Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.46 no.2 Santa Marta July/Dec. 2017
https://doi.org/10.25268/bimc.invemar.2017.46.2.728
Research Articles
Spaciotemporal variation in the coral fish larvae assembly of Gorgona Island, Colombian Pacific
1 Universidad del Valle, Facultad de Ciencias Naturales y Exactas, Departamento de Biología. Calle 13# 100-00. Cali. Colombia. isabel.calle@correounivalle.edu.co
2 Universidad del Valle, Facultad de Ciencias Naturales y Exactas, Departamento de Biología. Calle 13# 100-00. Cali. Colombia. alan.giraldo@correounivalle.edu.co
3 Universidad del Valle, Facultad de Ciencias Naturales y Exactas, Departamento de Biología. Calle 13# 100-00. Cali. Colombia. andres.cuellar.chacon@correounivalle.edu.co
The spatial and temporal variation in the assemblages of the larval stages of fishes associated with coralline formations in the eastern sector of Gorgona Island was evaluated considering its relationship to local oceanographic conditions, particularly the instantaneous surface circulation field. Oceanographic and biological information was recorded in September 2014 and February 2015 from a grid of 16 stations, which were classified into three sectors based on their distance to the largest coral formation in the study area, La Azufrada coral reef. A total of 1185 larvae were collected from 29 species of fish associated with coralline formations, and the abundance, richness and diversity of the ensemble was significantly higher in February than in September, with Coryphopterus urospilus dominant in September and C. urospilus and Stegastes sp. dominant in February. In September, species richness and diversity were significantly lower in the area near La Azufrada coral reef, while richness and dominance were significantly lower in the outermost region of the study area in February. During September, the general trend of the instantaneous circulation field was towards the island, and a center of the larval concentration was identified in the sector containing La Azufrada coral reef. However, during February, it was away from the island, and a greater abundance of larvae was registered in the external sector of the study area. According to the evidence, the structure and composition of the fish larvae assembly in the study area varied temporally and spatially and was probably modulated by the local surface circulation pattern.
Keywords: Coral reefs; Circulation field; Fish larvae
Se describió la variación espacial y temporal del ensamble de larvas de peces asociados a formaciones coralinas en el sector oriental de isla Gorgona, Pacífico colombiano y se evaluó la relación entre la variación espacial de la abundancia y las condiciones oceanográficas locales, particularmente el campo de circulación superficial instantáneo. Se registró información oceanográfica y biológica en septiembre 2014 y febrero 2015, siguiendo una malla de 16 estaciones clasificadas en tres sectores con base en la distancia a la formación coralina de mayor extensión en el área de estudio, el arrecife coralino de La Azufrada. Se capturaron 1185 larvas de 29 especies de especies de peces asociados a formaciones coralinas. La abundancia, riqueza y diversidad del ensamble fue significativamente mayor en febrero que en septiembre, estando el ensamble dominado en septiembre por Coryphopterus urospilus (Gobiidae) y en febrero por C. urospilus y Stegastes sp. (Pomacentridae). Durante septiembre, la riqueza y diversidad de especies fue significativamente menor en el sector cercano al arrecife coralino de La Azufrada, mientras que en febrero, la riqueza y la dominancia fueron significativamente menores en la zona más externa del área de estudio. Durante septiembre, la tendencia general del campo instantáneo de circulación fue en dirección a la isla, identificando un centro de agregación de larvas en el sector del arrecife coralino La Azufrada, mientras que durante febrero fue hacia afuera de la isla registrándose una mayor abundancia en el sector externo del área de estudio. De acuerdo con la evidencia se encontró que la estructura y composición del ensamble de larvas varió de forma temporal y espacial, probablemente modulada por el efecto de patrón local de circulación superficial.
Palabras clave: Arrecifes de coral; Circulación superficial; Larvas de peces
INTRODUCTION
Coral reefs are highly biodiverse ecosystems that harbor the most species-rich fish communities on the planet (Sale, 2004). Generally, the fish that inhabit coral reefs are characterized by a life cycle with two phases: a pelagic larval phase that allows them to be dispersed and a relatively sedentary demersal adult phase (Gerlach et al., 2007; Hamner et al., 2007; Hamner and Largier, 2012). Understanding the spatial and temporal dynamics of the larval phase is of great interest because of their importance to the population dynamics and connectivity, or the exchange of individuals, and the dispersal patterns of species (Cowen et al., 2000, 2006, Lefèvre and Bellwood, 2015). Within plankton, fish larvae are perhaps the best documented taxonomic group, and although research into this group has been focused on taxonomy, physiology, ethology, composition, abundance and distribution, there is a growing interest in the marine scientific community to address questions related to the population dynamics of fish communities, especially connectivity and recruitment (Sale, 2004; Jones et al., 2005; Hogan et al., 2012; Salles et al., 2015) including strategies and trajectories (Irisson et al., 2004;Green et al., 2015).
Generally, studies of the dispersal and distribution of fish larvae support the idea that larvae are exported from coral reefs to nearby areas and then return to “self-recruit” into their population of origin, or they may be directed to nearby sites (Harrison et al., 2012, Almany et al., 2013). This information is relevant for determining the important geographic areas in the life cycle of the species in a particular fish community, and it contributes to knowledge of genetic exchange, the colonization of new habitats and the status of populations (Buston et al., 2012).
It has been widely documented that the processes involved in the transport of larvae and eggs operate at different spatiotemporal scales with notable changes due to surface circulation patterns and thermal or saline fronts, among influences, as well as biological factors such as the natural predation rate. Specifically, these elements are responsible for the survival, transport and maintenance of larvae in habitats suitable for their development or recruitment (Paris and Cowen, 2004; Hamner and Largier, 2012; Wolansky et al., 2014). In the Eastern Pacific, research into factors involved in fish larvae dispersal processes has been mainly occurred in Hawaii, the Gulf of California and the Juan Fernández Archipelago (Chile) (Bogan, 1994; Landaeta and Castro, 2004; Eble et al., 2011). Therefore, there are still information gaps and a lack of knowledge about the spatial and temporal dynamics of ichthyoplankton in coastal environments of the Colombian Pacific despite their ecological importance and the economic and social interest in fishing activities; these gaps are even greater for the coral reef ecosystems in this region of the Eastern Tropical Pacific.
To date, some research on adult fish communities in coral environments has been conducted in the Colombian Pacific, mainly on Gorgona Island (Rubio, 1990; Zapata and Morales, 1997; Zapata, 2001) and in Utría Cove (Estupiñan et al. al., 1990; Gómez and Viera, 1996). However, the main focus of these investigations has been the species composition, abundance and diversity of the assemblies, so there is little knowledge of the ichthyoplankton at these sites. However, it has been suggested that the spatial distribution of zooplankton, including ichthyoplankton, would be primarily influenced by the local circulation pattern (Escarria et al., 2007; Giraldo et al., 2008, 2014, Giraldo and Valencia, 2012).
According to Giraldo (2008), there are two annual contrasting oceanographic periods around Gorgona Island that modulate the environmental conditions of the pelagic system including changes in the prevailing direction of the surface circulation that tend to favor local advective processes (Giraldo et al., 2008). However, the presence of the island mass in the surface circulation field could favor the formation of currents that could modulate the spatial distribution pattern of fish larvae, as has been suggested for other island locations (Boehlert et al., 1992; Cowen and Castro, 1994; Landaeta and Castro, 2004; Eble et al., 2011), promoting the permanence of short-lived larvae in the area such as those of fish species that live in association with the coral formations (Searcy and Sponaugle, 2000, Zapata and Herron, 2002, Bergenius et al., 2005, Leahy et al., 2015). The present study analyzed the temporal and spatial variation in the ensemble of larval instars of fishes whose main habitat is the coral formations of the eastern sector of Gorgona Island (where coral formation development is high), considering the potential effect of local oceanographic conditions on the composition and spatial distribution of the community.
MATERIALS AND METHODS
Study Area
Gorgona is a continental island located 30 km from the mainland in the Colombian Pacific Ocean (2°58’10’’N; 78°11’5’’W) (Figure 1). The average air temperature is 26 °C, and the annual precipitation is 7000 mm and bimodal with a low-precipitation period from December to March and two periods of high precipitation in April and November (Blanco, 2009). The transparency of the water column varies from 6 to 12 m; the surface temperature of the water is between 26 and 28 °C; and the average monthly salinity ranges from 28 to 33 practical units (Díaz et al., 2001, Giraldo, 2008; Giraldo et al., 2008).
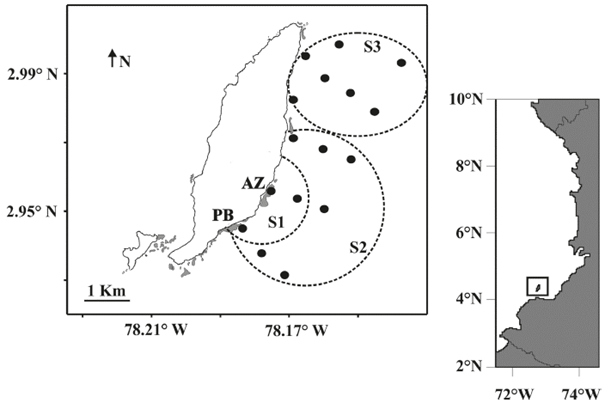
Figure 1 Location of Gorgona Island and the sampling mesh within the study area. In gray, locations of the coral formations around Gorgona Island with AZ indicating the La Azufrada reef and BL the Playa Blanca reef. The sectors were defined a priori based on their distance to the coral reef formations. S1 = Sector 1, S2 = Sector 2 and S3 = Sector 3.
Two contrasting oceanographic periods have been described for this locality: from May to December, when superficial salinity values are low and there is a deep thermocline (between 40 and 50 m in depth), and from January to April, when surface salinity is high and the thermocline is shallow (5-10 m deep). The tide is semidiurnal with a maximum height of 5.7 m (Giraldo, 2008; Giraldo et al., 2008a). This intra-annual variation in the local oceanographic conditions around Gorgona Island is closely related to the effect of the latitudinal displacement of the Intertropical Convergence Zone (ITCZ) on the Colombian Pacific (Rodríguez et al., 2003, Amador et al., 2016, Villegas et al., 2016). Specifically, the ITCZ has been identified as the main source of climatic variability in the region (Poveda et al., 2011; Hoyos et al., 2013), and it modulates the pattern of oceanic and coastal circulation in the eastern sector of the eastern Pacific, including the Colombian Pacific (Fiedler and Talley, 2006, Kessler, 2006, Villegas and Malikov, 2006, Rodríguez et al., 2007).
The main coral formations in the Colombian Pacific are located on the eastern coast of Gorgona Island (Figure 1), with the La Azufrada (AZ) reef stretching 15 ha and the Playa Blanca (PB) reef extending 9.6 ha (Glynn and Ault, 2000). As coral reefs are strategic ecosystems, the coralline formations of Gorgona Island have been included as conservation objects in the management plan for the Gorgona Natural National Park (UAESPNN, 2004, Muñoz and Zapata, 2013), and they have been one of the main research subjects in this locality over the last 20 years (Giraldo et al., 2014).
Sample Collection And Analysis
To develop this research, a grid consisting of 16 oceanographic stations was established in the eastern sector of Gorgona Island (Figure 1), and two sampling periods were selected: September 2014 and February 2015, which include the intra-annual variation in oceanographic conditions that have been described for this locality (Giraldo, 2008). At each sampling station, horizontal (0-10 m) zooplankton samplings were performed using a 30 cm-diameter bongo net with Hydrobios® flowmeters attached to each mouth and 250-μm pore collecting nets. Prior to sampling the zooplankton, discrete water samples were collected with a Niskin bottle at depths of 1 and 10 m, and the temperature and salinity at each depth was recorded using a YSIpro® multiparameter probe. In addition, the instant field of the surface current was constructed at a depth of 1 m from the velocity and flow direction registered at each station by tracking a modified Davis passive diverter (Joseph, 2014; Jerez et al., 2017), which consisted of a 30 cm-diameter inflatable buoy attached with a main line to a stainless-steel shunt with a dead weight. The instrument was allowed to drift for at least 20 minutes in each of the stations, and the initial and final geographic drift coordinates were recorded using a Garmin GPS.
The captured zooplankton were preserved in a mixture of formaldehyde and 4% sea water buffered with sodium borate. In the laboratory, fish larvae were separated and identified to the lowest possible taxonomic level using the ichthyoplankton guides of Moser (1996) and Beltrán and Ríos (2000). For the analyses, the larvae of fish species defined as associated with the coral formations of the Tropical Eastern Pacific by Rubio and Angulo (2003) and Robertson and Allen (2015) were selected.
Data Analysis
Three sectors (S1, S2 and S3) were established a priori in the study area based on the distance to La Azufrada coral reef (Figure 1). Temperature and salinity conditions as well as the abundance of fish larvae were compared between periods by a non-parametric Mann-Whitney test and between the study sectors for each sampling period by a Kruskal-Wallis non-parametric test with Bonferroni correction and a post-hoc Tukey’s range test.
Sampling representativeness was established as a proportion of the expected value of the wealth of the study area as defined by a non-parametric, first-order Jackknife wealth estimator. We compared species richness, dominance and alpha diversity between periods and between sectors using a bootstrap resampling comparison analysis of 10,000 random interactions. In addition, the inter-sectoral turnover rate was established from the Morisita-Horn beta diversity index and was represented by a similarity dendrogram constructed from the average linking function (Vellend, 2001; Jost et al., 2011; Calderón-Patrón et al., 2012), considering the coefficient of co-behavior as an indicator of the significance of the established grouping (Herrera- Moreno, 2000). Finally, we used the inverse distance to power grid interpolation method of the program Surfer11® to perform the vector graphic representation of the instant field of the surface current and the spatial variation in the larval abundance of fish species associated with the coralline formations in the study area.
RESULTS
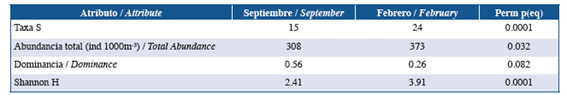
The temperature and salinity conditions in the study area differed between the sampling periods. During September 2014, the temperature at the surface and 10 m in depth was significantly higher than in February 2015 (Table 1). In contrast, the salinity at the surface and 10 m in depth during February 2015 was significantly higher compared to September 2014 (Table 1). When evaluating the variation in these oceanographic parameters between the study sectors, no significant differences were recorded considering each sampling period independently (Table 2). This result suggests that the thermal and saline conditions were consistent across study sectors during each of the sampling periods.
Table 1 Temperature and salinity records in the study area during September 2014 and February 2015. T = temperature, S = salinity, Z = Z statistic equivalent to calculated Mann-Whitney U estimator, p = p value, n = 9.
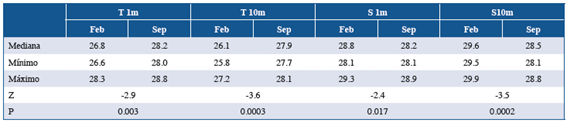
Table 2 Result of the comparison of the temperature (T) and salinity (S) between the study sectors defined during each sampling period. H = estimated Kruskal-Wallis, p = p value.
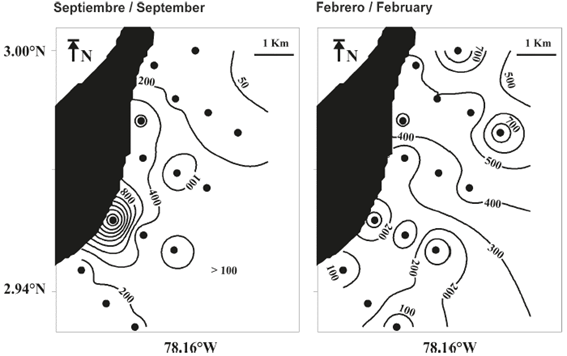
The surface pattern of the instant field of the surface current during September exhibited a predominantly westward direction with a maximum velocity of 21 cm s-1, whereas during February, the predominant direction was towards the northeast with a maximum velocity of 52 cm s-1 (Figure 2). These hydrodynamic conditions suggest that the local circulation pattern could be influencing the spatial location of planktonic organisms because the movement of the current in September trends towards the coastal zone of the island while it trends offshore in February (Figure 2).
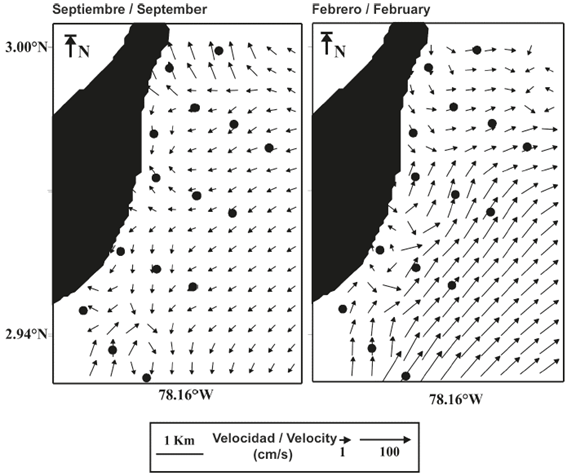
Figure 2 Instant field of the surface current in the eastern part of Gorgona Island during September 2014 and February 2015.
Sampling representativeness was greater than 74% in both study periods. In total, 1,185 larvae (277 in September and 908 in February) of 29 species of fish associated with coralline formations were captured in the study area (Table 3). The abundance, richness and diversity of the assemblage was significantly higher in February than in September (Table 4), being dominated by Coryphopterus urospilus (Gobiidae) in September and by C. urospilus and Stegastes sp. (Pomacentridae) in February (Table 3).
Table 3 Taxonomic listing of fish larvae associated with coral reefs caught in the eastern sector of Gorgona Island. The relative abundance (Ab) and catch frequency (Fr) values are presented for the September 2014 and February 2015 sampling periods. Sampling stations per period: 16.
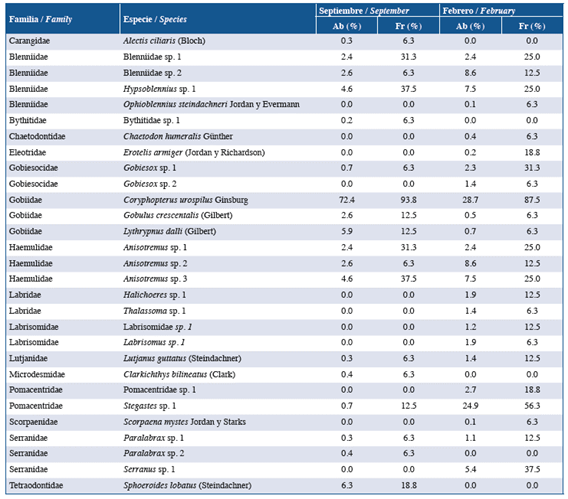
Table 4 Comparison of community attributes between September 2014 and February 2015. Perm p: significance value of the bootstrap resampling comparison from 10,000 permutations.
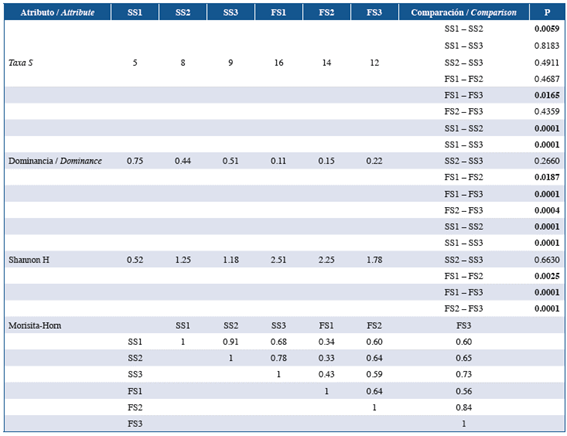
There were also significant differences in community attributes between study sectors during both sampling periods (Table 5). During September, there were fewer taxa in the sector closest to the coral reefs, and this sector exhibited significantly greater dominance and species diversity and richness. During February, by contrast, dominance and richness were significantly lower in the outermost sector of the study area (Table 5).
Table 5 Attributes of the fish larvae community associated with coralline formations in the eastern sector of Gorgona Island during September 2014 and February 2015 considering three sectors during each study period. SS1: September sector 1, SS2: September sector 2, SS3: September sector 3, FS1: February sector 1, FS2: February sector 2 and FS3: February sector 3 (significant differences are highlighted in bold).
Some species were only recorded during one of the study periods (Figure 3), causing notable differences in the composition of the assemblies, and the replacement rate between the periods was 64% (Figure 3). This suggests seasonal variation in the reproductive processes of the fish populations associated with coral reefs in the study area.
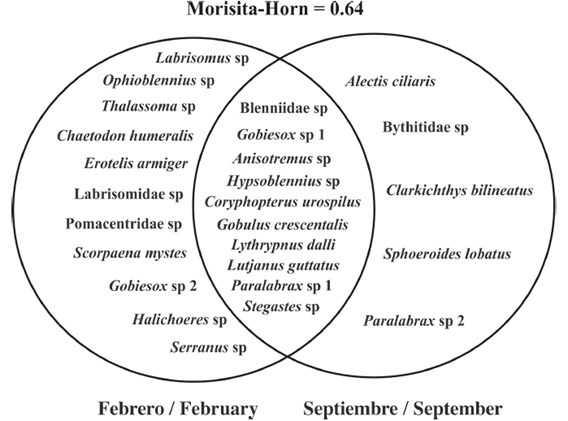
Figure 3 The taxonomic composition of the larval assemblages of fish species associated with coral reefs in the study area during September 2014 and February 2015. Both species recorded exclusively in one of the periods and shared species (overlap zone) are indicated. Anisotremus sp. and Blenniidae sp. include the nominal species established for each of the genera.
When assessing the variation in the diversity of the assemblage along the defined spatial gradient (beta diversity), the highest rate of species exchange was found between the outermost sector (S3) and the middle and inner sectors in September, while the highest replacement rate in February occurred between the inner sector (S1) and the middle and external sectors (Figure 4, Table 5). This result suggests that the larval assemblage of fish species associated with coral formations on Gorgona Island varies both temporally and spatially during each of the study periods.
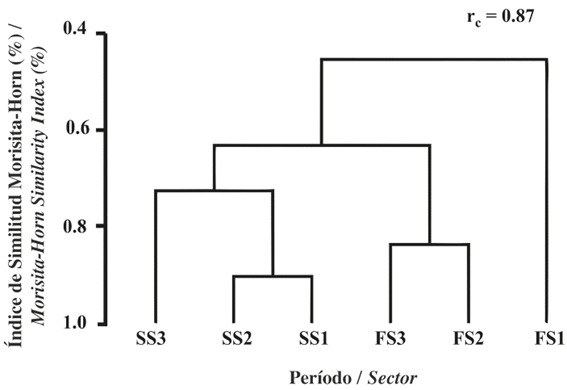
Figure 4 Dendrogram of the similarity of the larval assemblages of fish species associated with coral formations by study sector around Gorgona Island considering the periods of September 2014 and February 2015. rc: cophenetic correlation coefficient.
The spatial variation in the total abundance of the larvae of fish species associated with coral formations differed between the sampling periods (Figure 5). During September, the greatest abundances were recorded in the La Azufrada coral reef (1,925 larvae / 1,000 m-3), forming a center of aggregation (Figure 5). During February, the abundance in the La Azufrada reef sector was less (68 larvae / 1,000 m3) than in September, with a tendency towards an increase in abundance northeast of the study area in this period, specifically towards the sectors most distant to the coral formations with records on the order of 700 larvae / 1,000 m3 (Figure 3).
DISCUSSION
Continental and oceanic environments have been widely recognized as strategic for the development of fish larvae (Lobel and Robinson,1986, Boehlert et al., 1992, Cowen and Castro, 1994, Paris and Cowen, 2004, Sale et al., 2005). In particular, Gorgona Island has been identified as a strategic marine protected area for the Colombian Pacific because of its biological and ecosystem diversity and its potential role as a site for local fishery resources (Franke and Acero, 1992; 1995; Rojas and Zapata, 2006; Giraldo et al., 2014).
Although knowledge of the biology and ecology of larval instars is key to understanding the population dynamics of fish (Lehodey et al., 2006; Harrison et al., 2012), such information is scarce for Gorgona Island. Recently, Escarria et al. (2007) recorded the presence of larvae of 35 fish taxa around Gorgona Island, of which only two (Lythrypnus dalli and Paralabrax sp.) were recorded in the present investigation. Therefore, the records made during our study increased the count of fish larvae to 62 taxa.
It has been widely documented that temporal variation in environmental conditions can modulate the distribution, abundance and general structure of fish larvae assemblages in the pelagic environment (Franco et al., 2002; Funes et al., 2002; Aceves et al., 2003, 2008). During the study period, the oceanographic conditions coincided with those previously described for this location with a higher temperature and lower surface salinity during September (a period of increased precipitation) and a lower temperature and higher salinity during February (a period of lower precipitation) (Giraldo, 2008; Giraldo et al., 2008, 2012). In the present study, differences were found between the study periods in the predominant direction and mean velocity of the instant field of the surface current as well as differences in the community attributes of the larval assemblages of fish species associated with coral formations. Therefore, local oceanographic conditions could be conditioning the structure and composition of the fish larvae assemblage associated with coralline formations in this locality, either by modulating the reproductive processes of the adults or by influencing the spatial location of the eggs and larvae (Leis, 1993; Sponaugle et al., 2003; Fabricius et al., 2005; Srinivasan and Jones, 2006; Treml et al.,2012.).
It is important to note that the larval abundance of the fish species associated with coral formations in the eastern sector of Gorgona Island also varied spatially within each of the study periods. This “intraperiod” spatial variation could be related to the surface circulation pattern, which would favor aggregation towards the sector containing the coral formations of La Azufrada and Playa Blanca during September, but during February, dispersion would be favored throughout the entire study area. These results agree with what Cowen and Castro (1994) described on an island scale in Barbados, Lesser Antilles. At this locality, the spatial variation in the abundance of the fish larvae associated with the coral reefs was modulated by the local circulation field with the larvae being actively transported by the current, forming areas of aggregation, and this has been proposed as a mechanism used by coral larvae to colonize surrounding areas or even return to their spawning grounds (Jones et al., 2005; Sundelöf and Jonsson, 2012).
In dispersion studies, larvae in their early stages of development are considered passive particles, whose mobility in insular pelagic environments would be closely related to the physical conditions of the water column, such as currents, thermohaline fronts or small- scale eddies (Paris and Cowen, 2004; Hogan et al., 2012). It seems that the interaction of these physical mechanisms on a local scale could favor the recirculation of fish larvae, thus reducing the probability of individual mortality due to transport to inappropriate settlement sites by favoring the return of the larvae to their original spawning sites within days (Jones et al., 2005, 2009, Hamner et al., 2007, Wolanski and Kingsford, 2014). However, this mechanism to promote site fidelity could be further favored if the sensory (Lecchini et al., 2005; Mouritsen et al., 2013; Paris et al., 2013) and swimming capacities (Fisher et al., 2005; Irisson et al., 2015) exhibited by most coral fish larvae are considered, as they allow larvae to orient themselves and reach locations that offer a greater probability of survival.
Although the results of this research can be considered a synoptic view of the study area, significant evidence was generated showing that the abundance of the larvae of fish species associated with coral formations varies temporally and spatially in the eastern sector of Gorgona Island. Even this variability seems to be related to the temporal variation in the oceanographic conditions described for the study area, particularly the island-wide surface circulation conditions. However, it is necessary to continue to delve into this issue, incorporating the factors that modulate the movement capacity of fish larvae, such as their swimming and sensory abilities (Wolanski and Kingsford, 2014), to understand how they impact the fish populations that inhabit the coral formations in this locality.
ACKNOWLEDGEMENTS
The authors thank Felipe Muriel, Marisol Rivera, María Moreno, Andrés Carmona, Stephania Rojas, José Ortiz, Ana Méndez, Juan Carvajal, David Friedrich, María Isabel Ospina, Kevin Mendoza, Felipe López, Yolanda Otero and Harold Cuenca for their valuable support during sampling as well as the officials of the Gorgona Island PNN, especially Ximena Zorrilla, Luis Fernando Payán, Hector Chirimía, Justino Bonilla, Filiberto Paredes and Abad Ruiz, for their willingness to facilitate and support the development of this research. This study was partially funded by the Oceanography Research Group of the University of Valle, the Department of Biology of the University of Valle and the Gorgona Island PNN within the framework of the program to monitor the conservation value of the pelagic environment component of Gorgona Park. This contribution represents the work of the first author to obtain the academic degree of Biologist from the Department of Biology of the University of Valle.
REFERENCES
Aceves, G., S.P. Jiménez, A. Hinojosa, R. Funes, R.J. Saldierna, D. Lluch, PE. Smith and W. Watson. 2003. Fish larvae from the Gulf of California. Scientia Mar., 67(1): 1-11. [ Links ]
Aceves, G., R. Saldierna, A. Hinojosa, S.PA. Jiménez, M.E. Hernández and R. Morales. 2008. Vertical structure of larval fish assemblages during diel cycles in summer and winter in the southern part of Bahía de La Paz, Mexico. Est. Coast. Shelf Sci., 76: 889-901. [ Links ]
Almany, G.R., R.J. Hamilton, M. Bode, M. Matawai, T. Potuku, P. Saenz and G.R. Russ. 2013. Dispersal of grouper larvae drives local resource sharing in a coral reef fishery. Curr. Biol., 23(7): 626-630. [ Links ]
Amador, J.A., E.R. Rivera, A.M. Durán, G. Mora and F. Sáenz. 2016. The easternmost tropical Pacific. Part I: A climate review. Rev. Biol. Trop., 64: 1-22. [ Links ]
Beltrán, B.S. y R. Ríos. 2000. Estadios tempranos de peces del Pacífico colombiano. Instituto Nacional de Pesca y Acuicultura INPA, Buenaventura, Colombia. 727 p. [ Links ]
Bergenius, M.A., M.I. McCormick, M.G. Meekan and D.R. Robertson. 2005. Environmental influences on larval duration, growth and magnitude of settlement of a coral reef fish. Mar. Biol., 147(2): 291-300. [ Links ]
Blanco, J. F. 2009. The hydroclimatology of Gorgona Island: seasonal and ENSO-related patterns. Actual. Biol., 31(91): 111-121. [ Links ]
Boehlert, G.W., W. Watson and C. Sun. 1992. Horizontal and vertical distributions of larval fishes around an isolated oceanic island in the tropical Pacific. Deep Sea Res., 39(3/4): 439-466. [ Links ]
Bogan, M. 1994. Distribution and retention of larval fishes near reefs in the Gulf of California. Mar. Ecol. Prog. Ser., 115: 1-13. [ Links ]
Buston, P.M., P.J. Geoffrey, S. Planes and S.R. Thorrold. 2012. Probability of successful larval dispersal declines fivefold over 1 km in a coral reef fish. Proc. Royal Soc. B., 279: 1883-1888. [ Links ]
Calderón-Patrón, J.M., C.E. Moreno e I. Zuria. 2012. La diversidad beta: medio siglo de avances. Rev. Mex. Biodivers., 83(3): 879-891. [ Links ]
Cowen, R. K. and L.R. Castro. 1994. Relation of coral reef fish larval distributions to island scale circulation around Barbados, West Indies. Bull. Mar. Sci., 54(1): 228-244. [ Links ]
Cowen, R.K., K.M. Lwiza, S. Sponaugle. C.B. Paris and D.B. Olson. 2000. Connectivity of marine populations: open or closed? Science, 287: 857-859. [ Links ]
Cowen, R.K., C.B Paris and A. Srinivasan. 2006. Scaling of connectivity in marine populations. Science, 311: 522-527. [ Links ]
Díaz, J.M., J.H. Pinzón, A.M. Perdomo, L.M. Barrios y M. López. 2001. Generalidades: 17-26. En (Eds.). Gorgona Marina: Contribución al conocimiento de una isla única. Invemar Ser. Publ. Esp., 7: 160 p. [ Links ]
Eble, J., R. Toonen, L. Sorenson, L. Basch, Y. Papastamatiou and B. Bowen. 2011. Escaping paradise: larval export from Hawaii in an Indo-Pacific reef fish, the yellow tang Zebrasomaflavescens. Mar. Ecol. Prog. Ser., 428: 245-258. [ Links ]
Escarria, E., B. Beltrán, A. Giraldo and F.A. Zapata. 2007. Ichthyoplankton in the Nacional Natural Park Isla Gorgona (Pacific Ocean of Colombia) during September 2005. Invest. Mar. Valparaíso, 35: 127-133. [ Links ]
Estupiñán, F., H.v. Prahl y E. Rubio. 1990. Ictiofauna de la ensenada de Utría, Pacífico colombiano. Rev. Cienc., 2: 65-75. [ Links ]
Fiedler, P.C. and L. Talley. 2006. Hydrography of the eastern tropical Pacific: A review. Progr. Oceanogr., 69: 143-180. [ Links ]
Fisher, R., J.M. Leis, D.L. Clark and S.K. Wilson. 2005. Critical swimming speeds of late-stage coral reef fish larvae: variation within species, among species and between locations. Mar. Biol., 147(5): 1201-1212. [ Links ]
Fabricius, K., G. De'ath, L. McCook, E. Turak and D.M. Williams. 2005. Changes in algal, coral and fish assemblages along water quality gradients on the inshore Great Barrier Reef. Mar. Pollut. Bull., 51(1): 384-398. [ Links ]
Franco, C., E. Godínez and E. Suárez. 2002. Larval fish assemblages in waters off the central Pacific coast México. J. Plankton Res., 24 (8): 775-784. [ Links ]
Franke, R. y A. Acero P. 1992. Peces lutjánidos del Parque Gorgona Pacifico colombiano (Osteichthyes: Lutjanidae). Rev. Biol. Mar. Valparaiso, 27: 59-71. [ Links ]
Franke, R. y A. Acero P. 1995a. Peces óseos comerciales del Parque Gorgona, Pacífico colombiano (Osteichthyes: Muraenesocidae. Hemiramphidae. Belonidae. Scorpaenidae. Triglidae. Malacanthidae. Gerreidae. Sparidae. Kyphosidae. Sphyraenidae e Istiophoridae). Rev. Biol. Trop., 44(2): 763-770. [ Links ]
Franke, R. y A. Acero P 1995b. Peces serránidos del Parque Gorgona. Pacífico colombiano (Osteichthyes: Serranidae). Rev. Acad. Colomb. Cienc., 19(74): 593-600. [ Links ]
Funes, R., C. Flores, A. Esquivel, M.A. Fernández and A. Gracia. 2002. Larval fish community structure along the west coast of Baja California during and after the El Niño event (1983). Bol. Cienc. Mar., 70(1): 41-54. [ Links ]
Gerlach, G., J. Atema, M.J. Kingsford, K. Black and V.P. Miller. 2007. Smelling home can prevent dispersal of reef fish larvae. PNAS, 104(3): 858-863. [ Links ]
Giraldo, A. 2008. Variabilidad espacial de temperatura, salinidad y transparencia en el ambiente pelágico del PNN Gorgona durante septiembre 2007 y marzo 2008. Bol. Cient. CIOH, 26: 157-163. [ Links ]
Giraldo, A. y B. Valencia 2012. Condiciones oceanográficas en la Ensenada de Utría y su potencial influencia sobre los ecosistemas marinos objetos valor de conservación del PNN Utría. Informe Técnico Final US-191. Universidad del Valle, Cali. 67 p. [ Links ]
Giraldo, A., E. Rodríguez y F. Zapata. 2008. Condiciones oceanográficas en isla Gorgona, Pacífico Oriental Tropical de Colombia. Lat. Am. J. Aq. Res., 36: 121-128. [ Links ]
Giraldo, A., B. Valencia, J. Acevedo y M. Rivera. 2012. Columna de agua del Parque Nacional Natural Gorgona: 27-44. En Giraldo, A. y B. Valencia (Eds) Isla Gorgona, paraíso de biodiversidad y ciencia. Universidad del Valle, Cali. 222 p. [ Links ]
Giraldo, A., M.C. Diazgranados y C.F. Gutiérrez. 2014. Isla Gorgona, enclave estratégico para los esfuerzos de conservación en el Pacífico Oriental Tropical. Rev. Biol. Trop., 62: 1-12. [ Links ]
Glynn, PW. and J.S. Ault. 2000. A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs, 19: 1-23. [ Links ]
Gómez, F. y C. Vieira. 1996. Ictiofauna asociada a los arrecifes coralinos hermatípicos de la ensenada de Utría, Chocó, Pacifico colombiano. Universitas Scientiarum, 3(1-2): 53-61. [ Links ]
Green, A.L., A.P. Maypa, G.R. Almany, K.L. Rhodes, R. Weeks, R.A. Abesamis and A.T. White. 2015. Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biol. Rev., 90(4): 1215-1247. [ Links ]
Hamner, W.M. and J.L. Largier. 2012. Oceanography of the planktonic stages of aggregation spawning reef fishes: 159-190. En de Sadovy, Y. y P.L. Colin (Eds.). Reef fish spawning aggregations: Biology, research and management. Fish Fish. Series. Springer. New York. 622 p. [ Links ]
Hamner, W.M., P.L. Colin and P.P. Hamner. 2007. Export-import dynamics of zooplankton on a coral reef in Palau. Mar. Ecol. Prog. Ser. , 334: 83-92. [ Links ]
Harrison, H. B., D.H. Williamson, R.D. Evans, G.R. Almany, S.R. Thorrold, G.R. Russ, K.A. Feldheim, L. van Herwerden, S. Planes, M. Srinivasan, L.M Berumen and G.P. Jones. 2012. Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr. Biol., 22: 1023-1028. [ Links ]
Herrera-Moreno, A. 2000. La clasificación numérica y su aplicación en la ecología. Inst. Tecnol. Santo Domingo, República Dominicana. 88 p. [ Links ]
Hogan, J. D., R.J. Thiessen, P.F. Sale and D.D. Heath. 2012. Local retention, dispersal and fluctuating connectivity among populations of a coral reef fish Oecologia, 168(1): 61-71. [ Links ]
Hoyos, N., J. Escobar, J.C. Restrepo, A.M. Arango and J.C. Ortiz. 2013. Impact of the 2010-2011 La Niña phenomenon in Colombia, South America: The human toll of an extreme weather event. Ap. Geogr., 39: 16-25. [ Links ]
Irisson, J.O., A. LeVan, M. de Lara and S. Planes. 2004. Strategies and trajectories of coral reef fish larvae optimizing self-recruitment. J. Theor. Biol., 227: 205-218. [ Links ]
Irisson, J.O., C.B. Paris, J.M. Leis and M.N. Yerman. 2015. With a little help from my friends: group orientation by larvae of a coral reef fish. PloS One, 10(12): e0144060. [ Links ]
Jones, G. P., S. Planes and S.R. Thorrold. 2005. Coral reef fish larvae settle close to home. Curr. Biol. , 15(14): 1314-1318. [ Links ]
Jones, G.P., G.R. Almany, G. R. Russ, P.F. Sale, R.S. Steneck, M.J.H. Van Oppen and B.L. Willis. 2009. Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs, 28(2): 307-325. [ Links ]
Joseph, A. 2014. Measuring ocean currents: tools, technologies and data. Elsevier. Waltham, USA. 448 p. [ Links ]
Jost, L., A. Chao and R.L. Chazdon. 2011. Compositional similarity and ß (beta) diversity: 66-87. En Magurran, A. y B.J. McGill (Ed.). Biological diversity: frontiers in measurement and assessment. Oxford, New York. 368 p. [ Links ]
Kessler, W. S. 2006. The circulation of the eastern tropical Pacific. A review. Prog. Oceanogr., 69: 181-217. [ Links ]
Landaeta, M. y L. Castro. 2004. Zonas de concentración de ictioplancton en el archipiélago de Juan Fernández, Chile. Cienc. Tecnol. Mar., 27(2): 43-53. [ Links ]
Leahy, S.M., G.R. Russ and R.A. Abesamis. 2015. Pelagic larval duration and settlement size of a reef fish are spatially consistent, but post-settlement growth varies at the reef scale. Coral Reefs , 34(4): 1283-1296. [ Links ]
Lecchini, D., J. Shima, B. Banaigs and R. Galzin. 2005. Larval sensory abilities and mechanisms of habitat selection of a coral reef fish during settlement. Oecologia, 143(2): 326-334. [ Links ]
Lefèvre, C. D. and D.R. Bellwood. 2015. Disturbance and recolonization by small reef fishes: the role of local movement versus recruitment. Mar. Ecol. Progr. Ser., 537: 205-215. [ Links ]
Lehodey, P., J. Alheit, M. Barange, T. Baumgartner, G. Beaugrand, K. Drinkwater, J. Fromentin, S. Hare, G. Ottersen, R. Perry, C. Roy, C. van der Lingen and F. Werner. 2006. Climate variability, fish and fisheries. J. Clim., 19: 5009-5030. [ Links ]
Leis, J.M. 1993. Larval fish assemblages near Indo-Pacific coral reefs. Bull. Mar. Sci., 53(2): 362-392. [ Links ]
Lobel, P. S. and A.R. Robinson. 1986. Transport and entrapment of fish larvae by ocean mesoscale eddies and currents in Hawaiian waters. Deep Sea Res. Part A, Oceanogr. Res., 33(4): 483-500. [ Links ]
Moser, H. G. 1996. The early stages of fishes in the California Current Region. Calcofi Atlas. 33. Allen Press, Lawrence, USA. 1505 p. [ Links ]
Mouritsen, H., J. Atema, M.J. Kingsford and G. Gerlach. 2013. Sun compass orientation helps coral reef fish larvae return to their natal reef. PLoS One, 8(6): e66039. [ Links ]
Muñoz, C. G. y F. A. Zapata. 2013. Plan de Manejo de los Arrecifes Coralinos del Parque Nacional Natural Gorgona, Pacífico colombiano. Parques Nacionales Naturales y WWF-Colombia. Cali. 68 p. [ Links ]
Paris, C.B. and R.K. Cowen. 2004. Direct evidence of a biophysical retention mechanism for coral reef fish larvae. Limnol. Oceanogr., 49(6): 1964-1979. [ Links ]
Paris, C.B., J. Atema, J.O. Irisson, M. Kingsford, G. Gerlach and C.M. Guigand. 2013. Reef odor: a wake up call for navigation in reef fish larvae. PloS One, 8(8): e72808 [ Links ]
Poveda, G., D.M. Alvarez and O.A. Rueda. 2011. Hydro-climatic variability over the Andes of Colombia associated with ENSO: a review of climatic processes and their impact on one of the Earth's most important biodiversity hotspots. Clim. Dyn., 36: 2233-2249. [ Links ]
Robertson, D.R. y G.R. Allen. 2015. Peces costeros del Pacífico Oriental Tropical: Sistema de información en línea. Versión 2.0 Inst. Smithsonian Invest. Trop. Balboa, Panamá. [ Links ]
Rodríguez, E., W. Schneider and R. Abarca del Río. 2003. On the seasonal circulation within the Panama Bight derived from satellite observations of wind, altimetry and sea surface temperature. Geophys. Res. Let., 30(7): 1410. doi:10.1029/2002GL016794, 7. [ Links ]
Rodríguez, E., J.R. Ortiz y J.G. Rueda . 2007. Aspectos oceanográficos: 29-44. En DIMAR-CCCP y UAESPNN- DTSO (Eds.). Santuario de Fauna y Flora Malpelo: descubrimiento en marcha. DIMAR, Bogotá. 142 p. [ Links ]
Rojas, PA. y L.A. Zapata. 2006. Peces demersales del Parque Nacional Natural Gorgona y su área de influencia. Pacífico colombiano. Biota Colomb., 7(2): 211-244. [ Links ]
Rubio, E. 1990. Ictiofauna pacífica asociada a los corales de la isla de Gorgona. Rev. Cienc., 2: 97-106. [ Links ]
Rubio, E. y J. Angulo. 2003. Peces coralinos del Pacífico colombiano: incluye especies deportivas y comerciales de áreas adyacentes. Univ. Valle, Cali. 317 p. [ Links ]
Sale, P. F. 2004. Connectivity, recruitment variation, and the structure of reef fish communities. Integr. Comp. Biol., 44: 390-399. [ Links ]
Sale, P, R. Cowen, B. Danilowicz, G. Jones, J. Kritzer, K. Lindeman and R. Steneck. 2005. Critical science gaps impede use of no-take fishery reserves Trends Ecol. Evol., 20(2): 74-80. [ Links ]
Salles, O., J. Maynard, M. Joannides, C. Barbu, P. Saenz, R. Almany and S. Planes. 2015. Coral reef fish populations can persist without immigration. Proc. Roy. Soc. B, 282: 20151311. http://dx.doi.org/10.1098/rspb.2015.1311 20151311). [ Links ]
Searcy, S. P and S. Sponaugle. 2000. Variable larval growth in a coral reef fish. Mar. Ecol. Progr. Ser., 206: 213-226. [ Links ]
Sponaugle, S., J. Fortuna, K. Grorud and T. Lee. 2003. Dynamics of larval fish assemblages over a shallow coral reef in the Florida Keys. Mar. Biol., 143(1): 175-189. [ Links ]
Srinivasan, M and G.P. Jones. 2006. Extended breeding and recruitment periods of fishes on a low latitude coral reef. Coral Reefs, 25(4): 673-682. [ Links ]
Sundelöf, A. and P.R. Jonsson. 2012. Larval dispersal and vertical migration behavior-a simulation study for short dispersal times. Mar. Ecol., 33: 183-193. [ Links ]
Treml, E. A., J.J. Roberts, Y. Chao, P.N. Halpin, H.P. Possingham and C. Riginos. 2012. Reproductive output and duration of the pelagic larval stage determine seascape-wide connectivity of marine populations. Integr. Comp. Biol., 52(4): 525-537. [ Links ]
UAESPNN. 2004. Plan básico de manejo 2005-2009 Parque Nacional Natural Gorgona. Parques Nacionales Naturales, Dirección Territorial Suroccidente, Cali. 255 p. [ Links ]
Vellend, M. 2001. Do commonly used indices of ß-diversity measure species turnover? J. Veg. Sci., 12: 545-552. [ Links ]
Villegas, N.L. e I. Málikov. Modelación de la estructura dinámica de las aguas de la cuenca del Pacífico colombiano. Bol. Cient. CCCP., 13: 97-114. [ Links ]
Villegas, N.L., I. Málikov y D. Díaz. 2016. Variabilidad mensual de la velocidad de surgencia y clorofila a en la región del Panama Bight. Rev. Mutis, 6(2): 82-94. [ Links ]
Wolanski, E. and M. Kingsford. 2014. Oceanographic and behavioural assumptions in models of the fate of coral and coral reef fish larvae. J. Roy. Soc. Interface, 11: 20140209. http://dx.doi.org/10.1098/rsif.2014.0209. [ Links ]
Zapata, F. 2001. Ecología de peces arrecifales en Gorgona: Composición, abundancia, diversidad e historia de vida temprana: 111-122. En Barrios, L. M. y M López-Victoria (Eds.). Gorgona Marina: Contribución al conocimiento de una isla única. Invemar Ser. Publ. Esp. 7, 160 p. [ Links ]
Zapata, F. and Y. Morales. 1997. Spatial and temporal patterns of fish diversity in a coral reef at Gorgona Island, Colombia. Proc. 8th Internat. Coral Reef Symp., 1: 1029-1034. [ Links ]
Zapata, F. and P. Herrón. 2002. Pelagic larval duration and geographic distribution of tropical eastern Pacific snappers (Pisces: Lutjanidae). Mar. Ecol. Progr. Ser., 230: 295-300. [ Links ]
Received: January 27, 2017; Accepted: August 16, 2017











 text in
text in