Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.46 no.2 Santa Marta July/Dec. 2017
https://doi.org/10.25268/bimc.invemar.2017.46.2.731
Research Articles
Invasion of a native polychaete in an Eastern Tropical Pacific island
1 Departamento de Biología, Universidad del Valle. Calle 13 No. 100-00, A.A. 25360, Cali, Colombia. sprc392@gmail.com, wilmar.alexander.torres@correounivalle.edu.co, lizeth.lopez.molina.b@gmail.com, andresjlopez25@gmail.com, edgardo.londono@correounivalle.edu.co
Fluctuations in population dynamics, like demographic expansions and invasions, are relatively common in ecosystems, and in certain cases may affect biodiversity and a suite of other ecological attributes. In this paper, we report the appearance and population explosion of the reef-building polychaete (Sabellariidae) Idanthyrsus cf. cretus in Gorgona Island (Eastern Tropical Pacific), describing some ecological characteristics (abundance and coverage). The survey was carried out in three study areas of Gorgona Island, located in the Colombian Pacific. Sampling was performed randomly at low, mid and high intertidal levels, in order to measure density and coverage. Density was measured randomly in three study areas at low, mid and high intertidal levels collecting samples (N=37) of 100 cm2 from the colony. Coverage was measured using random transects (N=21) per locality and intertidal levels (20 m length × 1 m width). A total of 1,904 I. cf. cretus were collected with a mean density of 73 ind./100 cm2. Coverage was statistically different between intertidal zones, with the highest values in the mid-intertidal level (11%). Differences in coverage of I. cf. cretus colonies among study areas are probably due to differing intertidal physical characteristics: the availability of adequate substrate and building materials in the study areas sediments, which in turn might affect abundance and colony size. Suitable substrate and construction material might have favored the rapid spreading and local invasion of this species.
Keywords: Demographic explosion; Biological engineer; Invasion; Idanthyrsus
Las fluctuaciones en la dinámica de poblaciones como las explosiones demográficas e invasiones, son relativamente comunes en los ecosistemas, y en ciertos casos pueden afectar la biodiversidad y otra serie de atributos ecológicos. En este artículo, reportamos la aparición y explosión demográfica de Idanthyrsus cf. cretus (Chamberlin, 1919), un poliqueto constructor de arrecifes y describimos algunas de sus características ecológicas (abundancia y cobertura). Este estudio fue llevado a cabo en tres localidades de la Isla Gorgona, ubicada en el Pacífico colombiano. El muestro se realizó de forma aleatoria en los niveles intermareales bajo, medio y alto con el fin de medir la densidad poblacional y la cobertura. La densidad fue medida utilizando muestras de 100 cm2 de área de la colonia (N=37), mientras que la cobertura fue medida utilizando transectos aleatorios por localidad y nivel intermareal (20m largo x 2m ancho). Un total de 1904 individuos fueron colectados. La densidad media (73 ind./100cm2) fue estadísticamente similar entre las localidades. La cobertura fue estadísticamente diferente entre las zonas intermareales, reportando los valores más altos en el intermareal medio (11%). Las diferencias de cobertura de las colonias de I. cf. cretus entre las localidades se deben probablemente a las características físicas: disponibilidad adecuada de sustrato y sedimentos, los cuales pueden afectar la abundancia y el tamaño de la colonia. El sustrato y los materiales de construcción apropiados pueden favorecer una propagación rápida, llevando a la invasión local.
Palabras clave: Explosión demográfica; Ingenieros biológicos; Invasión; Idanthyrsus
INTRODUCTION
Ecological attributes, such as competition, predation, recruitment, colonization and species distribution in natural ecosystems might fluctuate over time and space (Wallentinus and Nyberg, 2007; Quintana et al., 2013). In addition, habitats become susceptible to ecological invasions by native and non-native species (Valéry et al., 2008). Furthermore, it has been demonstrated that native species can also exhibit demographic explosions, occupy wider ranges, and show an invasive behavior as a result of natural and human perturbations. Even though, “native invasive species” can cause several problems in ecosystems (e.g. ecosystem dominance, local extinctions and biodiversity reduction) (Parsons and Lalli, 2002; Olden, 2006; Valéry et al., 2009a, 2009b; Carey et al., 2012; Rilov et al., 2012), they have been poorly documented worldwide (Valéry et al., 2008, 2009a; Buczkowski, 2010; Carey et al., 2012; Simberloff et al., 2013).
Population explosions can be defined as a sudden increase in the number of individuals of a given species (Hawley, 1982), producing an imbalance in species abundances, decrease in local resources and modification of landscape features (Morelle et al., 2016). Native invasive species show the same rapid expansion behavior as foreign invasive species (Valéry et al., 2009b; Morelle et al., 2016). Therefore, it is important to investigate which are the causes influencing population explosions, and the consequences of high abundances of one species to ecosystems. Conversely, when an invasive species is considered an ecosystem engineer (Bazterrica et al., 2012), it can help improving an ecosystem, changing the structure of the actual habitat, modifying the physical conditions and modulating the availability of biotic and abiotic resources for other species (Jones et al., 1994; Barrios et al., 2009; Bazterrica et al., 2012, 2014). For example, ecosystem engineers can easily create new accessible habitats, prevent recruitment of other species or increase their own dispersal activity and reproduction within the new conditions they are creating (Cuddington and Hastings, 2004; Ruesink et al., 2006; Lambrinos and Bando, 2008). In general, the majority of previous studies have focused on foreign species invaders (Luppi and Bas, 2002; Parsons and Lalli, 2002; Bazterrica et al., 2012, 2014; Rilov et al., 2012; Quintana et al., 2013; Urban-Malinga et al., 2013; Shumka et al., 2014; Jaubet et al., 2015). However, reports of native invasive species in marine ecosystems are scarce (Valéry et al., 2009a; Buczkowski, 2010; Carey et al., 2012). Intertidal zones may be highly susceptible to invasion by a suite of different organisms, including reef-building polychaetes, bivalves, sea grasses, mussels and crabs (Bruschetti et al., 2009), due to their inherent conditions such as regular water motion, nutrients load and suitable substrates. The Colombian Pacific coast has a meso to macrotidal range (Ramírez-Martínez et al., 2016) that makes it particularly prone to biological invasions.
Polychaetes are a highly diverse common group in intertidal areas (Bouchet, 2006; Appeltans et al., 2012; Valencia et al., 2014). Sabellarids (honey-comb worms) can produce hard tubes to dwell and some groups may form colonies creating hard reef-like structures (Pawlik, 1988). Previous studies have reported the distribution of reef building polychaetes in the Pacific Ocean (Barrios et al., 2009; Bastida-Zavala and García- Madrigal, 2012; Alalykina, 2013): For example, Barrios et al. (2009) studied the distribution of Idanthyrsus cretus in Las Perlas Archipelago (Panama) and Gómez et al., (1997) reported the presence of this species in Oaxaca, Mexico. However, there are no studies in the Colombian Pacific coast concerning density and coverage of this polychaete. For example, Laverde-Castillo (1989) reported sabellarids, such as Phragmatopoma attenuata, I. cretus and I. armatus, in Bahia Cobita, Bahia Octavia, Ensenada de Utria and Gorgona Island. Although he did not measure coverage, that study do not report more than 11 individuals. In addition, previous studies of I. cretus [either when reported as I. pennatus in Monro (1933), Fauchald (1977) and Prahl (1979), or recently as I. cretus by Kirtley (1994) and Jaubet et al. (2015)] have found few individuals hardly building big colonies. According to this information, about four or five individuals were living in hard-single tubes, and several of them were together and attached to the stones. Posterior reports (Kirtley, 1994; Jaubet et al., 2015), agreed with this observation.
Idanthyrsus cretus Chamberlin (Sabellariidae), is a reef-building polychaete usually found in intertidal areas exposed to coastal currents and surf. Idanthyrsus cretus can also be found at intermediate depths below water on the continental platform associated with hard corals. These environments are perfect for the development of this polychaete due to the regular interchange of sand and sediments, allowing them to construct their tubes and colonies (Kirtley 1994; Bastida-Zavala and Becerril- Tinoco, 2009). This specie as other Sabellarids are filtering animals that feed on plankton, organic detritus, little parts of crustaceans and algae (Bastida-Zavala and Becerril- Tinoco., 2009). Usually, other organisms are associated with sabellarids reefs (Barrios et al., 2009) and with other families of polychaetes (Luppi and Bass, 2002; Cuddington and Hasting, 2004), using these colonies as shelter and food source.
Bastida-Zavala and Becerril-Tinoco (2009) also mentioned that little is known about phylogenetic relationships between sabellarids species. According to first reports of these polychaetes made by Monro (1933), Hartman (1940), Fauchald (1977) and Prahl (1979) at Gorgona Island, this polychaete was considered as I. pennatus. However, after taxonomic revision of sabellarids by Kirtley (1997), I. pennatus was corrected as I. cretus. So, according to bibliographic references, all previous reports correspond to I. cretus today. Even though there is no a taxonomic paper describing Idanthyrsus cretus yet in the Colombian Pacific coast, to identify correctly this species in our study we consulted an expert to corroborate its identification.
In this paper, we report for the first time, information about ecological attributes, such as coverage and density, of the recent population outbreak of the reef- building polychaete Idanthyrsus cf. cretus (Sabellariidae) at three localities in Gorgona Island, southern Colombian Pacific. This study is the first report of I. cf. cretus building colonies and creating reef-like formations in Gorgona Island.
STUDY AREA
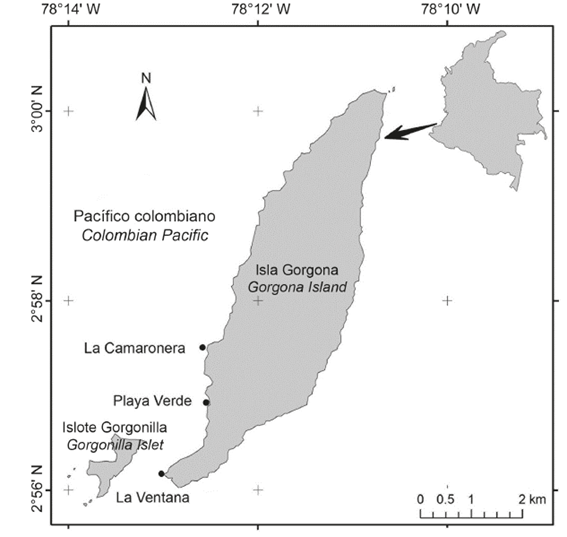
The Colombian Pacific basin is formed by sedimentary and igneous rocks deposited in ancient geological periods produced by volcanic eruptions (Cantera et al., 1998). Gorgona Island (2º58’ N - 78º11’ W), located 35 km off the coast in the Colombian Pacific basin, holds a variety of coastal and shallow water ecosystems like sand beaches, coral reefs and rocky reefs (Valencia et al., 2014), making it one of the highest biodiversity areas in the Colombian Pacific (Cantera et al., 1995; Díaz and Acero, 2003). Density and coverage of I. cf. cretus were measured at high (HI), middle (MI) and low (LI) intertidal zones of three areas (La Camaronera, Playa Verde and La Ventana) southwest Gorgona Island (Fig. 1). La Camaronera and Playa Verde are two rocky beaches composed by sand, gravel and some large intermingled blocks (high and middle intertidal zones); in addition, boulders, blocks, bare rock and tide pools can also be found (low intertidal zone). In contrast, La Ventana is a gently sloping rocky platform with large blocks (high intertidal), boulders (middle intertidal) and tide pools, bare rock and gravel (low intertidal).
MATERIALS AND METHODS
Density estimation
Before sampling, qualitative observations were made at each intertidal zone within each locality in order to describe colonies conforming to polychaete reefs, and the associated biota (e.g. algae, mollusks and crabs). Pictures were taken to better document and describe the study area, and some samples were collected to be stored at Universidad del Valle Museum (Cali, Colombia). At each area and intertidal zone (HI, MI and LI), a sample of 100 cm2 in the surface area of I. cf. cretus colonies were randomly sampled (N=37) by chisel and hammer. To make sure each colony was fully sampled we dig until the rock base was found; hence, volumes differed depending on colony development. For data analysis, area instead of volume was used. Colonies were carefully moldered in order to remove individuals, and only polychaete heads were counted to prevent over- estimation.
Coverage estimation
Seven random transects (20 m length × 1 m width) per area of study and intertidal zone were set along the coastline. 21 transects in total for each locality, resulting in a total of 63 study transects. Each transect consisted of a grid of 100 points separated every 5 cm in a quadrant of 50 cm. We used this grid on both sides of the transect to get 1 m of width (200 points), resulting in 4,000 points under which data on colony presence/absence was recorded (200 points×20 m).
Data Analysis
Density analyses (numbers of individuals per 100 cm2) considered only areas. Intertidal zones were excluded for the following reasons: 1) no colonies large enough to sample were found at HI and LI zones in Playa Verde and La Camaronera, and 2) when comparing intertidal zones for La Ventana, no significant differences were found; therefore, density is considered to be homogeneous in the three zones of this studied area. A generalized linear model with a quasi-Poisson probability distribution was used to assess the effects of locality on polychaete density, including the over-dispersion of data (Bilder and Loughin, 2014).
Coverage (absence/presence data) was assessed using a generalized linear model with a quasi-binomial probability distribution. The adjusted model included study area and intertidal zone as factors. It was necessary to remove the interaction between factors due to the presence of zeroes; therefore, factors were analyzed independently. All statistical analyses were conducted in R version 3.2.2 (R Core Team, 2015).
RESULTS
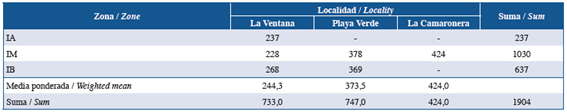
A total of 1,904 Idanthyrsus cf. cretus individuals (Fig. 2a-b) were collected from colonies found in the studied areas of Gorgona Island (Fig. 2c-e). These individuals were present in all intertidal zones in La Ventana. In Playa Verde, I. cf. cretus was present only in the MI and LI zones, and in La Camaronera only in the MI zone. The general lowest abundance was observed at HI and the highest at MI (Table 1). The MI at La Camaronera alone accounted for 22.3% (424 individuals) of the total number of individuals. The weighted mean of the total number of individuals per studied area showed the highest value at La Camaronera and the lowest at La Ventana. However, using the dispersion index (I), the number of individuals collected at La Ventana and Playa Verde were almost similar and higher than La Camaronera. Furthermore, it was found that La Ventana shows a random distribution pattern , (p=0.165) while in Playa Verde and La Camaronera a patchy distribution was observed (p≪0.001).
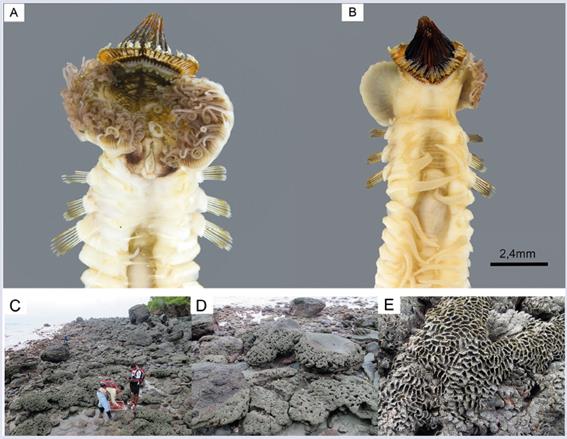
Figure 2 Ventral (A), dorsal (B), landscape general view (C), colony morphology (D) and colony close-up (E) of Idanthyrsus cf. cretus sampled at Gorgona Island.
Table 1 Total number of Idanthyrsus cf. cretus individuals per area of study and intertidal level at Gorgona Island.
Coverage at Playa Verde and La Camaronera was higher at MI zones, which reflected the general observed coverage pattern (Table 2). In addition, coverage at Playa Verde was the highest, compared to any other locality. Indeed, it was 1.6 and 2.0 times higher than La Camaronera and La Ventana, respectively (See table 1, Fig. 3). Moreover, the effect of intertidal zone on coverage was even more remarkable because coverage at MI was as much as 47.5 and 5.0 times higher than La Ventana and La Camaronera, respectively (See table 1, Fig. 4).
Table 2 Colony coverage (%) of Idanthyrsus cf. cretus per area of study and intertidal level at Gorgona Island.
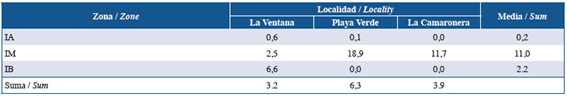

Figure 3 Total mean coverage (+SD) of I. cf. cretus colonies in (a) each study area, and (b) intertidal zone at Gorgona Island.
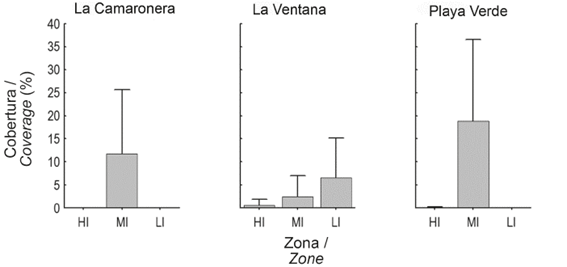
Figure 4 Mean coverage (+SD) of I. cf. cretus colonies for each study area in every intertidal level at Gorgona Island.
Studied areas had no significant effect on coverage (Fig. 3a). However, Playa Verde was the locality with the highest mean coverage, followed by La Camaronera and La Ventana (Fig. 3a). Statistical differences between intertidal zones were found . The high coverage at MI levels were significantly different to LI ( and HI levels while LI and HI were not statistically different between them ( (Fig. 3b). Although the interaction (locality × intertidal zone) was not included in the model, the aggregation of I. cf. cretus in the MI of Playa Verde and La Camaronera was evident. On the other hand, La Ventana showed an increasing tendency in coverage, from the lowest in the HI to the highest in the LI level (Fig. 4).
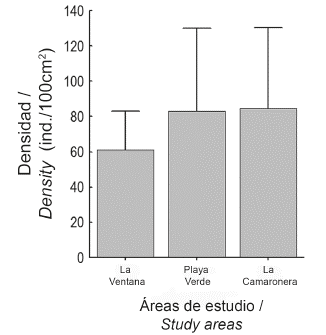
Mean density (ind./100cm2±SD) between studied areas was similar . However, the locality with the lowest mean density was La Ventana , while Playa Verde and La Camaronera had higher and similar densities (Fig. 5). These two localities also showed wider data dispersion (see also dispersion index above), which could have affected statistical results. In addition, density between intertidal levels was evaluated separately and only at La Ventana and Playa Verde. There were no statistical effects on I. cf. cretus density between levels neither at La Ventana nor at Playa Verde .
DISCUSSION
Reports of I. cf. cretus at Gorgona Island are rare. To the best of our knowledge, there are only reports of a few individuals made by Monro (I. pennatus and I. armatus, 1933). However, Hartman (1940), Fauchald (1977) and Prahl (1979) cited these reports in their studies, while Kirtley (1994) described I. pennatus as I. cretus. So, there are no more published reports of this specie besides the studies mentioned here, or reported colonies prior to ca. 2013. During our constant trips to the island (at least twice a year), we were unable to observe any signs of presence neither individuals nor colonies. Hence, it was evident that these colonies suddenly appeared along the coasts of La Ventana, La Camaronera and Playa Verde. Given the previous reasoning, we are confident to inform that there were no published reports of colonies prior to ca. 2013 (or even before). Therefore, our results showed high coverage and density of I. cf. cretus, suggesting that there has been a sudden outbreak in the demography of this species in the island at least since 2014.
As studies about density and coverage of Idanthyrsus cf. cretus (Sabellaridae) are scarce, it was difficult to compare our results with other geographic areas. However, I. cf. cretus reefs surveyed at Gorgona are similar in structure and general characteristics to those reported in areas nearby. For instance, Barrios et al. (2009) reported a density in Las Perlas, Panama of 72 ind./100cm2 (although in the results they report densities per 10 cm2, we firmly believe they are referring to 100 cm2 since in their methodology they state the use of 10×10 cm quadrats), a density almost equal to our results (73 ind./100cm2). In addition, Gómez et al (1997) also found this species in two of five sampled bays of Oaxaca, Mexico (La Entrega and Puerto Angel), but its density was very low compared to other sabellarids reported in this study (six individuals in 40 transects of 5 m×1 m). Reports of the abundance of I. cf. cretus in the Colombian Pacific include few or no information about density estimations (only presence of species). In general, polychaete reports for the Colombian Pacific coast are incomplete or do not have specific details about morphology (Salazar-Vallejo and Londoño-Mesa, 2004). Moreover, Londoño-Mesa (2017) also said that, in general, polychaetes in the Colombian Pacific remain little studied. Concerning the Colombian Pacific, our results provide important details about possible changes in the population ecology of this polychaete in Gorgona Island that demand further investigation.
Various organisms associated to this polychaete (e.g. algae, mollusks, crustaceans and other polychaetes) were also observed during our sampling expeditions. These organisms have already been reported to inhabit other reef-building polychaetes (Luppi and Bas, 2002; Cuddington and Hastings, 2004; Barrios et al., 2009; Bruschetti et al., 2009). Reefs provide an array of microhabitats (Hunter and Sayer, 2009) that are used by different organisms, this is the case of the colonies formed by I. cf. cretus; while this is true, ecosystem engineers (e.g. reef-building polychaetes; see Jones et al., 1994) tend to, rapidly, modify their habitat altering ecosystem functioning (Schwindt et al., 2001; Bruschetti et al., 2009). As a consequence, this might have severe negative or positive effects on community components (e. g. richness and diversity). Gribben et al., 2013, explains that ecosystem engineers can reduce population growth and dispersion of other organisms, especially invertebrates. Even though our knowledge about this reef-building polychaete is very poor, and we did not take any formal data of other invertebrates (e.g. mollusk, crabs), our results suggested that this apparent dominance in most mid to low intertidal levels of the Island (table 1 and figure 3), could change the development rates of other organisms as the colonies disperse and grow. In addition, ecosystem engineers can negatively affect the infaunal community components decreasing their number of individuals and taxa as well (Gribben et al., 2013).
The presence and coverage of Idanthyrsus cf. cretus colonies were different between localities and tidal zones. We hypothesize that this is due to physical characteristics where the colonies are constructed, including the slope, type and heterogeneity of substrate, wave energy, and sediment transport (Pawlik et al., 1991; Voulgaris et al., 1998; Barrios et al., 2009). The bottom portion of the MI in Playa Verde and La Camaronera is formed by large boulders and blocks that provide suitable places where worms can safely settle and grow (cracks, pits and the undersides of rocks). Also, there is a constant supply of construction material (sand and cracked shells and corals) produced by the strong surf that puts in motion sediments from beaches at the high intertidal, allowing colonies to grow thicker. The absence of suitable substrates at the high intertidal range of Playa Verde and La Camaronera hindered the presence of colonies. Even though we observed a different pattern in La Ventana where colonies were found at all intertidal levels (Fig. 4), those colonies were thin and less abundant. This could be due to the gently sloping platform of the substrate with only small rocks and gravel present. As the largest sand beach of the island is located next to this area, this might restrict the sand supply for the formation of colonies. In addition, the presence of a smaller island (Gorgonilla Islet) located in front of this locality and separated only for a narrow channel, might serve as a barrier to water motion impacting the shore and recirculating the sediments. In general, although conditions at La Ventana can be severe for the proper formation of colonies, these conditions (e.g. humidity, sand supply) in LI are slightly more suitable than in HI and MI. As a consequence, density and coverage were higher at this intertidal level (LI) at La Ventana. Another possible explanation for the difference in abundance and colony sizes and the fact that density of polychaetes did not display any statistical differences between intertidal levels could be the dispersion pattern of I. cf. cretus in La Camaronera and Playa Verde. The demographic explosion seemed to begin at these sites because density and coverage of colonies were higher in these localities and had a random distribution pattern. On the other hand, La Ventana had a patchy distribution pattern, and colonies and density values were poor compared to the other sites. Therefore, these results may be suggesting that the colonization and population explosion began at these two localities (Playa Verde and La Camaronera) and is underway to La Ventana. It is known that reef- building sabellarids like I. cf. cretus expand through marine habitats depending on suitable conditions and marine currents, but this process takes time (e.g. Cuddington and Hastings, 2004). Cuddington and Hastings (2004) also described that biological engineer’s invasions occur in two phases: 1) when the species just settles and its growth is very slow (as it is happening at La Ventana), and 2) when the expansion becomes explosive (as it has already happened at La Camaronera-Playa Verde).
CONCLUSIONS
We are reporting for the first time a notable demographic expansion of I. cf. cretus (Sabellariidae) for Gorgona Island. Our results are in concordance with reports of I. cf. cretus elsewhere (Barrios et al., 2009), where these polychaetes have colonized benthic environments with high levels of water motion and bare rocks. Idanthyrsus cf. cretus might be modifying intertidal zones, due to its rapid spreading and high coverage, monopolizing space, therefore, affecting biodiversity. Ecosystem engineers, like polychaetes, can serve as host for other intertidal species (Gutierrez et al., 2003; Bruschetti et al., 2009). Even though we only measured abundance and coverage for this polychaete, several individuals of other species (e.g. small crabs, mollusks and other crustaceans) were widely observed either inside or outside of colonies. So, the effect of this quick invasion on the local biodiversity is uncertain and need to be further tested. In addition, it is important to acknowledge that this polychaete has been previously found inhabiting coral reefs, even in Gorgona Island (Monro, 1933); Gorgona seems to be a unique and proper place for polychaetes to settle given its water temperature conditions (Monro, 1933). So, since coral reefs are under marginal conditions at this Island, it is important to keep monitoring this native invading species, which could be a possible threat due to its dominance and rapid spreading (Barrios et al., 2009). Most importantly, as there are no other studies reporting the sudden growth and spreading of I. cf. cretus, it is necessary to further investigate this specie in the Colombian Pacific coasts in order to get conclusive remarks on this hypothesis. Specifically, it is needed to also study water motion and currents, sediment content, temperature, etc. as these factors are essential for the development and growth of Idanthyrsus species (Barrios et al., 2009). Thus, this might help explain what changes occurred since the last report from Monro (1933) and what could cause the sudden invasion of this native species to develop colonies of these sizes 80 years after.
ACKNOWLEDGEMENTS
We would like to thank the Universidad del Valle for supporting our field activities during the Marine Population Ecology course, which allowed us to conduct field work and collect the necessary data for this study. We want to thank J. Cupitra for his assistance during field work, to Dr. J. Tavera for the revision of a previous draft and to Dr. G. Toro-Farmer for the correction and improvement of English grammar in the final version. We would also like to thank the personnel at National Natural Park of Gorgona for allowing this research, especially M.X. Zorrilla and L. Payan. This research was not supported by any specific grant from funding agencies in the public, commercial, or not-for-profit sectors; hence, the authors declare that they have no conflict of interest.
REFERENCES
Alalykina, I. 2013. Preliminary data on the composition and distribution of polychaetes in the deep-water areas of the north-western part of the Sea of Japan. Deep-Sea Res. Part II: Top. Stud. Oceanogr., 86-87: 164-171. [ Links ]
Appeltans, W., S.T. Ahyong, G. Anderson, M.V. Angel, T. Artois, N. Bailly, R. Bambe, A. Barber, I. Bartsch, P Bock, G. Boxshall, C.B. Boyko, N.L. Bruce, S.D. Cairns, T. Chan, M. Curini-Galletti, F. Dahdouh-Guebas, W. Decock, S. De Grave., N.J. De Voogd, A. Gittenberger, S. Gofas, L. Go, D.P. Gordon, M.D. Guiry, F. Hernandez, B.W. Hoeksema, R.M. Kristensen, A. Kroh, M. Longshaw, J. Lowry and E. Macpherson. 2012. The magnitude of global marine species diversity. Curr. Biol., 22: 1-14. [ Links ]
Barrios, L.S., N. Chambers, H. Ismail, J.M. Guzman and J.M. Mair. 2009. Distribution of Idanthyrsus cretus (Polychaeta: Sabellariidae) in the Tropical Eastern Pacific and application of PCR-RAPD for population analysis. Zoosymposia, 2(May): 487-503. [ Links ]
Bastida-Zavala, R. and S. García-Madrigal. 2012. First record in the Tropical Eastern Pacific of the exotic species Ficopomatus uschakovi (Polychaeta, Serpulidae). ZooKeys, 238: 45-55. [ Links ]
Bazterrica, M.C., F. Botto and O. Iribarne. 2012. Effects of an invasive reef-building polychaete on the biomass and composition of estuarine macroalgal assemblages. Biol. Inv., 14(4): 765-777. [ Links ]
Bazterrica, M.C. , C.M. Bruschetti, M.F. Alvarez, O. Iribarne andF. Botto. 2014. Effects of macroalgae on the recruitment, growth, and body condition of an invasive reef forming polychaete in a south-western Atlantic coastal lagoon. J. Sea Res., 88: 121-129. [ Links ]
Bilder, C.R. and T.M. Loughin. 2014. Analysis of categorical data with R. CRC Press Taylor & Francis Group. [ Links ]
Bouchet, P. 2006. The magnitude of Marine biodiversity. In: C.M. Duarte (Ed.). The exploration of marine biodiversity scientific and technological challenges. Fundación BBVA. París, 64 p. [ Links ]
Bruschetti, M., C. Bazterrica, T. Luppi and O. Iribarne. 2009. An invasive intertidal reef-forming polychaete affect habitat use and feeding behavior of migratory and locals birds in a SW Atlantic coastal lagoon. J. Exp. Mar. Biol. Ecol., 375(1-2): 76-83. [ Links ]
Buczkowski, G. 2010. Extreme life history plasticity and the evolution of invasive characteristics in a native ant. Biol. Invasions, 12(9): 3343-3349. [ Links ]
Cantera, J.R. 1995. Biodiversidad en el ecosistema de acantilados rocosos en el Pacífico colombiano: 195-208. En: Cantera J.R. y J.D. Restrepo (Eds.). Delta del Río San juan, Bahías de Málaga y Buenaventura, Pacífico colombiano. Colciencias, Univ. EAFIT, Univ. Valle, Cali. [ Links ]
Cantera, J., R. Neira y C. Ricaurte. 1998. Bioerosión en la costa Pacífica colombiana: un estudio de la biodiversidad, la ecología y el impacto de los animales destructores de acantilados rocosos sobre el hombre. Fondo Fen Colombia, Bogotá. 89 p. [ Links ]
Carey, M.P., B.L. Sanderson, K.A. Barnas and J.D. Olden. 2012. Native invaders - Challenges for science, management, policy, and society. Front. Ecol. Environ., 10(7): 373-381. [ Links ]
Chamberlin, R.V. 1919. The Annelida Polychaeta of the Albatross Tropical Pacific Expedition, 1891-1905. Mem. Mus. Comp. Zool., 48: 1-514. [ Links ]
Cuddington, K. and A. Hastings. 2004. Invasive engineers. Ecol. Modell., 178(3): 335-347. [ Links ]
Díaz, J.M. and A. Acero P. 2003. Marine biodiversity in Colombia: Achievements, status of knowledge, and challenges. Gayana (Concepción), 67(2): 261-274. [ Links ]
Fauchald, K. 1977. Polychaetes from intertidal areas in Panama, with a review of previous shallow-water records. Smithson. Contrib. Zool., 221 p. [ Links ]
Gómez, P., J.A. Mercado, L.M. Mitchel y S. Salazar-Vallejo. 1997. Poliquetos de fondos duros (Polychaeta) de bahías de Huatulco Puerto Ángel, Oaxaca, México. Rev. Biol. Trop., 45(3): 1067-1074. [ Links ]
Gribben, P.E., J.E. Byers, J.T. Wright and T.M. Glasby. 2013. Positive versus negative effects of an invasive ecosystem engineer on different components of a marine ecosystem. Oikos, 122: 816-824. [ Links ]
Gutierrez, J.L., C.G. Jones, D.L. Strayer and O.O. Iribarne. 2003. Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos, 101(1): 79-90. [ Links ]
Hartman, O. 1940. The polychaetous annelids collected by the Presidential Cruise of 1938. Smithson. Misc., 98: 1-22. [ Links ]
Hawley, H. 1982. Ecology. International encyclopedia of population. New York, N.Y. Free Press. [ Links ]
Hunter, W.R. and M.D.J. Sayer. 2009. The comparative effects of habitat complexity on faunal assemblages of northern temperate artificial and natural reefs. ICES J. Mar. Sci., 66 (4): 691-698. [ Links ]
Jaubet, M.L., G.V. Garaffo, E.A. Vallarino and R. Elías. 2015. Invasive polychaete Boccardia proboscidea Hartman, 1940 (Polychaeta: Spionidae) in sewage-impacted areas of the SW Atlantic coasts: Morphological and reproductive patterns. Mar. Ecol., 36(3): 611-622. [ Links ]
Jones, C.G., J.H. Lawton and M. Shachak. 1994. Organisms as ecosystem engineers. Oikos, 69(3): 373-386. [ Links ]
Kirtley, D. 1994. A review and taxonomic revision of the family Sabellariidae Johnston, 1865 (Annelida: Polychaeta). Sabecon Press, Science Series, Florida. 223 p. [ Links ]
Lambrinos, J.G. and K.J. Bando. 2008. Habitat modification inhibits conspecific seedling recruitment in populations of an invasive ecosystem engineer. Biol. Inv., 10(5): 729-741. [ Links ]
Laverde-Castillo, J.J. 1986. Lista anotada de los poliquetos (Annelida) registrados para el Pacífico colombiano, con notas preliminares sobre su zoogeografía. Actual. Biol., 15(58): 13-22. [ Links ]
Londoño-Mesa, M.H. 2017. Poliquetos de Colombia: un reto para la megadiversidad. Poliquetos de Sudamérica. 71-88 p. [ Links ]
Luppi, T.A. and C.C. Bas. 2002. The role of the invasive polychaete Ficopomatus enigmaticus Fauvel 1923 (Polychaeta : Serpulidae) reefs in the recruitment of Cyrtograpsus angulatus Dana 1851 (Brachyura : Grapsidae), in the Mar Chiquita coastal lagoon, Argentina. Cienc. Mar., 28(4): 319-330. [ Links ]
Monro, C.C.A. 1933. The Polychaeta Sedentaria collected by Dr. C. Crossland at Colón, in the Panama Region, and the Galapagos Islands during the Expedition of the S.Y. 'St. George'. Proc. Zool. Soc. Lond., 2: 1039-1092. [ Links ]
Morelle, K., J. Fattebert, C. Mengal and P. Lejeune. 2016. Invading or recolonizing? Patterns and drivers of wild boar population expansion into Belgian agroecosystems. Agric. Ecosyst. Environ., 222: 267-275. [ Links ]
Olden, J.D. 2006. Biotic homogenization: A new research agenda for conservation biogeography. J. Biogeogr., 33(12): 2027-2039. [ Links ]
Parsons, T.R. and C.M. Lalli. 2002. Jellyfish population explosions: Revisiting a hypothesis of possible causes. La Mer, 40(3): 111-121. [ Links ]
Pawlik, J.R. 1988. Larval settlement and metamorphosis of Sabellariid Polychaetes, with special reference to Phragmatopoma lapidosa, a reef-building species, and Sabellaria floridensis, a non-gregarious species. Bull. Mar. Sci., 43(1): 41-60. [ Links ]
Pawlik, J.R., C.A. Bumani, V.R. Starczak, C.A. Butman and V.R. Starczak. 1991. Hydrodynamic facilitation of gregarious settlement of a reef-building tube worm. Science, 251(4992): 421-424. [ Links ]
Prahl, H.v., F. Guhl y M. Grögl. 1979. Poliquetos de Gorgona: 131-140. En: H. V. Pral., F. Guhl y M. Grögl (Eds.). Gorgona. Futura, Bogotá. [ Links ]
Quintana, C.O., E. Kristensen and T. Valdemarsen. 2013. Impact of the invasive polychaete Marenzelleria viridis on the biogeochemistry of sandy marine sediments. Biogeochemistry, 115(1-3): 95-109. [ Links ]
R Development Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org . 16/06/2015. [ Links ]
Ramirez-Martínez, G.A., G.A. Castellanos-Galindo and U. Krumme. 2016. Tidal and diel patterns in abundance and feeding of a marine-estuarine-dependent fish from macrotidal mangrove creeks in the Tropical Eastern Pacific (Colombia). Estuaries Coast, 39(4): 1249-1261. [ Links ]
Rilov, G., R. Mant, D. Lyons, F. Bulleri, L. Benedetti-Cecchi, J. Kotta, A.M. Queirós, E. Chatzinikolaou, T. Crowe and T. Guy-Haim. 2012. How strong is the effect of invasive ecosystem engineers on the distribution patterns of local species, the local and regional biodiversity and ecosystem functions? Environ. Evid., 1-8. [ Links ]
Ruesink, J.L., B.E. Feist, J.S. Hong, A.C. Trimble and L.M. Wisehart. 2006. Changes in productivity associated with four introduced species: ecosystems transformation of a "pristine" estuary. Mar. Ecol. Prog. Ser., 311: 203-215. [ Links ]
Salazar-Vallejo, S.I. y M.H. Londoño-Mesa. 2004. Lista de especies y bibliografía de poliquetos (Polychaeta) del Pacífico Oriental Tropical. An. Inst. Biol., Univ. Nal Aut. México, Ser. Zool., 75(1):9-97. [ Links ]
Schwindt, E., A. Bortolus and O.O. Iribarne. 2001. Invasion of a reef-builder polychaete: Direct and indirect impacts on the native benthic community structure. Biol. Inv., 3(2): 137-149. [ Links ]
Shumka, S., L. Kashta and A. Cake. 2014. Occurrence of the nonindigenous tubeworm Ficopomatus enigmaticus (Fauvel, 1923) (Polychaeta: Serpulidae) on the Albanian coast of the Adriatic Sea. Turk. Zool. Derg., 38: 519-521. [ Links ]
Simberloff, D., J.L. Martin, P. Genovesi, V. Maris, D.A. Wardle, J. Aronson, F. Courchamp, B. Galil, E. García-Berthou, M. Pascal, P. Pysek, R. Sousa, E. Tabacchi and M. Vilà. 2013. Impacts of biological invasions: What's what and the way forward. Trends Ecol. Evol., 28(1): 58-66. [ Links ]
Urban-Malinga, B., J. Warzocha and M. Zalewski. 2013. Effects of the invasive polychaete Marenzelleria spp. On benthic processes and meiobenthos of a species-poor brackish system. J. Sea Res., 80: 25-34. [ Links ]
Valencia, B., L. Herrera y A. Giraldo. 2014. Estructura de la comunidad y distribución vertical de la macrofauna de fondos blandos en isla Gorgona, Pacífico Colombiano. Rev. Biol. Trop., 62: 169-188. [ Links ]
Valéry, L., H. Fritz, J.C. Lefeuvre and D. Simberloff. 2008. In search of a real definition of the biological invasion phenomenon itself. Biol. Inv., 10(8): 1345-1351. [ Links ]
Valéry, L ., H. Fritz, J.C. Lefeuvre and D. Simberloff. 2009a. Ecosystem-level consequences of invasions by native species as a way to investigate relationships between evenness and ecosystem function. Biol. Inv., 11(3): 609-617. [ Links ]
Valéry, L., H. Fritz, J.C. Lefeuvre and D. Simberloff. 2009b. Invasive species can also be native. Trends Ecol. Evol., 24(11): 584-585. [ Links ]
Voulgaris, A.G., D. Simmonds, D. Michel, H. Howa, M.B. Collins, D.A. Huntley and F. Winter. 1998. Measuring and modelling sediment transport on a macrotidal ridge and runnel beach: an intercomparison. J. Coast. Res., 14(1): 315-330. [ Links ]
Wallentinus, I. and C.D. Nyberg. 2007. Introduced marine organisms as habitat modifiers. Mar. Pollut. Bull., 55(7-9): 323-32. [ Links ]
Received: May 02, 2017; Accepted: August 29, 2017











 text in
text in