Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.46 no.2 Santa Marta July/Dec. 2017
https://doi.org/10.25268/bimc.invemar.2017.46.2.732
Research Articles
Diet overlap between lionfish Pterois volitans (Teleostei: Scorpaenidae) and native fishes at a similar trophic level in Cuba
1 Acuario Nacional Cuba: Calle 1ra, esquina 60, #608. Playa. La Habana. Cuba. lali.pantoja1990@gmail.com
2 Parque Nacional Guanahacabibes (ECOVIDA): La Bajada, Municipio Sandino. Pinar del Río. Cuba
3 Instituto de Oceanología: Avenida 1ra, entre 184 y 186 #18406. Reparto Flores. Playa. La Habana. Cuba
The spread of the Indo-Pacific lionfish has been one of the most rapid marine fish invasions in the history of the tropical and subtropical western Atlantic, and populations of this species pose a threat to native fishes feeding at similar trophic levels due to competition for food. To determine dietary overlap, 899 lionfish and 377 native fishes of the families Haemulidae, Holocentridae, Serranidae and Lutjanidae were caught in three Cuban localities, and their stomach contents were analyzed based on the number of items, frequency, volume and index of relative importance. Diet composition was similar in the studied localities; Lutjanidae, Serranidae and lionfish mainly fed on fish while Haemulidae and Holocentridae primarily consumed small benthic invertebrates. Mantel correlations showed that the consumption of fish and crustaceans did not increase as lionfish increased in size. The degree of dietary overlap between lionfish and some native fishes depends on locality, ecological zone and the characteristics of each family, confirming the opportunistic nature of the invader. It is likely that native fishes are not affected by competition with lionfish for food because they feed on the most abundant and available organisms; therefore, food availability is not a limiting factor for these groups.
Keywords: Diet composition; Trophic ecology; Invasive species
El pez león, originario del Indo Pacífico, ha protagonizado una de las invasiones de peces marinos más rápida de la historia en el Atlántico occidental tropical y subtropical. Sus poblaciones representan una amenaza para los peces nativos de nivel trófico similar en la competencia por los recursos alimentarios. Con la finalidad de comparar sus dietas, se capturaron 899 peces león y 377 peces nativos pertenecientes a las familias Haemulidae, Holocentridae, Serranidae y Lutjanidae en tres localidades de Cuba. Se analizó el contenido de sus estómagos mediante las variables número, frecuencia, volumen y el índice de importancia relativa. La composición general de las dietas fue similar en las tres localidades estudiadas. El pez león y las familias Lutjanidae y Serranidae se alimentaron principalmente de peces, mientras que en las familias Haemulidae y Holocentridae predominaron los pequeños invertebrados bentónicos. En las correlaciones realizadas a partir de la prueba de Mantel se obtuvo que el consumo de peces y crustáceos no varió significativamente a medida que la talla del pez león aumenta. El nivel de superposición entre la dieta del pez león y los peces nativos varió según la localidad, la zona ecológica y las características propias de cada familia, confirmándose el carácter oportunista de estas especies. Los peces nativos probablemente no se vean afectados por el pez león en la competencia por los recursos alimenticios ya que consumen los organismos más abundantes y disponibles en el hábitat por lo que la disponibilidad de alimento no constituye un factor limitante para estos grupos.
Palabras clave: Composición de la dieta; Ecología trófica; Especie invasora
INTRODUCTION
Invasive species establish outside their native range (Molnar et al., 2008) and have negative effects on invaded ecosystems (Crooks, 2002). Lionfish (Pterois volitans) (Linnaeus, 1758), which are originally from the Indo-Pacific region, have spread into the tropical and subtropical western Atlantic, constituting one of the most rapid marine fish invasions in history (Morris et al., 2009). In Cuba, this species was first recorded along the southeast coast in 2007 by Chevalier et al. (2008), and by 2009, it was widely distributed throughout the waters of the island (Chevalier et al., 2014). This species may pose a serious threat to the native fishes and invertebrates of which it feeds, drastically impacting the trophic structure of reefs (Albins and Hixon, 2008; Morris and Akins, 2009; Alexander and Haynes, 2011; Cabrera, 2011; Valdez-Moreno et al., 2012; Morris, 2013; Cabrera- Guerra, 2014). Additionally, it may pose a serious threat to native fishes at a similar trophic level due to competition for resources, food and habitat (Morris, 2013, Morris and Green, 2013) because it exhibits novel behavioral characteristics and habits to the invaded ecosystem, which can confer some degree of predatory efficiency (Albins and Hixon, 2011, Albins and Lyons, 2012). Some studies (Whitfield et al., 2007; Albins and Hixon, 2008; Morris and Akins, 2009; Green and Côté, 2010; Cure et al., 2012; Green et al., 2012; Albins, 2013; Benkwitt, 2014) suggest that lionfish may outcompete some native predators for food in invaded habitats and decrease the abundance of prey native fishes and invertebrates (Albins, 2013). Considering the potential impact of lionfish on native species, the present research aimed to characterize the diet composition and structure of lionfish and native fishes at a similar trophic level, evaluate the relationship between the diet compositions of lionfish as their size increases and calculate the dietary overlap between lionfish and native fishes at a similar trophic level.
STUDY AREA
The study was conducted from March 2012 to February 2016 at three Cuban localities (Figure 1): Guanahacabibes (Pinar del Río) (21°46’35.6’’-55’77.9’’N and 84°32’93.8’’-27’91.8’’W), La Habana (23°06’98.5’’N and 82°26’20.3’’W) and Cayo Las Brujas (Villa Clara) (22°32’54.8’’-38’12.3’’N and 79°10’17.3’’-15’46.6’’W). Field observations were performed on coral reefs (Guanahacabibes and La Habana) and in seagrass (Cayo Las Brujas) to determine if the dietary overlap between lionfish and native fishes varies according to the reef type and marine biotope.
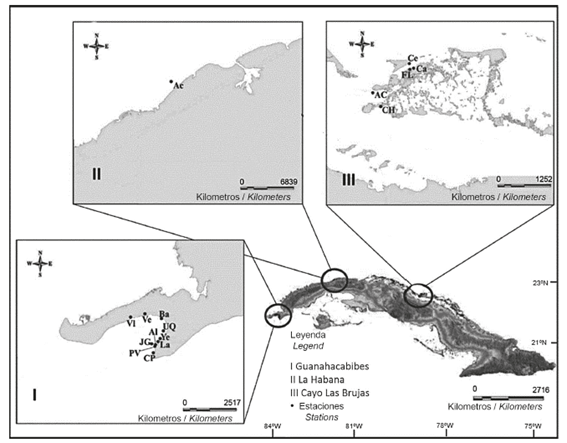
Figure 1 Study localities and capture stations of lionfish (Pterois volitans) and native fishes at a similar trophic level. I: Guanahacabibes (Pinar del Río), II: La Habana, III: Cayo Las Brujas (Villa Clara). Capture stations include Acopio Cobo: AC, Acuario: Ac, Almirante: Al, Bajada: Ba, Canal del Hielo: CH, Carey: Ca, Cerco: Ce, Cuevas de Pedro: CP, Delfinario: De, Farallón de Lali: FL, Jardín de las Gorgonias: JG, Laberinto: La, Patio de Vanesa: PV, Uvero Quemado: UQ, Veral: Vl, Verraco: Ve and Yemayá: Ye.
Guanahacabibes
The Guanahacabibes National Park is a marine protected area in the province of Pinar de Río with an area of 15,950 ha (Cobián and Chevalier, 2009). Marginal or coastal reefs are found in this zone, and their typical profile is one ledge culminating in a deep escarpment with a varied structure. The reefs of the area are considered among the most beautiful and best preserved in Cuba (Alcolado et al., 2003; Gotera, 2005; González- Ferrer et al., 2007).
La Habana
This location is in a sector facing the western coast of La Habana province. The growth of corals, gorgonians and sponges is limited mainly to the reef shelf (Guardia and González-Sansón, 2000) and there are no crests or patch reefs (peaks). Instead, the entire coastal strip up to approximately 7 m deep is a rocky ledge with few refuges (Aguilar and González- Sansón, 2007). There are no large areas of soft bottoms and those that exist are unstable, so there are practically no seagrasses (Aguilar and González- Sansón, 2007).
Caibarién
Cayo Las Brujas is located near the municipality of Caibarién in the province of Villa Clara. In this area, many mangrove cays and islets rest on a common platform, separated from each other by canals and fairways that allow the sea to access the Cuban coast (Martínez-Daranas, 2007). Seagrasses predominate, particularly the phanerogam Thalassia testudinum, and a dense row of red mangrove (Rhizophora mangle) borders the keys.
MATERIALS AND METHODS
Specimen collection
In the localities of Guanahacabibes and La Habana, individual members of the families Lutjanidae, Serranidae, Haemulidae and Holocentridae were collected, but in Cayo Las Brujas, only Lutjanidae and Haemulidae were collected due to low abundance of Serranidae and Holocentridae species similar in size to lionfish. The fish species of the family Serranidae were Cephalopholis cruentata and C. fulva; those of Holocentridae were Holocentrus adscensionis and H. rufus; and those of Haemulidae were Haemulon sciurus and H. flavolineatum. In La Habana, individual members of the Lutjanidae family belonged to Lutjanus synagris, while they belonged to L. griseus and L. apodus in Guanahacabibes and Cayo Las Brujas. In addition to being similar in size to lionfish, all the native fishes were the most abundant carnivorous predators in each locality. Three times a year, specimens were caught between 8:00 am and 10:00 am using hand nets and Hawaiian spears.
Laboratory processing
Lionfish and native fishes were analyzed soon after collection; when this was not possible, they were frozen for later identification. Specimens were sacrificed by cervical sectioning of the spine using scissors, and the dorsal, pelvic and anal spines of lionfish were removed to prevent injury. The total length (cm) and weight (g) of all individuals were measured using a ruler with an accuracy of 0.1 mm and an analytical scale with an accuracy of 0.5 g, respectively.
Prey items found in the stomachs were identified to the lowest possible taxonomic level. Fish were identified according to Böhlke and Chaplin (1968), Guitart (1974), Guitart (1975) and Claro (2001), and crustaceans were identified according to Gómez (1980), Martínez-Iglesias and Gómez (1986) and Ortiz et al. (2010). Volumetric analysis was performed using graduated beakers and cylinders with an accuracy of 1 ml and 0.2 ml, respectively.
Data analysis
An accumulated curve of prey items was constructed by plotting the total number of prey items observed vs. the number of stomachs analyzed (sampling units); the stomachs were randomly combined and ordered. The items found in the stomachs of each species were analyzed by calculating their proportion (%N), frequency (%F) and volume (%V). From these results, the index of relative importance (IRI) was calculated (Pinkas et al., 1971) using the formula IRI=(%N+%V) x%F; percent IRI was also calculated as %IRI=100xIRIi/ Σni=IRIi (Hyslop, 1980).
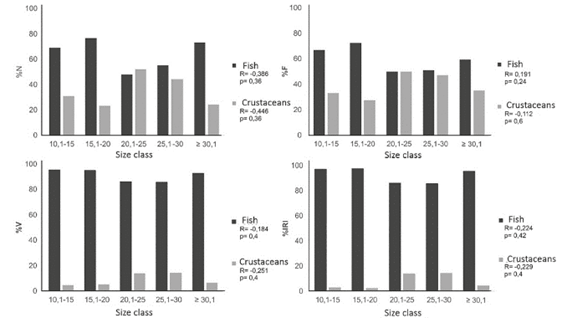
In La Habana, diets were analyzed relative to the size of the lionfish as this was the only locality where all sizes of lionfish were captured. These lionfish specimens were grouped into five (5) total length classes: 10-15 cm, 15.1-20 cm, 20.1-25 cm, 25.1-30 cm and greater than 30 cm. The consumption of fish and crustaceans in relation to size was determined from the variables %N, %F, %V and %IRI to consider whether the diet composition of lionfish varied with growth. To determine changes in the food spectrum from one stage of development to another, the degree of trophic overlap between each size class of lionfish and native fishes was analyzed.
The Monte Carlo method (Metropolis and Ulam, 1949) was used to compare the mean values of %N, %F, %V and %IRI for prey items found in the stomachs of lionfish and native fishes (Metropolis and Ulam, 1949). Ten thousand (10,000) random resamples of the original data were performed with replacement, and a Mantel test (Mantel, 1967) was performed to determine whether there was a correlation between the consumption of fish and crustaceans (%N, %F, %V and %IRI) by lionfish and their size. Data were converted to Euclidean distance matrices; 999 permutations were performed; and the matrices were compared by calculating the Z statistic and testing its significance against a null distribution obtained by permutations of the second matrix. To assess the trophic overlap between lionfish and native fishes, confidence intervals were calculated for Morisita-Horn indices (Horn, 1956). A Monte Carlo simulation was also performed using 10,000 random resamples of the original data with replacement, and confidence limits were calculated by bootstrapping. The programs Microsoft Excel, Pop Tools version 3.23, First version 6.0, R version 3.2.2 and MapInfo version 10.5 were used for data processing and statistical analysis.
RESULTS
Diet composition
Eight hundred ninety-nine (899) lionfish and 377 native fishes at a similar trophic level were captured, of which 74 belonged to the family Serranidae, 146 to Haemulidae, 71 to Holocentridae and 86 to Lutjanidae.
The asymptotic trend in the curve of cumulative items vs. stomachs analyzed indicated that the sample size was appropriate (Figure 2).
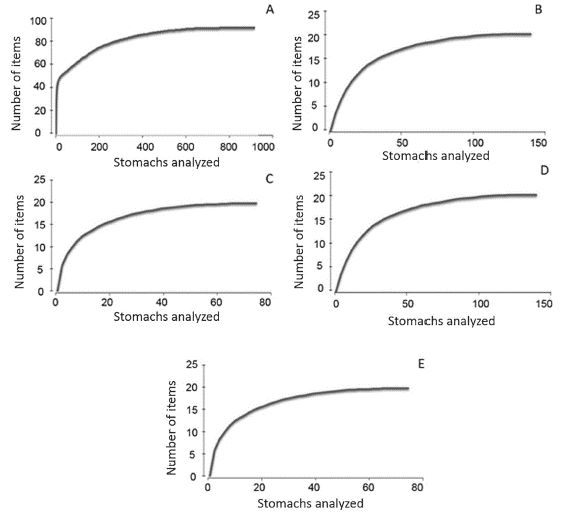
Figure 2 Accumulated curves of prey items. A: lionfish, B: Lutjanidae, C: Serranidae, D: Haemulidae E: Holocentridae.
Eighty-one (81) food items were identified from the stomach contents of lionfish that were grouped into ten orders and 30 families (Tables 1, 2, 3, 4 and 5). The families Pomacentridae (%IRI=1.41), Gobiidae (%IRI=0.82), Scaridae (%IRI=0.33) and Labridae (%IRI=0.22) were the most important. Forty-four (44) items were identified from the stomach contents of native fishes that were grouped into 11 orders and 21 families.
Table 1 List of items found in the stomach contents of lionfish (Pterois volitans) from the three studied localities: Guanahacabibes, La Habana and Cayo Las Brujas. Number: N, frequency: F, volume: V and index of relative importance: IRI
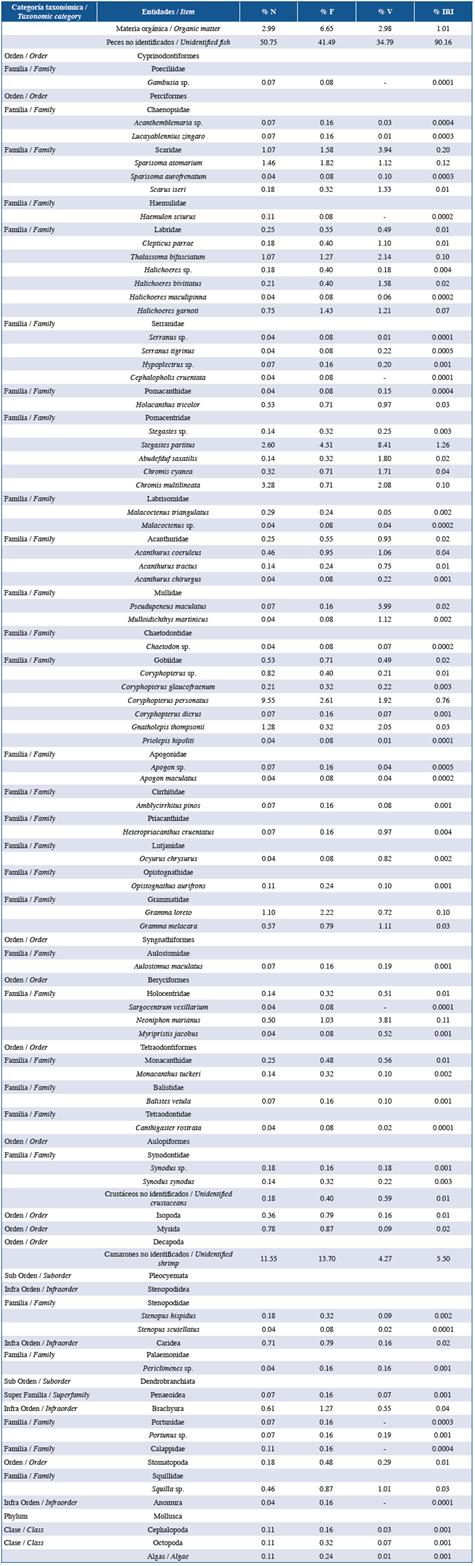
Table 2 List of items found in the stomach contents of the family Haemulidae in the three studied localities: Guanahacabibes, La Habana and Cayo Las Brujas. Number: N, frequency: F, volume: V and index of relative importance: IRI.
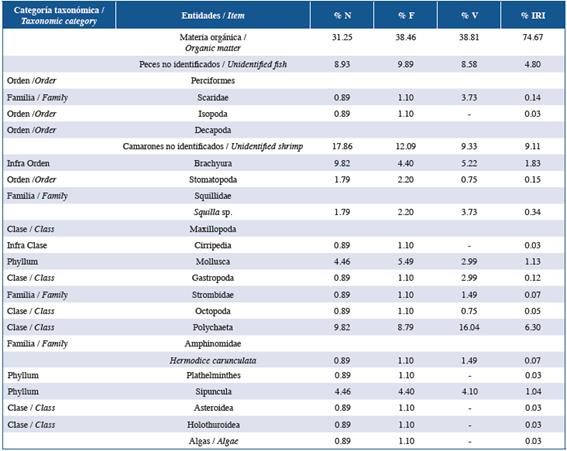
Table 3 List of items found in the stomach contents of the family Serranidae in the three studied localities: Guanahacabibes, La Habana and Cayo Las Brujas. Number: N, frequency: F, volume: V, and index of relative importance: IRI.
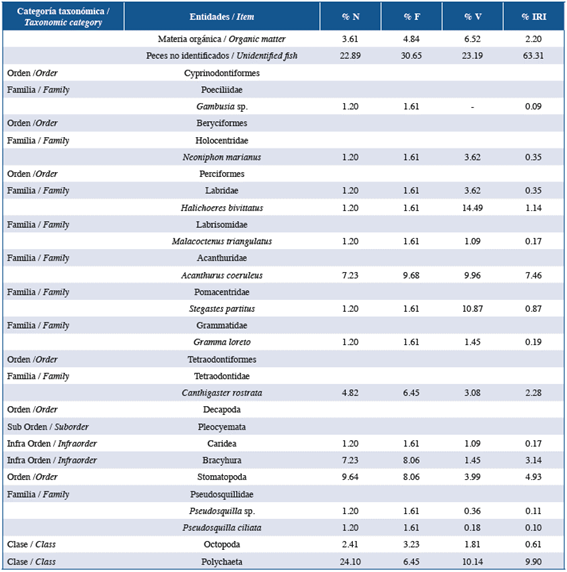
Table 4 List of items found in the stomach contents of the family Holocentridae in the three studied localities: Guanahacabibes, La Habana and Cayo Las Brujas. Number: N, frequency: F, volume: V and index of relative importance: IRI.
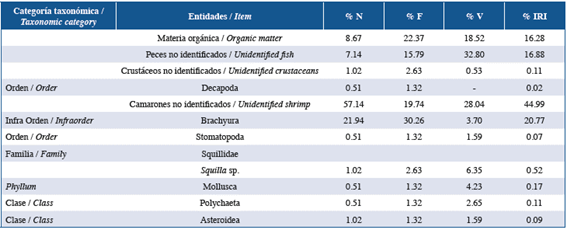
Table 5 List of items found in the stomach contents of members of the family Lutjanidae in the three studied localities: Guanahacabibes, La Habana and Cayo Las Brujas. Number: N, frequency: F, volume: V and index of relative importance: IRI.
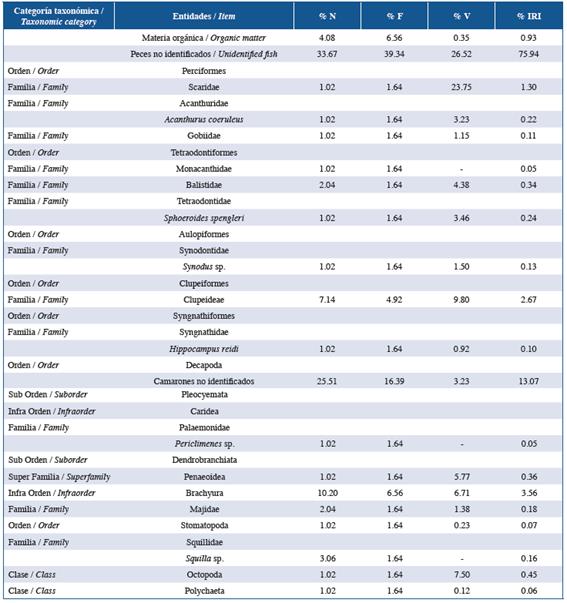
In order of abundance according to %IRI, lionfish and species of the families Lutjanidae and Serranidae mainly fed on fish and crustaceans. Species belonging to the family Holocentridae mainly fed on crustaceans while Haemulidae fed on small benthic invertebrates. In the family Lutjanidae, nine fish families were identified in the diets, of which Acanthuridae (%IRI=9.49), Clupeidae (%IRI=2.67) and Scaridae (%IRI=1.30) were predominant. Serranidae had eight fish families in its diet, of which Acanthuridae (%IRI=7.45) and Labridae (%IRI=1.48) were the most important. In the family Holocentridae, shrimps (infraorders Stenopodidea and Caridea, the superfamily Penaeoidea (%IRI=44.99) and the infraorder Brachyura (%IRI=20.77)) were the most important items. Although fish were the second best- represented group, identification to the family level was not possible (%IRI=16.88). The diet of the family Haemulidae included crustaceans (shrimp and crabs), polychaetes, mollusks, platyhelminths, sipunculids, holothuroids and asteroids; the proportion of each component varied according to locality.
Diet analysis in relation to lionfish size in La Habana
There was no significant correlation between the size of lionfish and the consumption of fish and crustaceans in the locality of La Habana based on any of the methods used (Figure 3). Therefore, juvenile and adult diets are similar in the proportion of fish and crustaceans consumed.
Trophic overlap index
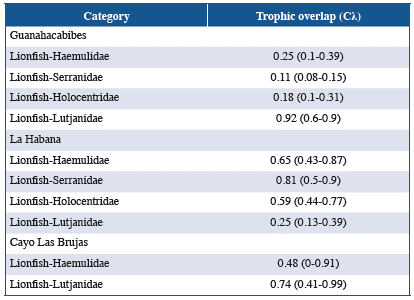
Mean Morisita-Horn index values suggested low dietaryoverlapbetweenlionfishandthefamilies Haemulidae, Serranidae and Holocentridae in Guanahacabibes; but this index was high for Lutjanidae. In La Habana, dietary overlap was high between Serranidae and lionfish; Haemulidae and Holocentridae exhibited moderate overlap; and Lutjanidae showed low overlap. In Cayo Las Brujas, moderate and high overlap was observed when comparing the diets of members of the families Haemulidae and Lutjanidae to that of lionfish, respectively (Table 6).
DISCUSSION
The asymptotic trends observed in the accumulated curves show that the number of stomachs analyzed were sufficient to characterize the diets of lionfish and the families of native fishes. The quantity of food items found in the stomach contents of lionfish and members of the families Lutjanidae, Serranidae, Haemulidae and Holocentridae confirms that all are active generalist predators (Sierra et al., 2001; Morris et al., 2009), and the lionfish diet composition in the three localities was similar to that found in other regions of the Caribbean (Albin and Hixon, 2008; Cabrera, 2011; McCleery, 2011). Fish and crustaceans were the main groups in the lionfish diet, although fish were the most abundant, which is consistent with results obtained in the Atlantic, the Bahamas, Cuba, the Caribbean, the southeastern United States and Mexico (Albins and Hixon, 2008; Morris and Akins, 2009; Cabrera, 2011; McCleery, 2011; Muñoz et al., 2011; Green et al., 2012; Côté et al., 2013; Chevalier et al., 2014; Cabrera-Guerra, 2014; Dahl and Patterson, 2014; Cobián et al., 2016). The fish families with the highest percentage IRI in the lionfish diet were similar to those recorded by other authors in Cuba and the Caribbean (Morris and Akins, 2009; McCleery, 2011; Chevalier et al., 2014; Cabrera- Guerra, 2014; Cobián-Rojas et al., 2016); therefore, we conclude that their diet composition is similar throughout the Caribbean region (Morris and Akins, 2009; Cabrera, 2011; McCleery, 2011; Cabrera-Guerra, 2014).
Lionfish are known to be low-mobility species that prefer rocky pits (Morris and Akins, 2009), and their diet is influenced by their behavior. This explains that the best-represented families in their diet included Gobiidae, Scaridae and Labridae, which live on the bottom and are among the most abundant in Cuban reefs (Sierra et al., 2001; Chevalier and Cárdenas, 2005, 2006; Cobián and Chevalier, 2009; Cobián et al. 2011), making them easy lionfish prey. In Pomacentridae, representatives of the genus Chromis are usually found in the water column, so greater mobility is required for their capture, especially adults, which explains why they are not among the most abundant in the lionfish diet despite being highly abundant in the studied reefs. However, species of the genus Stegastes have benthic habits and constituted one of the most important items in the lionfish diet in Guanahacabibes and La Habana, confirming that in addition to abundance, prey habits are also important (Green et al., 2011).
The diet composition of each family of native fishes was similar among localities. The families Lutjanidae and Serranidae are carnivores that consume large amounts of fish and a wide variety of benthic organisms, mainly crustaceans (Sierra et al., 2001; Claro and Robertson, 2010), and the species in these families exhibit bottom-feeding habits (Sierra et al., 2001), explaining why Labridae and Scaridae, which are also associated with the bottom, are the most important items in their diets, respectively. In contrast, Haemulidae and Holocentridae are carnivorous, but they rarely consume fish and generally feed on small benthic organisms (Sierra et al., 2001; Claro and Robertson, 2010).
In Guanahacabibes, although fish were the most important items in the diet of the family Serranidae, it was not possible to identify food items at the generic and specific levels, and in Cayo Las Brujas, only one fish genus and two species were identified in the diet of the family Lutjanidae. In both cases, the inability to identify food items was due to the degradation of the stomach contents because catches occurred too long after feeding. In contrast, the family Clupeidae may have been overestimated by the volumetric method in Guanahacabibes because some large specimens were found in the stomachs of snappers (Lutjanidae). Large schools of sardines (Clupeidae) were observed near the sampled reefs in this locality, where different predators could be seen feeding on them.
The broadest trophic spectrum was observed in Haemulidae due to the wide variety of invertebrates found in the diet, but the observed food items showed high degradation. This might be because members of this family grind their food with pharyngeal tooth plates so that only fragments or heterogeneous masses are found in their intestines, which makes it difficult to identify prey organisms and measure their weight or volume (Sierra et al., 1994).
Although C. cruentata and H. sciurus were considered predators at a similar trophic level of lionfish, juveniles were found in lionfish stomach contents. Therefore, in addition to possible population impacts through competition for food, lionfish could affect these families through direct predation.
The analysis of diet in relation to lionfish size found no correlation between the consumption of fish and crustaceans and increased size, which is consistent with the results of Cabrera-Guerra (2014) in Cuba. However, this result does not correspond to those recorded in the Bahamas (Morris and Akins, 2009), the Caribbean (McCleery, 2011) and by other studies in Cuba (García, 2015). These previous studies suggest that there is a proportional increase in the consumption of fish as lionfish grow; therefore, the consumption of crustaceans declines. Most authors suggest that the increase in the size of lionfish increases the consumption of fish and decreases that of crustaceans. However, other authors such as Chevalier et al. (2014) captured large lionfish specimens on Thalassia meadows and mangroves, where shrimp of the infraorder Penaeidae reach large sizes, and these shrimp were found to be very common in the diet of lionfish at these locations based on the number, frequency and volume of items. That lionfish diets do not vary with growth suggests that the degree of overlap between the diets of lionfish and those of native fishes will not depend on the size of this invasive species, at least in La Habana. Thus, trophic-level competition will not be influenced by lionfish size because juvenile and adult lionfish have similar diets in La Habana.
The Morisita-Horn index comparing the diets of lionfish and native fishes varied according to locality, marine biotope (reef and seagrasses) and the characteristics of each family. In Guanahacabibes, dietary overlap between lionfish and the families Haemulidae, Holocentridae and Serranidae was low, and for Haemulidae and Holocentridae, this is attributable to these families feeding mainly on small benthic invertebrates while lionfish feed mainly on fish. Although Serranidae species feed on large numbers of fish, the inability to classify food items to the lowest taxonomic level could be the main explanation for the low dietary overlap.
In La Habana, Serranidae had high dietary overlap with lionfish; both were found to mainly feed on fish in this research. The families Haemulidae and Holocentridae exhibited moderate overlap because the fish and crustacean taxa present in the diets of these families coincided with those in the lionfish diet. In contrast, there was low dietary overlap between lionfish and members of the family Lutjanidae. Most fish captured in this locality belonged to the species L. synagris, which had many crustaceans in its diet; this is consistent with the results obtained by Sierra et al. (2001). Thus, the large number of crustaceans present in the food spectrum of L. synagris determines the low dietary overlap between this family and lionfish. Additionally, La Habana is subject to habitat deterioration and high levels of pollution caused by anthropization of the environment (Guardia and González-Sansón, 2000; Alcolado-Prieto et al., 2012); therefore, the diversity of fish species, both predators and prey, and their abundance in the natural environment are lower than in other localities (Betancourt and González- Sansón, 2007; Cobián and Chevalier, 2009).
In Cayo Las Brujas, there was moderate dietary overlap between the family Haemulidae and lionfish. Members of this family mainly feed on small invertebrates, but fish in this locality were caught in seagrasses, where the abundance and diversity of the available species differ from that on reefs. In contrast, in this locality and in Guanahacabibes, there was high dietary overlap between lionfish and members of the family Lutjanidae because most of the captured individuals were Lutjanus apodus and L. griseus, which similar to lionfish, mainly feed on fish.
CONCLUSIONS
Lionfish and members of the families Lutjanidae and Serranidae mainly fed on fish, whereas those of Haemulidae and Holocentridae mainly consumed small benthic invertebrates.
Lionfish size had no influence on diet composition in La Habana.
The degree of dietary overlap between lionfish and native fishes varied according to locality and ecological zone, the availability and abundance of prey in the natural environment and the characteristics of each family.
There was no evidence of trophic competition between lionfish and native fishes, so food availability is not a limiting factor for these groups.
BIBLIOGRAFÍA
Aguilar, C. y G. González-Sansón. 2007. Composición de la ictiofauna costera de Ciudad de La Habana y evaluación preliminar de los factores que la determinan. Rev. Invest. Mar., 28(1): 43-56. [ Links ]
Albins, M. A. 2013. Effects of invasive Pacific red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish communities. Biol. Inv., 15: 29-43. [ Links ]
Albins, M. A. and M. A. Hixon. 2008. Invasive Indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reef fishes. Mar. Ecol. Prog. Ser., 367: 233-238. [ Links ]
Albins, M. A. and M. A. Hixon. 2011. Worst case scenario: potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ. Biol. Fish., Doi 10.1007/s10641-011-9795-1. [ Links ]
Albins, M. A. and P. J. Lyons. 2012. Invasive red lionfish Pterois volitans blow directed jets of water at prey fish. Mar. Ecol. Prog. Ser., 448: 1-5. [ Links ]
Alcolado, M. P., B. Martínez-Daranas, G. Menéndez-Macía, R. del Valle, M. Hernández and T. García. 2003. Rapid assessment of coral communities of María la Gorda, southeast Ensenada de Corrientes, Cuba (part 1: stony corals and algae). 268-277 p. In: Status of Coral Reefs in the Western Atlantic: Results of Initial Surveys, Atlantic and Gulf Rapid Reef Assessment (AGRRA), Judith C. Lang (Ed.). Atoll Research. Bulletin. 496, 630 p. [ Links ]
Alcolado-Prieto, P. 2012. Efectos de agentes estresantes múltiples sobre el reclutamiento de corales pétreos al noroeste de Cuba. Tesis Maestría Cienc. Biol. Mar. Acuicultura, Centro Invest. Mar., Univ. La Habana, Cuba. 111 p. [ Links ]
Alexander, A. K. and J. M. Haynes. 2011. Red lionfish (Pterois volitans) invade San Salvador, Bahamas: No early effects on coral and fish communities. Int. J. Bahamian Stud., 17(2): 50-66. [ Links ]
Benkwitt, C. E. 2014. Non-linear effects of invasive lionfish density on native coral-reef fish communities. Biol. Inv., Doi: 10.1007/s10530-014-0801-3. [ Links ]
Betancourt, C. A. y G. González-Sansón. 2007. Composición de la ictiofauna costera de Ciudad de la Habana, y evaluación preliminar de los factores que la determinan. Rev. Invest. Mar., 28(1): 43-56. [ Links ]
Böhlke, J. E. and C. G. Chaplin. 1968. Fishes of the Bahamas and adjacent tropical waters. Academy Natural Sciences of Philadelphia, 771 p. [ Links ]
Cabrera, E. 2011. Abundancia y dieta de Pterois volitans/miles (Teleostei: Scorpaenidae) en varias localidades de Cuba. Tesis Lic. Biol., Centro Invest. Mar., Univ. La Habana, Cuba. 46 p. [ Links ]
Cabrera-Guerra, D. 2014. Caracterización de la dieta del pez león (Teleostei: Scorpaenidae: Pterois sp.) en cuatro localidades de Cuba. Tesis Lic. Biol., Centro Invest. Mar., Univ. La Habana, Cuba. 78 p. [ Links ]
Chevalier, P. P. y A. L. Cárdenas. 2005. Variación espacio temporal de las asociaciones de peces en arrecifes costeros de la costa oriental de la bahía de Cochinos. I. Abundancia y diversidad. Rev. Invest. Mar., 26(1): 45-57. [ Links ]
Chevalier, P. P. y A. L. Cárdenas. 2006. Estudio diagnóstico del arrecife coralino del Rincón de Guanabo, Ciudad de La Habana, Cuba. Rev. Invest. Mar., 27: 2. [ Links ]
Chevalier, P. P., E. Gutiérrez, D. Ibarzabal, S. Romero, V. Isla y E. Hernández. 2008. Primer registro de Pterois volitans (Pisces: Scorpaenidae) para aguas cubanas. Solenodon, 7: 37-40. [ Links ]
Chevalier, P. P, E. Cabrera, H. Caballero, R. Corrada, A. Fernández, D. Cobián y A. García-Rodríguez. 2014. Distribución, abundancia y relaciones ecológicas del pez león (Pterois volitans/miles: Scorpaenidae) en Cuba. Proc. Gulf Carib. Fish. Inst., 66: 178-179. [ Links ]
Claro, R. y D. R. Robertson. 2010: Los peces de Cuba (CD-ROM). La Habana, Cuba. Instituto de Oceanología de Cuba, CITMA. ISBN. 978-959-298-019-8. [ Links ]
Cobián, D. y P.P. Chevalier. 2009. Evaluación de las asociaciones de peces de los arrecifes coralinos del Centro Internacional de Buceo María la Gorda, Parque Nacional Guanahacabibes, Cuba. Rev. Invest. Mar., 1(009): 111-125. [ Links ]
Cobián, D., R. Claro, P. C. Chevalier, S. Perera y H. Caballero. 2011. Estructura de las asociaciones de peces en los arrecifes coralinos del Parque Nacional Guanahacabibes, Cuba. Rev. Mar. Cost., 3: 153-169. [ Links ]
Cobián, D. , P. Chevalier, J. J. Schmitter-Soto, R. I. Corrada, H. Salvat, E. Cabrera, A. García, A. Fernández Osorio, L. Espinosa, D. Cabrera Guerra, L. M. Pantoja, H. Caballero and S. Perera. 2016. Biology and ecology of lionfish (Pterois volitans) in Guanahacabibes National Park. Aquat. Biol., 24: 219-226. [ Links ]
Côté, I. M., S. J. Green and M.A. Hixon. 2013. Predatory fish invaders: Insights from Indo-Pacific lionfish in the western Atlantic and Caribbean. Biol. Conserv., 164: 50-61. [ Links ]
Crooks, J. A. 2002. Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos, 97: 153-166. [ Links ]
Cure, K., C. E. Benkwitt, T. L. Kindinger, E. A. Pickering, T. J. Pusack, J. L. Mcilwain and M. A. Hixon. 2012. Comparative behavior of red lionfish Pterois volitans on native Pacific versus invaded Atlantic coral reefs. Mar. Ecol. Prog. Ser., 467: 181-192. [ Links ]
Dahl, K. A. and W. F. Patterson. 2014. Habitat-specific density and diet of rapidly expanding invasive red lionfish, Pterois volitans, populations in Northern Gulf of Mexico. PLoS ONE, 9(8), e105852, Doi:10.1371/journal.pone.0105852. [ Links ]
García, A. 2015. Relación de Pterois volitans/miles (Teleostei: Scorpaenidae) con la ictiofauna de arrecifes del oeste de La Habana, Cuba. Tesis Maestría Cienc. Biol. Mar. Acuic., Centro Invest. Mar., Univ. La Habana, Cuba. 138 p. [ Links ]
Gómez, O. 1980. Sistemática de los brachiuros (Crustacea, Decapoda, Brachiura) de Cuba. Tesis Doct. Cienc. Biol., Centro Invest. Mar., Univ. La Habana, Cuba. 240 p. [ Links ]
González-Ferrer, S., H. Caballero, P. M. Alcolado, A. Jiménez, F. Martín, y D. Cobián. 2007. Diversidad de corales pétreos en once sitios de buceo recreativo de "María la Gorda", Cuba. Rev. Invest. Mar., 28(2): 121-130. [ Links ]
Gotera, G. G. 2005. Buceando en Cuba/ Diving in Cuba. Ediciones Niocia, S. L. Catalunya. Barcelona. [ Links ]
Green, S.J. and I.M. Côté. 2010. Consumption potential of invasive lionfish (Pterois volitans) on Caribbean coral reefs. Proc. Gulf Carib. Fish. Inst., 358- 359. [ Links ]
Green, S. J., J. L. Akins and I.M. Côté. 2011. Foraging behaviour and prey consumption in the Indo-Pacific lionfish on Bahamian coral reefs. Mar. Ecol. Prog. Ser., 433: 159-167. [ Links ]
Green, S. J., J. L. Akins, A. Maljkovic and I. M Côté. 2012. Invasive lionfish drive Atlantic coral reef fish declines. PLoS ONE, 7(3), e32596, Doi:10.1371/journal.pone.0032596. [ Links ]
Guardia, E. y G. González-Sansón. 2000. Asociaciones de corales, gorgonias y esponjas del sublitoral habanero al oeste de la bahía de La Habana. II Índices ecológicos. Rev. Invest. Mar., 21: 9-16. [ Links ]
Guitart, D. 1974. Sinopsis de los peces marinos de Cuba, Volumen 1. Academia de Ciencias de Cuba, Instituto de Oceanología, 881 p. [ Links ]
Guitart, D. 1975. Sinopsis de los peces marinos de Cuba, Volumen 2. Academia de Ciencias de Cuba, Instituto de Oceanología, 881 p. [ Links ]
Horn, H. 1966. Measurement of "overlap" in comparative ecological studies. Am. Nat., 100: 419-424 p. [ Links ]
Hyslop, E. J. 1980. Stomach contents analysis. A review of the methods and their application. J. Fish. Biol., 7(4): 411-430. [ Links ]
Mantel, N. 1967. The detection of disease clustering and a generalized regression approach. Cancer Res., 27: 209-220. [ Links ]
Martínez-Daranas, B.R. 2007. Características y estados de conservación de los pastos marinos en áreas de interés del archipiélago Sabana-Camagüey, Cuba. Tesis Doct. Cienc. Biol., Centro Invest. Mar., Univ. La Habana, Cuba. 136 p. [ Links ]
Martínez-Iglesias, J.C. y O. Gómez. 1986. Los crustáceos decápodos del Golfo de Batabanó. Brachyura. Poeyana, 332: 1-91. [ Links ]
McCleery, C. 2011. A comparative study of the feeding ecology of invasive lionfish (Pterois volitans) in the Caribbean. J. Mar. Sci., 9: 38-43. [ Links ]
Metropolis, N. and S. Ulam. 1949. The Monte Carlo Method. J. Am. Stat. Assoc., 44: 247. [ Links ]
Molnar, J.L., R.L. Gamboa, C. Revenga and M.D. Spalding. 2008. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ., 6(9): 485-492. [ Links ]
Morris, J.A. 2013. La invasión del pez león: pasado, presente y futuro. In: El pez león invasor: guía para su control y manejo (1-2), Gulf and Caribbean Fisheries Institute, Special Publication Series Number 2, Marathon. [ Links ]
Morris, J.A. and J.L. Akins. 2009. Feeding ecology of invasive lionfish (Pterois volitans) in the Bahamian archipelago. Environ. Biol. Fish, 86: 389-398. [ Links ]
Morris, J. A. y S. J. Green. 2013. Las investigaciones sobre el pez león: resultados alcanzados y cuestiones pendientes. In J.A. Morris Jr. (Eds.), El pez león invasor: guía para su control y manejo (3-15), Gulf and Caribbean Fisheries Institute, Special Publication Series Number 2, Marathon, EE.UU. [ Links ]
Morris, J. A., J. L. Akins, A. Barse, D. Cerino, D. W. Freshwater, S. J. Green, R. C. Muñoz, C. Paris and P. E. Whitfield. 2009. Biology and ecology of the invasive lionfishes, Pterois miles and Pterois volitans. 409-414. [ Links ]
Muñoz, R. C., C. A. Currin and P. E. Whitfield. 2011. Diet of invasive lionfish on hard bottom reefs of the Southeast USA: Insights from stomach contents and stable isotope. Mar. Ecol. Prog. Ser., 432: 181-193. [ Links ]
Ortiz, M., R. Lalana y C. Varela. 2012. Guía ilustrada para la identificación de los camarones comerciales (Decapoda, Dendrobranchiata, Penaeoidea) de Cuba. Rev. Cub. Cien. Biol., 1 (1): 49-69. [ Links ]
Pinkas, L., M. S. Oliphant and J.L.K. Iverson. 1971. Food habits of albacore, Bluefin tuna and bonito in California waters. California. Fish. Bull., 152: 1-105. [ Links ]
Sierra, L.M., R. Claro y O.A. Papova. 1994. Ecología de los peces marinos de Cuba. In R. Claro (Eds.). Alimentación y relaciones tróficas Inst. Oceanol. y CIQRO, México. [ Links ]
Sierra, L.M., R. Claro and O.A. Papova. 2001. Ecology of the marine fishes of Cuba. In R. Claro, K. C. Lindeman and L. R. Parenti. (Eds.) Trophic biology of the marine fishes of Cuba. Smithsonian Institution Press, Washington. [ Links ]
Valdez-Moreno, M., C. Quintal-Lizama, R. Gómez-Lozano and M. C. García-Rivas. 2012. Monitoring an alien invasion: DNA barcoding and the identification of lionfish and their prey on coral reefs of the Mexican Caribbean. Plos One, 7(6): 1-8. [ Links ]
Whitfield, P.E., J.A. Hare, A. W. David, S. L. Harter, R. C. Muñoz and C. M. Addison. 2007. Abundance estimates of the Indo-Pacific lionfish Pterois volitans/miles complex in the western North Atlantic. Biol. Inv., 9: 53-64. [ Links ]
Received: March 14, 2017; Accepted: August 29, 2017











 text in
text in