reinés et al., 2013).
Remarks. It was found in vegetation zones (macrophytes, mangrove) and was the most abundant freshwater cyclopoid in the surveyed area.
Microcyclops anceps pauxensi Herbst, 1962
Synonymy. Microcyclops anceps var minor Reid, 1985
Material examined. One adult female undissected, one male undissected. UARC180M.
Distribution. According to Silva (2008), this primarily freshwater subspecies has been known to occur only in the Neotropical region. In Colombia it has been recorded in the Magdalena Department (Fuentes-Reinés et al., 2012).
Remarks. It was found in littoral (macrophytes) and limnetic areas (0-28), its presence at higher salinity areas could be explained by passive transportation by local riverine currents.
Microcyclops ceibaensis (Marsh, 1919)
Synonymy. Dussart and Defaye, 2006, 103.
Material examined. 4 adult females, undissected. UARC179M.
Distribution. According to Suárez-Morales et al. (2004) and Silva (2008), this freshwater species is distributed mainly in the Neotropical region and it is widely distributed in Mexico (Mercado-Salas and Suárez-Morales, 2011). In Colombia it has been reported in Córdoba and Magdalena Departments (Gaviria and Aranguren, 2007; Fuentes-Reinés et al., 2013).
Remarks. It was found in the macrophytes-related area in freshwater.
Genus Mesocyclops G.O. Sars, 1914
Mesocyclops ellipticus Kiefer, 1936
Material examined. 2 adult males undissected. UARC181M.
Distribution. It is considered a Neotropical species (Silva, 2008); its taxonomic status has been discussed in different works (Gutiérrez-Aguirre and Suárez-Morales, 2001). It has been synonymized to M. reidae Petkovski, 1986 (Fimia Duarte et al., 2004), but some differences with the Venezuelan specimens (Dussart, 1984) suggest that it is a valid species and thus its distribution appears to be restricted to an area including Venezuela and Brazil (Gutiérrez-Aguirre et al., 2006). Recently, it was recorded from Colombia by Fuentes-Reinés et al. (2013) in the Magdalena Department.
Remarks. Mesocyclops ellipticus is considered a freshwater and oligohaline species (Rocha and Botelho, 1998). It was found only in the macrophytes vegetation zone, in freshwater.
Genus Thermocyclops Kiefer, 1927
Thermocyclops decipiens (Kiefer, 1929)
Synonymy. Chaicharoen et al., 2011, 796.
Material examined. One adult female, dissected. UARC185M-191M.
Distribution. It is considered a pantropical species (Chaicharoen et al., 2011). In Colombia this species has been recorded in different areas including Amazonia, Antioquia, Atlántico, Cundinamarca, Huila, Nariño, and Tolima Departments (Gaviria and Aranguren, 2007)
Remarks. This species was found only among macrophytes in freshwater. Thermocyclops decipiens is considered the most abundant species of the genus in the southern Neotropical region (Silva, 2008) and it has not been recorded from Central America (Mercado-Salas and Suárez-Morales, 2011). This species dwells in meso and eutrophic waters (Perbiche-Neves et al., 2010). Its resemblance with T. crassus (Sivakumar et al., 2013) suggests that some regional records of this species are actually assignable to T. decipiens (Sendacz and Kubo, 1982; Gutiérrez-Aguirre and Suárez-Morales, 2000).
Subfamily Eucyclopinae Kiefer, 1927
Genus Eucyclops Claus, 1893
Eucyclops titicacae Kiefer, 1957
Synonymy. Fuentes-Reinés and Suárez-Morales, 2013, 2.
Material examined. 3 adult females, dissected. UARC88M-95M.
Distribution. It has been recorded from Colombia, Venezuela, Perú and Bolivia (Fuentes-Reinés and Suárez-Morales, 2013).
Remarks. This primarily freshwater species was recorded only in the macrophytes-related areas.
Subfamily Halicyclopinae Kiefer, 1927
Genus Halicyclops Norman, 1903
Halicyclops venezuelaensis Lindberg, 1954
Material examined. 2 adult females, undissected. UARC151M. Distribution. It has been recorded in South and Central America: Venezuela (Lindberg, 1954), Belize (Rocha, 1995), Mexico (Suárez-Morales and Reid, 2003) and Colombia (Fuentes-Reinés et al., 2013).
Remarks. Halicyclops venezuelaensis was the least abundant species among its congeners in samples from the macrophytes-related areas. It was originally described by Lindberg (1954) from Venezuela and redescribed by Rocha (1995) from additional specimens from Sittee River estuary (Belize). It has been reported from brackish and freshwater (Suárez-Morales and Reid, 2003; Fuentes-Reinés et al., 2012) and appears to be more frequent in freshwater (Fuentes-Reinés et al., 2012).
Halicyclops exiguus Kiefer, 1934
(Figures 1 to 3, Table 2)
Material examined. 12 adult females undissected, 5 males undissected. UARC152M, UARC182M.
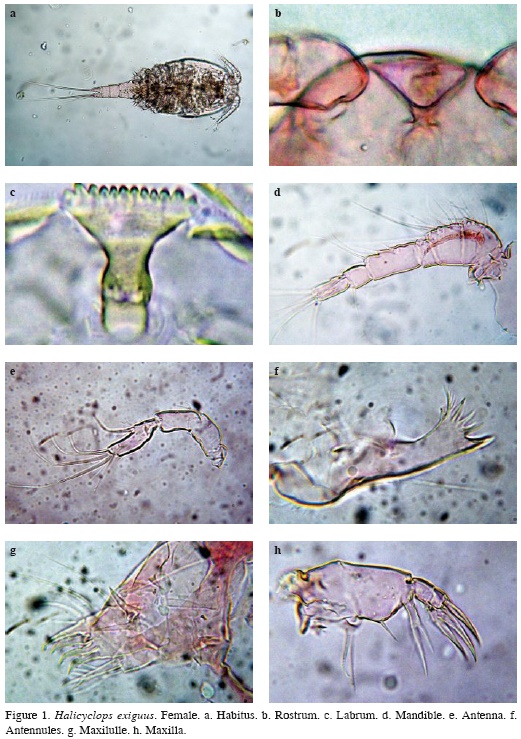
Female. The specimens examined bear the diagnostic features of H. exiguus as described by Kiefer (1934). Habitus in ventral position as in figure (Figure 1a), body wide and compact anteriorly, cephalothorax length/wide ratio about 1.0, with rounded integumental window dorsally, body length, excluding caudal setae = 348476 μm (average = 462 μm, n = 12). Rostrum triangular (Figure 1b), labrum with 12 equally sized teeth (Figure 1c).
Antennules 6-segmented, setal formula as: 1(8), 2(11), 3(4+ 1 spine), 4(6), 5(2), 6(10 + ae). Fourth segment about 1.66 times as long as wide (Figure 1d). Ornamentation on the first segment was not observed.
Antenna consisting of four segments, coxa reduced and unarmed, basis with 2 setae inserted at inner corner; seta representing exopod present. Proximal endopodal segment naked. Terminal endopodal segment with 5 setae on inner margin and 7 setae around apex. Length/wide ratio of distal segment about 3 (Figure 1e).
Mandible. Praecoxa with 5 teeth and 2 plumose setae. Palp with long smooth seta (Figure 1f).
Maxillule. Praecoxal arthrite with 4 spines; inner surface armament consisting of 3 strong elements. Palp basis bearing 3 setae on inner margin and 1 proximal outer seta representing exopodite; endopodite 1-segmented, with 3 setae (Figure 1g)
Maxilla 5-segmented. Praecoxa fused to coxa on posterior surface with 2 setae on endite. Coxa with proximal and 2 distal setae on endite; seta fused to endite ornamented with 4 long setules. Basis forming claw armed with 2 setae. Endopod 2-segmented, bearing 2 strong setae (Figure 1h).
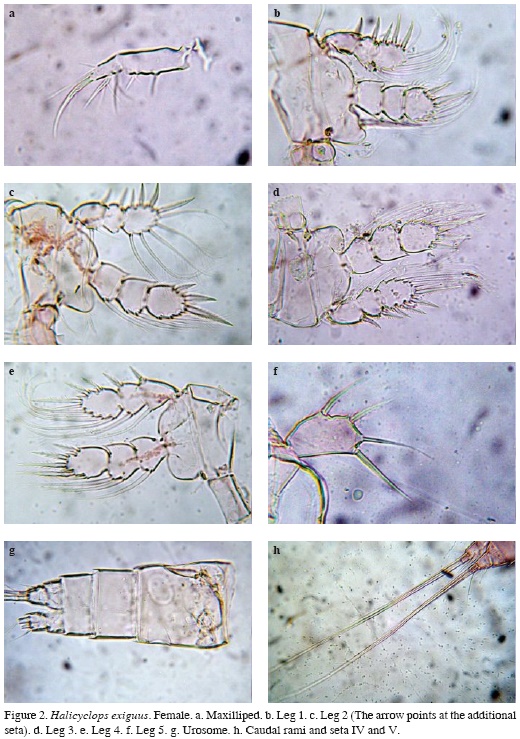
Maxilliped 2-segmented, armed with 1 proximal and 2 subdistal short setae on basal segment and 5 setal elements on distal segment, 3 of which are ornamented with conspicuous stout setules. Apical seta being curved and thicker than the others (Figure 2a).
P1-P4 exopods and endopods 3-segmented (Figures 2b-e), armed as in Table 2. Inner basal spine of P1 reaching midlength of P1 ENP3 (Figure 2b). P2P3 similar to each other (Figure 2c-d). P4 ENP3 about 1.4 times as long as wide; inner apical spine about 1.1 as long as segment and 1.2 times as long as outer apical spine. Proximal inner seta not reaching tip of inner apical spine (Figure 2e). P5 unsegmented, exopod elongate, 1.66-1.75 times as long as wide, bearing 3 spines and 1 seta; inner apical spine and seta longer than segment, relative length of elements from inner to outer margin as follows 1; 1; 0.66; 0.85 (Figure 2f).
Urosome with four segments, genital double somite as long as wide, lateral margins not produced (Figure 2g), caudal rami as long as wide, outer seta two times as long as ramus, inner apical seta two times as long as dorsal apical seta and three times as long as ramus; middle inner seta about 1.85 times as long as middle outer seta (Figure 2h).
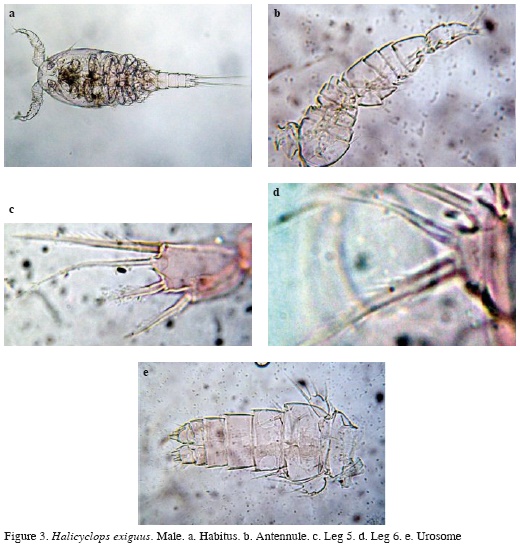
Male. Habitus similar to female (Figure 3a), body length excluding caudal setae = 350-378 μm (average = 364 μm, n = 6). Antennule 14-segmented (Figure 3b), antenna, mandible, maxilla, maxilliped, and P1-P4 as in female. P5 segment 1.5 times as long as wide, with 3 spines and one seta, inner spine being longest. Relative length of elements from inner to outer margin as follows: 1; 0.63; 0.5; 0.54 (Figure 3c). P6 with one spine and two setae (Figure 3d). Urosome with 5 somites; caudal rami as in female (Figure 3e).
Differential diagnosis. Halicyclops exiguus belongs to the group of species recognized by Rocha (1991) as sharing the following characters: 1) Last segment of antenna with five lateral setae, length/wide ratio about three times as long as wide, 2) fourth segment of female antennules less than twice as long as wide, 3) inner spine of the second basipodite of P1 reaching at least midlength of the third endopod of that swimming P1, 4) third endopodal segment of P4 with proximal inner seta not reaching tip of inner apical spine, 5) female P5 exopod elongate, inner spine and apical seta as long as or longer than the segment. 6) male P5 with three spines and one seta, the inner spine the longest. In addition, it shares with many species of this group the spine formula 3-4-4-3 for the last exopodal segment of P2-P4.
Variability. One of the females collected in the surveyed area was observed to possess two setae on P2EXP instead of one (Figure 2c).
Distribution. This species has been reported from different countries of the Neotropical region including Haiti (Kiefer, 1934) (but see Rocha et al., 1998), Costa Rica (Collado et al., 1984), French Guiana (Defaye and Dussart, 1988), and Brazil (Rocha and Iliffe, 1993). This is the first record of this halicyclopine in Colombia.
Remarks. It was found in both the limnetic and vegetation zones and is considered a meso and oligohaline species (Rocha and Botelho, 1998). Morphological data on this species have been provided by Kiefer (1934, 1936), Collado et al. (1984), and Defaye and Dussart (1988). Our specimens agree with the description by Kiefer (1934) and Defaye and Dussart (1988), but Rocha et al. (1998) suggested that the Haitian specimens could represent a separate species. The Colombian specimens differs from those from Costa Rica (Collado et al., 1984) in the length of the innermost terminal caudal seta; it is relatively longer in our specimens (three times as long as the caudal ramus in Colombia specimens whereas it is shorter than the caudal ramus in the Costa Rican specimens) and in the length of the inner spine of the female P5 which is shorter than the segment in specimens from Costa Rica, while in our specimens from Colombia the spine is as long as or longer than the segment. On the other hand, our specimens differ from French Guiana individuals (Defaye and Dussart, 1988) in the spine formula of the third exopodal segment of P1P4, which is 3-4-3-3 in the French Guiana specimens vs 3-4-4-3 in our specimens. Further comparative morphological studies should be developed to determine the actual extent of the variability of this species. The specimens of Halicyclops sp. (Table 1) found in the surveyed area probably represent an undescribed species.
Order Poecilostomatoida
Family Corycaeidae Dana, 1852
Genus Corycaeus Dana, 1845
Corycaeus (Corycaeus) clausi F. Dahl, 1894
Synonymy. Campos-Hernández and Suárez-Morales, 1994, 265.
Material examined. One adult female, dissected. UARC157M-162M.
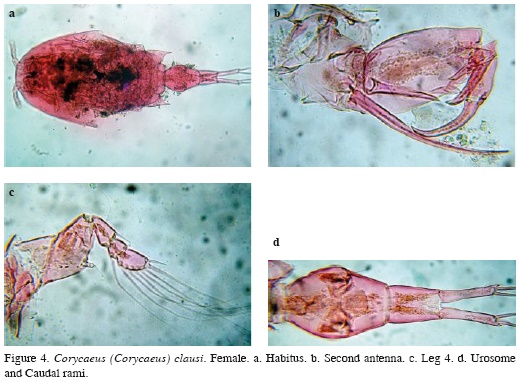
Female. Body length, excluding caudal setae = 1.65 mm. Body robust, cephalic area with large ocular lenses (Figure 4a). This species can be differentiated from its congeners by its possession of: 1) second antenna with one basal seta longer than the other (Figure 4b), 2) fourth leg exopod 3-segmented and endopod transformed in a small button bearing a smooth seta (Figure 4c), 3) female urosome 2-segmented and 4) divergent caudal rami representing almost ½ the length of the urosome (Figure 4d).
Distribution. It has been reported in the tropical and subtropical region of the Atlantic, Indian and Pacific oceans (Tanaka, 1957; Razouls et al., 2005-2013). This is the first report of C. clausi in continental brackish water environments of Colombia. It has been previously reported in the Colombian Caribbean coast (Medellín-Mora and Navas, 2009).
Remarks. Only one specimen was found in the limnetic region of the surveyed coastal system. It is an epiplanktonic, neritic species (Vives and Shmeleva, 2010). Its occurrence in the surveyed system could be related to passive transportation by tidal currents. This is the first illustrated record confirming its presence in Colombia.
DISCUSSION
The planktonic copepods found in the estuarine system Laguna Navío Grande are all tropical forms; most of them have been found in other Colombian estuaries (Fuentes-Reinés et al., 2013). In the surveyed area, the littoral zone with submerged vegetation showed a slightly higher species richness (12 species) than the limnetic area (10 species). The littoral vegetation represents a wide variety of microhabitats for copepods, providing food and shelter (Iversen et al., 1985; Lima et al., 1998). The local fauna is a mixture of typically littoral or epibenthic forms like species of Halicyclops and Microcyclops, considered as phytophylic (Lansac-Tôha et al., 2002), and the strictly planktonic forms represented by species of Acartia and Temora. The mixed community of copepods from Laguna Navío Quebrado is also representative of the salinity gradient from the innermost reaches of the system to the marine front. This system favors the presence of freshwater forms like species of Eucyclops, Mesocyclops, Thermocyclops, and Microcyclops (Suárez-Morales and Reid, 2003; Fuentes-Reinés et al., 2013); they were collected at salinities of 0 in the surveyed area. Other species like C. clausi, T. turbinata, A. lilljeborgi, and O. oswaldocruzi were found in the limnetic zone with salinities up to 28 and are considered marine species (Campos-Hernández and Suárez-Morales, 1994). Their occurrence in the lagoon system represents the marine influence featuring the outer sector of the lagoon. A third group including Halicyclops sp., H. exiguus, P. marshi, A. tonsa, A. lilljeborgi, and A. panamensis, found at a wider salinity range in both littoral and limnetic zones (0-28), are deemed as euryhaline species (Kiefer, 1936; Mauchline, 1998; Rocha and Botelho, 1998; Suárez-Morales et al., 2004; Lance, 2007) that appear to be related to transitional salinity conditions in the surveyed area. The knowledge of the basic composition and expected distribution of these species in this protected lagoon will be an important tool in studying and monitoring the local ecology.
The information presented in this paper increase the total number of free-living cyclopoid copepods reported for Colombia (Gaviria and Aranguren, 2007; Fuentes-Reinés et al., 2013) to 47 species. With these new records, the number of species belonging to the genera Halicyclops and Eucyclops increase to two and eight, respectively (Fuentes-Reinés and Suárez-Morales, 2013). Up to eight species are new records for La Guajira Department and one for Colombia (see Table 1). We found fewer taxa than expected; Fuentes-Reinés et al. (2012) reported 21 species of planktonic copepods from Ciénaga Grande de Santa Marta in contrast to 16 species found in this study, which is probably a result of the sampling method, which emphasized littoral habitats at the Ciénaga.
In summary, the copepod fauna found in the Laguna Navío Quebrado is clearly a typical coastal-estuarine community, but it is expected that further studies with different sampling methods and gear will reveal additional new records of copepods from different environments (see Fuentes-Reinés and Suárez-Morales, 2014). Studies of copepods in the area should be continued with a strong taxonomic base; only reliable determinations of species will lead to an adequate understanding of the Colombian aquatic biodiversity.
ACKNOWLEDGEMENTS
We express our gratitude to Drs. Evelyn Zoppi de Roa (Instituto de Zoología y Ecología Tropical, Universidad Central de Venezuela, Caracas), and Rony Huys (Natural History Museum, London) for kindly providing useful literature during the development of this work. The comments from two anonymous reviewers greatly improved an earlier version of this work; helpful comments were received from Santiago Gaviria (Dept. of Limnology & Technisches Büro für Biologie, University of Vienna).
LITERATURE CITED
Ara, K. 2002. Temporal variability and production of Temora turbinata ( Copepoda: Calanoida) in the Cananéia Lagoon estuarine system. Sao Paulo Brazil. Sci. Mar., 66 (4): 399-406. [ Links ]
Beck, M.W., K.L. Heck, Jr.K. Able, D. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, C.G. Hays, K. Hoshino, T.J. Minello, R.J. Orth, P.F. Sheridan and M.P. Weinstein. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience, 51: 633-641. [ Links ]
Begon, M., C.R. Townsend and J.L. Harper. 2006. Ecology. From individuals to ecosystems. 4th edition, Blackwell Publ., Liverpool. 746 p. [ Links ]
Björnberg, T.K.S. 1981. Copepoda 587-680. In: Boltovskoy, D. (Ed.). Atlas del zooplancton del Atlántico sudoccidental y métodos de trabajo con zooplancton marino. Instituto Nacional de Investigaciones para el Desarrollo Pesquero, Mar del Plata, Argentina. 936 p. [ Links ]
Bouley, P. and W.J. Kimmerer. 2006. Ecology of a highly abundant, introduced cyclopoid copepod in a temperate estuary. Mar. Ecol. Progr. Ser., 324: 219-228. [ Links ]
Boxshall, G. and D. Defaye. 2008. Global diversity of copepods (Crustacea: Copepoda) in freshwater. Hydrobiologia, 595:195-207. [ Links ]
Bradford, J.M. 1977. Distribution of the pelagic copepod Temora turbinata in New Zealand coastal waters, and possible trans-Tasman population continuity. N.Z.J. Mar. Fresh. Res., 11(1): 131-144. [ Links ]
Campos-Hernández, A. and E. Suárez-Morales. 1994. Copépodos pelágicos del Golfo de México y mar Caribe. I. Sistemática y biología. CIQRO/Conacyt, Mexico D.F. 374 p. [ Links ]
Chaicharoen, R., L. Sanoamuang and M. Hołyńska. 2011. A review of the genus Thermocyclops (Crustacea: Copepoda: Cyclopoida) in Cambodia. Zool. Stud., 50(6): 780-803. [ Links ]
Chih-Hao, H. and C. Tai-Sheng. 1997. Copepod abundance and species composition of Tanshui River Estuary and adjacent waters. Acta Zool. Taiwan., 8(2): 75-83. [ Links ]
Collado, C., D. Defaye, B.H. Dussart and C.H. Fernando. 1984. The freshwater Copepoda (Crustacea) of Costa Rica with notes on some species. Hydrobiologia, 119: 89-99. [ Links ]
Cordell, J.R. and S.M. Morrinson. 1996. The invasive Asian copepod Pseudodiaptomus inopinus in Oregon, Washington and British Columbia estuaries. Estuaries, 19: 629-638. [ Links ]
Decker, A.H.B. and D.J. Coetzee. 1979. Indicator copepods and oil yield fluctuations in pelagic fish in the Benguela current system. Ann. South Afr. Mus., 78(7): 69-79. [ Links ]
Defaye, D. and B.H. Dussart. 1988. Compléments à la faune des Crustacés Copépodes des eaux intérieures de Guyane française. Rev. Hydrobiol. Trop., 21: 109-125. [ Links ]
Dole-Olivier, M.J., D.M.P. Galassi, P. Marmonier and M. Creuzé Des Châtelliers. 2000. The biology and ecology of lotic microcrustaceans. Freshw. Biol., 44: 63-91. [ Links ]
Dussart, B. 1984. Some Crustacea Copepoda from Venezuela. Hydrobiologia, 113: 25-67. [ Links ]
Dussart, B. and D. Defaye. 2006. World directory of Crustacea Copepoda of inland waters. II-Cyclopiformes. Backhuys Publishers. Leiden, The Netherlands. 354 p. [ Links ]
Escamilla, J.B., E. Suárez-Morales and R. Gasca. 2001. Distribución del zooplancton durante flujos de marea opuestos en el complejo lagunar de Chelem, Yucatán, México. Rev. Biol. Trop., 49(1): 47-52. [ Links ]
Escamilla, J.B., U. Ordóñez-López and E. Suárez-Morales. 2011. Spatial and seasonal variability of Acartia (Copepoda) in a tropical coastal lagoon of the southern Gulf of Mexico. Rev. Biol. Mar. Oceanogr., 46(3): 379-390. [ Links ]
Eskinazi-Sant'Anna, E.M. and J.G. Tundisi. 1996. Zooplâncton do estuário do Pina (Recife-Pernambuco-Brasil): composição e distribução temporal. Rev. Bras. Oceanogr., 44(1): 23-33. [ Links ]
FADA. 2013. Crustacea-Copepoda Checklist. http://fada.biodiversity.be/CheckLists/CrustaceaCopepoda.pdf. 10/11/2013. [ Links ]
Ferrari, F.D. and T.E. Bowman. 1980. Pelagic copepods of the family Oithonidae (Cyclopoida) from the east Coast of Central and South America. Smithson. Contr. Zool., 3(12): 1-72. [ Links ]
Fimia Duarte, R., J.W. Reid and Z. Menéndez Díaz. 2004. Nuevos reportes de copépodos del género Mesocyclops (Crustacea: Copepoda) para Cuba. Gaceta Médica Espirituana, 6(3): 1. [ Links ]
Fuentes-Reinés, J.M. and E. Suárez-Morales. 2013. First record of the freshwater copepod Eucyclops titicacae Kiefer, 1957, new rank (Copepoda, Cyclopoida) in Colombia. Biota Neotrop., 13(4): 1-8. [ Links ]
Fuentes-Reinés, J.M. and E. Suárez-Morales. 2014. A new subspecies of Nitokra affinis Gurney, 1927 (Copepoda, Harpacticoida) from the Caribbean coast of Colombia. ZooKeys, 378: 1-15. [ Links ]
Fuentes-Reinés, J.M., E. Zoppi and H. Piñango. 2012. Redescription of Paraergasilus longidigitus Yin, 1954 (Copepoda: Ergasilidae) and report of its presence in South America. Met. Ecol. Sist., 7(3): 1-10. [ Links ]
Fuentes-Reinés, J.M., E. Zoppi and R. Torres. 2013. Calanoida and Cyclopoida (Copepoda: Crustacea) from Ciénaga Grande de Santa Marta, Colombia. Met. Ecol. Sist., 8(2): 54-103. [ Links ]
Gaviria, S. 1993. Crustacean plankton of a high altitude tropical lake: Laguna de Chingaza, Colombia. Verh. Int. Ver. Limnol., 25: 906-911. [ Links ]
Gaviria, S. 1994. Los copépodos (Arthropoda, Crustacea) de vida libre de las aguas continentales de Colombia. Rev. Acad. Colomb. Cienc., 19(73): 361-385. [ Links ]
Gaviria, S. and N. Aranguren. 2007. Especies de vida libre de la subclase Copepoda (Arthropoda, Crustacea) en aguas continentales de Colombia. Biota Colomb., 8(1): 53-68. [ Links ]
Gutiérrez-Aguirre. M.A. and E. Suárez-Morales. 2000. The Eurasian Thermocyclops crassus (Fischer, 1853) (Copepoda, Cyclopoida) found in Southeastern Mexico. Crustaceana, 73: 705-713. [ Links ]
Gutiérrez-Aguirre, M.A. and E. Suárez-Morales. 2001. Distribution and taxonomy of the tropical American Mesocyclops Sars, 1914 (Copepoda, Cyclopoida). Crustaceana, 74: 477-487. [ Links ]
Gutiérrez-Aguirre, M.A., E. Suárez-Morales, A. Cervantes, M. Elías-Gutiérrez and D. Previatelli. 2006. The neotropical species of Mesocyclops (Copepoda, Cyclopoida): an upgraded identification key and comments on selected taxa. J. Nat. Hist., 40: 549-570. [ Links ]
Huys, R. and G.A. Boxshall, 1991. Copepod Evolution. The Ray Society, London. 468 p. [ Links ]
Iversen, T.M., J. Thorup, T. Hansen, J. Lodal and J. Olsen. 1985. Quantity estimates and community structure of invertebrates in a macrophytes rich stream. Arch. Hydrobiol., 102: 291-301. [ Links ]
Kiefer, F. 1934. Neue Ruderfusskrebse von der lnsel Haiti. Zool. Anz., 108: 227-233. [ Links ]
Kiefer, F. 1936. Freilebende Süss- und Salzwasser. Copepoden von der Insel Haïti, mit einer Revision der Gattung Halicyclops. Norman. Arch. Hydrobiol., 30: 263-317. [ Links ]
Kiefer, F. 1957. Freilebende Ruderfüsskrebse (Crustacea Copepoda) des Titicaca sees. Veröff. Zool. Staatssamml. Munchen, 4: 125-150. [ Links ]
Lance J. 2007. The salinity tolerance of some estuarine planktonic copepods. Limnol. Oceanogr., 8(4): 440-449. [ Links ]
Lansac-Tôha, F.A., L.F.M Velho, J. Higuti and E.M. Takahashi. 2002. Cyclopidae (Crustacea, Copepoda) from the upper Paraná River floodplain, Brazil. Braz. J. Biol., 62(1): 125-133. [ Links ]
Lima, A.F., F.A. Lansac-Tôha, L.F.M. Velho and L.M. Bini. 1998. Environmental influence on planktonic cladocerans and copepods in the floodplain of the upper river Paraná, Brazil. Stud. Neotrop. Fauna Environ., 33: 188-196. [ Links ]
Lindberg, K. 1954. Cyclopides (Crustacés: Copépodes) de l'Amerique de Sud. Arch. Zool., 7: 193-222. [ Links ]
Löffler, H. 1981. Copepoda. 14-19 In: Hurlbert, S.H., G. Rodriguez, N.D. dos Santos (eds.) Aquatic Biota of Tropical South America, Part I. Arthropoda. San Diego State University Press, San Diego. 323 p. [ Links ]
Martínez-Barragán, M., J. Medina-Calderón, A. Franco-Herrera y A. Santos-Martínez. 2009. La comunidad de copépodos (Crustacea) en las islas de Providencia y Santa Catalina (Caribe colombiano) durante el período lluvioso de 2005. Bol. Invest. Mar. Cost., 38(1): 85-103. [ Links ]
Mauchline, J. 1998. The biology of calanoid copepods. Adv. Mar. Biol., 33: 1-710. [ Links ]
Medellín-Mora, J. and G. Navas. 2009. Listado taxonómico de copépodos (Arthropoda: Crustacea) del mar Caribe colombiano. Bol. Invest. Mar. Cost., 39(2): 265-306. [ Links ]
Mercado-Salas, N.F. and E. Suárez-Morales. 2011. Morfología, diversidad y distribución de los Cyclopoida (Copepoda) de zonas áridas del centro-norte de México. I. Cyclopinae. Hidrobiológica, 21: 1-25. [ Links ]
Noodt, W. 1972. Drei neue Parastenocaris aus Kolumbien (Crustacea, Copepoda) 1. Mitteilung über Kolumbianischen Grundwasser-Crustaceen. Stud. Neotrop. Fauna Environ., 7: 101-112. [ Links ]
Oliveira, L.P.H. 1947. Distribução geográfica da fauna e flora de Baía de Guanabara. Mem. Inst. Oswaldo Cruz., 45(3): 709-735. [ Links ]
Perbiche-Neves, G., M.G. Nogueira and M.S. Serafim. 2010. Size variations in the morphological structures of Thermocyclops decipiens (Kiefer, 1929) (Copepoda: Cyclopoida) from south-southeast Brazilian rivers. Braz. J. Aquat. Sci. Tech., 14(2): 105-107. [ Links ]
Petkovski, T.K. 1986. Zur Taxonomie des genus Mesocyclops G.O. Sars 1914 (Crustacea, Copepoda, Cyclopoida) in der Neotropics. Acta Mus. Maced. Sci. Nat., 18: 47-79. [ Links ]
Petkovski, T.K. 1988. Zur Cyclopidenfauna Kolumbiens (Crustacea, Copepoda). Acta Mus. Maced. Sci. Nat., 19(2/155): 39-64. [ Links ]
Razouls, C., F. de Bovée, J. Kouwenberg and N. Desreumaux. 2005-2013. Diversity and geographic distribution of marine planktonic copepods. http://copepodes.obs-banyuls.fr/en/accessed21/12/2013. [ Links ]
Reid, J.W. 1987. Some cyclopoid and harpacticoid copepods from Colombia, including descriptions of three new species. Proc. Biol. Soc. Wash., 100: 262-271. [ Links ]
Reid, J.W. 1988. Cyclopoid and harpacticoid copepods (Crustacea) from Mexico, Guatemala and Colombia. Trans. Am. Microscop. Soc., 107: 190-202. [ Links ]
Reid, J.W. 1990. Continental and coastal free-living Copepoda (Crustacea) of Mexico, Central America and the Caribbean region. 175-213. In: Navarro, D. and J.G. Robinson (Eds.). Diversidad biológica en la Reserva de la Biósfera de Sian Ka'an. Centro de Investigaciones de Quintana Roo/University of Florida, México D.F. 471 p. [ Links ]
Rocha, C.E.F. 1991. A new species of Halicyclops (Copepoda, Cyclopidae) from California, and a revision of some Halicyclops material in the collections of the US Museum of Natural History. Hydrobiologia, 236: 29-37. [ Links ]
Rocha, C.E.F. 1995. Copepods of the genus Halicyclops (Cyclopidae) from Belize. Hydrobiologia 308:111. [ Links ]
Rocha, C.E.F. and M.J.C. Botelho. 1998. Maxillopoda-Copepoda-Cyclopoida. 129-166. In: Young, P.S. (Ed.). Catalogue of Crustacea of Brazil. Series book 6, National Museum, Rio de Janeiro. 717 p. [ Links ]
Rocha, C.E.F. and T.M. Iliffe 1993. New cyclopoids (Copepoda) from anchialine caves in Bermuda. Sarsia, 78: 43-56. [ Links ]
Rocha, C.E.F., T.M. Iliffe, J.W. Reid and E. Suárez-Morales. 1998. A new species of Halicyclops (Copepoda, Cyclopoida, Cyclopidae) from Cenotes of the Yucatan Peninsula, Mexico, with identification key for the species of the genus from the Caribbean region and adjacent areas. Sarsia, 83: 387-399. [ Links ]
Sendacz, S. and E. Kubo. 1982. Copepoda (Calanoida e Cyclopoida) de reservatórios do Estado de Sao Paulo, Brasil. Bol. Inst. Pesca, 9: 51-89. [ Links ]
Silva, V.M. 2008. Diversity and distribution of the free-living freshwater Cyclopoida (Copepoda: Crustacea) in the Neotropics. Braz. J. Biol., 68(4, Suppl.): 1099-1106. [ Links ]
Sivakumar, K., R. Anandan, P. Muthupriya, M. Gopikrishna and K. Altaff. 2013. Phylogenetic analysis of Thermocyclops decipiens with reference to 18S rDNA. Ind. J. Sci. Tech., 6(12): 5585-5592. [ Links ]
Suárez-Morales, E. 1994. Copépodos pláncticos de la Bahía de Chetumal, México (1990-1991). Caribb. J. Sci., 30(3-4): 181-188. [ Links ]
Suárez-Morales, E. 2003. Historical biogeography and distribution of the freshwater calanoid copepods (Crustacea: Copepoda) of the Yucatan Peninsula, Mexico. J. Biogeogr., 30: 1851-1859. [ Links ]
Suárez-Morales, E. and R. Gasca. 1996. Planktonic copepods of Bahía de la Ascensión, Caribbean coast of Mexico: a seasonal survey. Crustaceana, 69(2): 162-174. [ Links ]
Suárez-Morales, E. and A. León-Oropeza. 1999. An illustrated geographical record and range extension of Corycaeus giesbrechti Dahl, 1894 (Copepoda Poecilostomatoida) in the Gulf of Mexico. Crustaceana, 72(7): 705-710. [ Links ]
Suárez-Morales, E. and J.W. Reid. 2003. An updated checklist of the continental copepod fauna of the Yucatan Península., Mexico, with notes on its regional associations. Crustaceana, 76(8): 977-992. [ Links ]
Suárez-Morales, E., J.W. Reid, F. Fiers and T.M Iliffe. 2004. Historical biogeography and distribution of the freshwater cyclopine copepods (Copepoda, Cyclopoida, Cyclopinae) of the Yucatan Peninsula, Mexico. J. Biogeogr., 31: 1051-1063. [ Links ]
Tanaka, O. 1957. On Copepoda of the family Corycaeidae in Japanese water. J. Fac. Agr. Kyushu Univ., 11(1): 77-97. [ Links ]
Thiébaud, M. 1912. Copépodes de Colombie et des Cordillères de Mendoza. 160-175. In: Fuhrmann, O. and E. Mayor (Eds.). Voyage d'exploration scientifique en Colombie. Mém. Soc. Neuchâtel. Sci. Nat., 5. Neuchâtel, Switzerland. 1306 p. [ Links ]
Vives, F. and A. Shmeleva. 2010. Crustacea. Copépodo Marinos II. Non Calanoida. Fauna ibérica. Vol. 33. Museo Nacional de Ciencias Naturales, CSIC, Madrid. 1486 p. [ Links ]
Walter, C. 1989. Review of the new world species of Pseudodiaptomus (Copepoda: Calanoida) with a key to species. Bull. Mar. Sci., 45(3): 590-628. [ Links ]
RECEIVED: 06/03/2014 ACCEPTED: 26/09/2015