INTRODUCTION
The patterns of diversity and the spatial distribution of organisms are the result of evolution, biogeography, and interactions among local biotic and abiotic factors operating at different spatial and temporal scales (Berasategui, 2003). Zooplankton is one of the most important biotic factors in understanding the structure of pelagic trophic networks; specifically, it occupies an intermediate trophic level and transfers energy generated from primary production to higher levels (González, 1988). Therefore, the Zooplankton community is representative and determinative of the productivity of a region as it provides pathways for energy transfer and, accordingly, is closely associated with the level of secondary production in a locality (Franke et al., 2005).
In the field of biological oceanography, the initial development phases of marine organisms are of great interest because they are important to the recovery of populations and the connectivity or exchange of individuals between populations (Cowen et al., 2000, 2006; Lefevre and Bellwood, 2015). Within the zooplankton, fish larvae and eggs (ichthyoplankton) are also of continuing research interest because they are a key element for understanding the biology, ecology, and distribution patterns of fish species in their adult stages and are essential to energy flow processes in and the stability of aquatic ecosystems (Köster et al., 2001; Frederiksen et al., 2006; Montagnes et al., 2010; Zhou et al., 2011). However, despite the importance of ichthyoplankton, several gaps remain in the knowledge of their taxonomy and ecology in several regions of the world, including the tropical coastal zone, due to their taxonomic complexity (Leis and Thrnski, 1989; Leis, 1991; Neira et ah, 1998).
In coastal marine environments, variations in environmental conditions at different time scales can modulate the distribution and abundance of fish larvae (Kingsford, 2001). The distribution, abundance, and composition of fish assemblages will respond to an annual cycle of varying hydrographic conditions, leading to variations in fish composition and abundance (Leis and McCormick, 2002).
The magnitude of seasonal fluctuations depends on the distance from the equator, yet intra-annual variations in climate patterns can strongly affect the biological communities in tropical marine environments (Longhurst and Pauly, 1987; Alongi, 1990). Overall, temporal and spatial variation in physical factors, which are among the most influential, significantly influence the biological response of marine communities. For example, fluctuations in circulation, the stability of the water column, turbidity, salinity, oxygen, or temperature determine the viability of individual species (Blaxter, 1991; Lamber et al, 2003; Perry et al., 2010; Villamizar et al., 2011).
Furthermore, the ichthyoplankton and its spatio-temporal variation are of interest scientifically because of the interest in harvesting commercial species. The first record of the ichthyoplankton of the Colombian Caribbean dates to the 1970s, when Mercado (1970) described the taxonomy of the larvae of the Atlantic tarpon (Megalops atlanticus), an important commercial species. Later, Ávila de Tabarés (1978) taxonomically described the ichthyoplankton associated with Ciénaga Grande de Santa Marta between January 1970 and May 1972. The INPAVECEP program (1994-1999) evaluated the fish resources of the outer continental shelf and the upper continental slope of the Colombian Caribbean up to an isobath of 200 m, generating the largest quantity of scientific information on the ichthyoplankton of the region (Manjarrés et al., 1994; 1997; 1998a; 1998b; 1998c; INPA, 1999; Vergara and Arteaga, 1999; Vergara et al., 1999).
Other ichthyoplankton studies have been carried out in the Colombian Caribbean, including those of Escobar and Manjarrés (1987), Rodríguez (1996), Vergara (1997), and Medellín et al. (2013). However, the available information about this developmental stage of the ichthyofauna is scarce for the northern coastal environments of the Colombian Caribbean, especially in the Alta Guajira region of which Bahía Portete is a hypersaline bay of great ecological importance (Fajardo, 1979; Cabrera and Donoso, 1993; Gutiérrez-Moreno et al., 2008) where adults of 224 fish species belonging to 70 families have been recorded (Garzón-Ferreira, 1989). This locality was declared a National Natural Park (Parque Nacional Natural [PNN]) in December 2014 (MADS, 2014), and one of the conservation objectives is to "contribute to the generation of the ecosystem services provided by maritime and coastal ecosystems and their associated species to favor the productivity of fishing activities in the Alta Guajira by protecting areas used by hydrobiological species to incubate eggs and rear juvenile individuals."
Considering the biological, ecological, and economic importance of understanding the taxonomic composition, abundance, and spatio-temporal variation of fish in their early developmental stages, the objectives of the present study were to describe the composition and quantify the abundance of the ichthyoplankton assemblage in Bahía Portete. In addition, the relationship between this assemblage and the local temperature, salinity, chlorophyll-a, dissolved oxygen, and transparency conditions as well as the instantaneous field of surface currents (circulation) was established. Two contrasting climate periods described by Guzmán-Alvis et al. (2006) for this locality were considered: the windy or dry season from June to August and the rainy season from September to November.
MATERIALS AND METHODS
Study area
Bahía Portete is in the northern region of La Guajira in the continental Colombian Caribbean between the cape of Vela and Punta Gallinas at 12°07' N-72° 02' W (Figure 1). It is a shallow bay ranging from 3 to 9 m in depth that covers an approximate area of 125 km2 with a width of 13 km, and it meets the sea through a mouth with a 2-km opening. Coral formations are found at the southern and western portions of the bay, and seagrass meadows and mangrove forests border the southeastern littoral zone (Garzón, 1989; Solano, 1994; Gutiérrez-Moreno et al., 2008).
Sample collection and analysis
Eighty-four zooplankton samples were collected during two oceanographic sampling campaigns. The first was performed in July 2015 during the windy season, when rainfall is below average, and the second was performed in November 2015, immediately after the period of maximum rainfall (Guzmán-Alvis et al., 2006). The sampling grid was composed of 21 stations systematically distributed throughout the bay to cover all the subtidal and littoral habitats described for the locality (Figure 1).
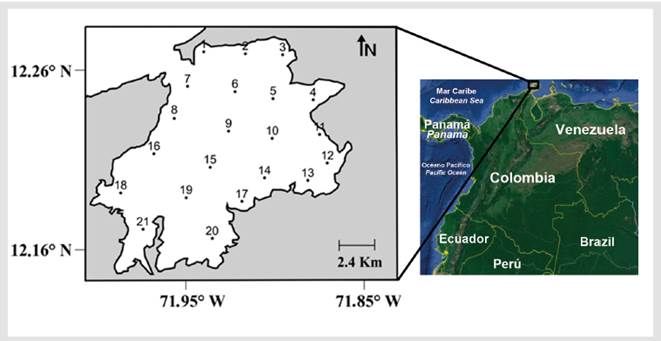
Figure 1 Locations of Bahia Portete in Alta Guajira of the northern Colombian Caribbean and the 21 stations sampled during two oceanographic campaigns in July and November 2015. Landsat/ Copernicus Image - Google Earth Pro®. Date: 31 March 2012, Height: 20 km, image centered at 12° 12' 56" N, 71° 54' 40" W.
At each station, vertical temperature and salinity measurements were taken with a Sea-Bird 19 CTD, and water transparency was measured using a Secchi disk. Direct water samples were taken with a Niskin bottle at a depth of 1 m to determine temperature, pH, and dissolved oxygen using a YSI 85® multiparametric meter, and the in vivo concentration of chlorophyll-a and turbidity were determined with a previously calibrated portable AquaFlour® fluorometer.
At each station, zooplankton were caught using a bongo net with a 30-cm opening equipped with 300- and 500-¿ím mesh nets via daily surface dragging (at a depth of 1 to 10 m) in a circular pattern for 10 min. A Hydrobios® flowmeter was attached to the opening of each net to quantify the volume of filtered water and to standardize the abundance per unit volume. The zooplankton samples were fixed in a formalin-sea water solution with a final concentration of 4% and transported to the Laboratory of Animal Ecology at the University of Valle (Universidad del Valle, UV) (Cali, Colombia), where each sample was reviewed and all fish larvae were separated, counted, and identified to the lowest possible taxonomic level following the guides of Beltrán and Herrera (2000) and Richards (2005). All identified material was deposited in the reference collection of the UV Marine Biology Unit (UNIVALLE: CIR-UV: 0150001 and 0150533).
To quantify the zooplankton biomass, half of the samples collected with the 300-/im mesh were concentrated on previously weighed cellulose filters. The samples were dried at 60 °C for 24 hours and then weighed using an analytical balance with a precision of 0.0001 g. The dry biomass was determined by calculating the difference between the weight after drying and the initial weight.
Data analysis
The representativeness of the samples was evaluated by constructing rarefaction curves for each study period, and the expected richness was calculated using the Chao 2 and second-order Jackknife estimators. The diversity and richness of the fish larvae assemblages were characterized by the Shannon and Margalef indices, which describe the community attributes. The two periods were compared by bootstrap resampling using 1000 repetitions, and kriging interpolation in Surferll® software was used to represent the spatial variation in the abundance of fish larvae, oceanographic conditions, and the instantaneous fields of surface currents (circulation) in the study area for both periods.
The structure of the assemblage was described using the Bray Curtis similarity index based on the abundance matrix of species with a frequency of occurrence greater than or equal to or 5%. Data were transformed using the log (x + 1) function to decrease the effect of dominant species on abundance. The group-average algorithm was used to construct a similarity dendrogram, and the similarity profile (SIMPROF) routine was used to determine the significance of the resulting groups. Finally, the contribution of individual species to the groups generated by the similarity analysis was established via a similarity percentage (SIMPER) routine run in PRIMER v6.0® software.
Lastly, the degree of correlation between the established structure of the fish larval assemblage and the oceanographic conditions of the study area was evaluated using a canonical correspondence analysis (CCA) in the free software PAST v3.0. This multivariate association test maximizes the degree of correlation between the abundance of fish larvae and physico-chemical parameters.
RESULTS
Composition and structure of the fish larval assemblage
A total of 2763 fish larvae belonging to 66 species and 37 families were caught (Table 1), and the representativeness of the sample varied from 91% to 96%. Fish larvae were collected at all sampling stations during both periods, and the species richness and diversity varied significantly between the study periods and was greater in November than in July (richness, d: July = 5.43 and November = 9.15, p = 0.001; diversity, H': July = 2.64 nats/ind and November = 3.17 nats/ ind, p = 0.001). However, the abundance of larvae (ind/1000 m3) was significantly higher in July than in November (Mann-Whitney p < 0.005, Figure 2).
Table 1 Taxonomic list of ichthyoplankton in Bahia Portete during July and November 2015. Relative abundance (Ra.) and catch frequency (FrC.) are indicated for each sampling season and for the entire sampling period. Data from FishBase® were used to establish the habitat, uses, and commercial interest of the species (Frose et al., 2017)
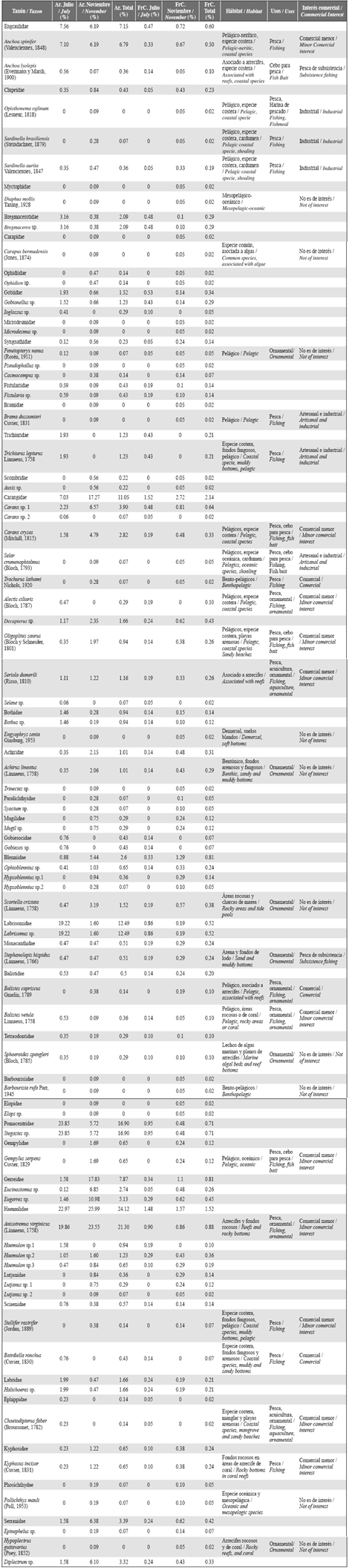
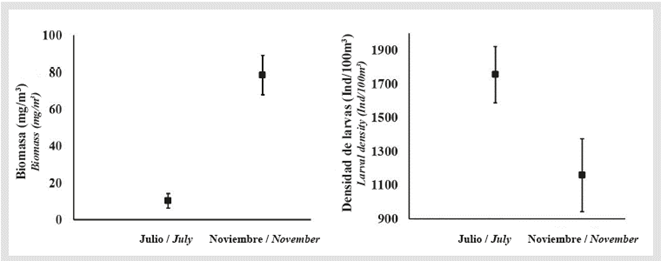
Figure 2 Zooplankton biomass and fish larval density in the water column of the study area during July and November.
The families most frequently caught in July were Haemulidae, Carangidae, and Pomacentridae, and those in November were Carangidae, Haemulidae, and Blenniidae. The species with the greatest catch frequency in July were Anisotremus virginicus, Stegastes sp., and Labrisomus sp., and those in November were Anchoa spinifer, Caranx sp. 1, and A. virginicus.
Considering both sampling periods, the most abundant families in Bahía Portete were Haemulidae (24.3%), Pomacentridae (16.9%), and Labrisomidae (12.5%), and the most abundant species were A. virginicus (21.3%) and Stegastes sp. (16.9%) (Table 1). Of the 66 species identified, 38% were of interest for fishing activities including A. virginicus (21.3%), which was highly abundant (Figure 3).
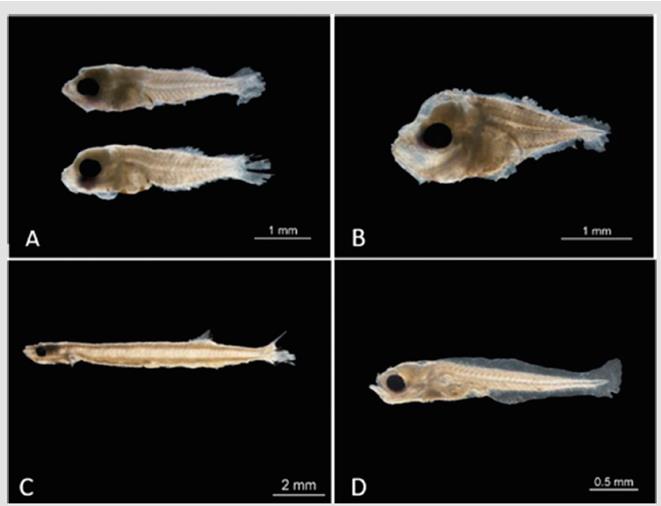
Figure 3 Larvae of the most abundant commercial fish species: A) Anisotremus virginicus, B) Caranx sp. 1, C) Sardinella aurita, and D) Trichiurus lepturus.
With a total of 10 identified species, the most diverse family was Carangidae, followed by Haemulidae and Blenniidae with 4 species each. Notably, the greatest number of species (57) was recorded in the month of November, whereas only 37 species were recorded in July.
Based on the similarity analysis between stations and periods, five significant groups (SIMPROF, p < 0.05) with dissimilarity percentages greater than 70% were established (Figure 4). The group with the most stations (group e) was formed by 18 stations in July; these were distributed across the entire study area except for one station in the southeastern portion and another 9 stations of November located south of the bay, both of which are home to mangrove forests, sandy shallows, and rocky reefs (Figure 4). This group was formed due to the contribution of Stegastes sp. 1, A. virginicus, and Labrisomus sp. 1 (SIMPER, 57.6% similarity).
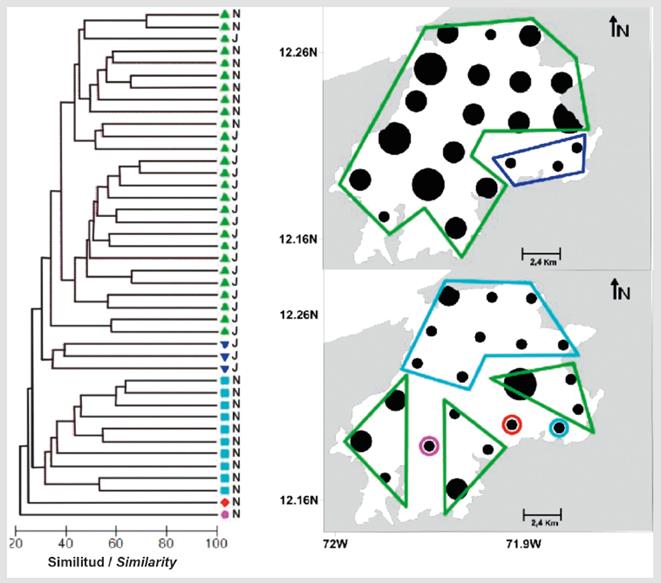
Figure 4 Similarity dendrogram for the fish larvae assemblage at Bahía Portete and the spatial variation in density (ind/100 m 3 ) during the two study seasons. The five significantly different groups (SIMPROF test, p < 0.05) are represented with black lines in the dendrogram, and their spatial locations are identified with colored polygons on the maps.
In July, three stations located at sites with seagrass formed group c (Figure 4) because of the contribution of Eugerres sp., Caranx sp. 1, Eucinostomus sp., and Diplectrum sp. (SIMPER, 76.5% similarity). In November, stations located at the central and northern portions of the study area, where rocky and sandy shallows are dominant, formed group d (Figure 4) because of the contribution of Eugerres sp., A. virginicus, and Stegastes sp. 1 (SIMPER, 38.5% similarity).
Finally, groups a and b were each composed from single stations in November. Group a was represented by station 19, where the most abundant species was Decapterus sp., and group b was represented by station 14 (Figure 4), where Caranx crysos was dominant. Both these species belong to the family Carangidae, which is characterized by pelagic spawning.
Environmental conditions
Water transparency varied between 0.7 and 3.0 m in July and between 0.8 and 3.3 m in November (Table 2); the lowest transparency during the two study periods was found at stations 13 and 14. Temperature, salinity, chlorophyll-a, and surface pH were significantly lower in July (Mann-Whitney, p < 0.001, Table 2, Figures 5 and 6), while surface dissolved oxygen was significantly lower in November (Mann-Whitney p < 0.001, Table 2, Figure 6). No significant differences in chlorophyll-a were found between July and November (Mann-Whitney p > 0.005, Figure 5).
Table 2 Physico-chemical parameters recorded in Bahía Portete during July and November 2015. n: number of stations at which physico-chemical patterns were recorded, ¡t: average, S.D.: standard deviation, Min: minimum recorded value, Max: maximum recorded value.
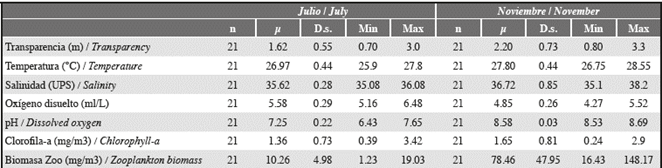
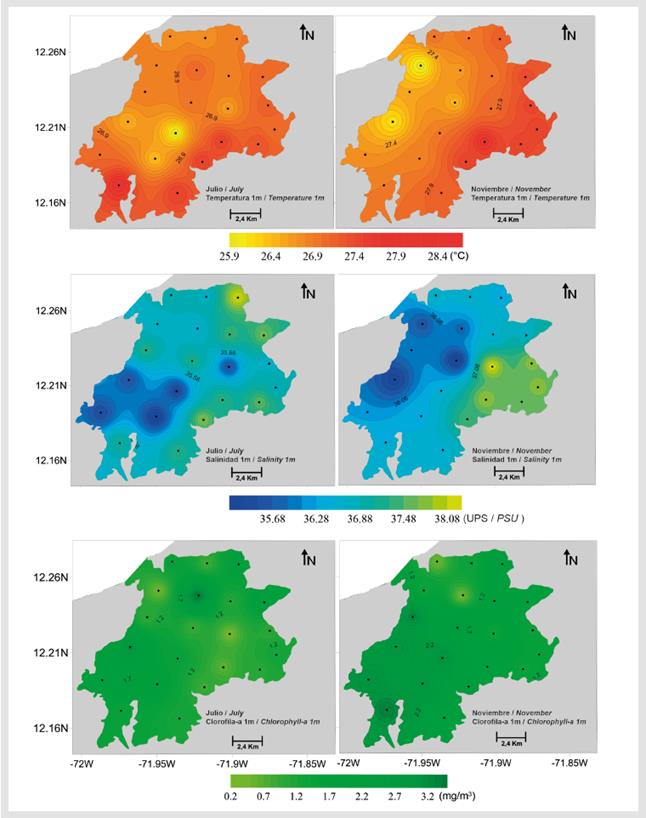
Figure 5 Spatial variation in surface (1 m) temperature, salinity, and chlorophyll-a in the study area.
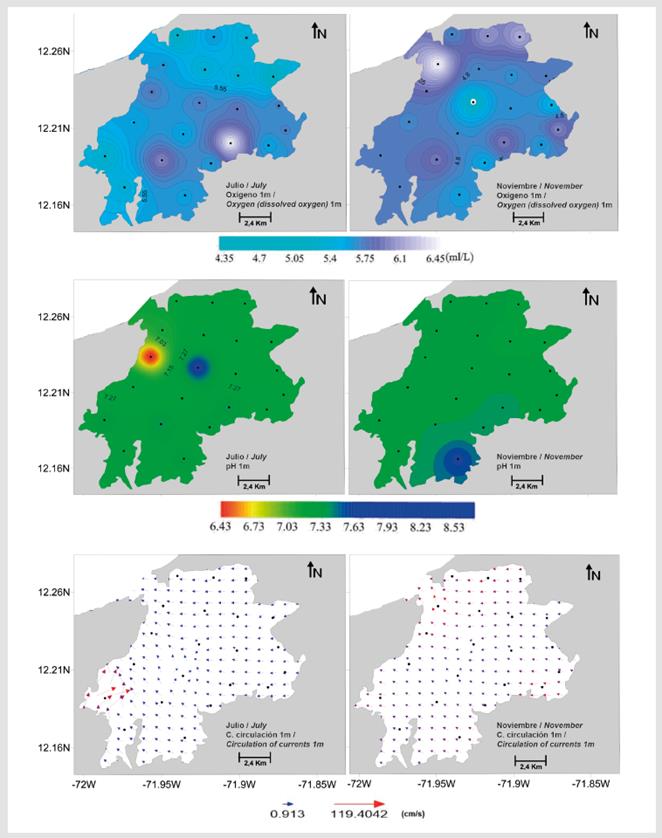
Figure 6 Spatial variation in dissolved oxygen, pH, and the instantaneous field of surface currents (circulation; 1 m) in the study area.
In July, surface water velocity varied from 0.1 to 1.1 m/s, and water predominantly moved in a northwest direction (Figure 6). The overall circulation pattern at the interior of Bahía Portete was maintained in November. Water circulated around a central circulation axis toward the entrance of the bay (Figure 6), yet the velocity was significantly lower and ranged from 0.05 to 0.3 m/s (Mann-Whitney, p < 0.001, Table 2). Significant differences in zooplankton biomass were found between the study periods (Mann-Whitney p < 0.001); greater biomass was recorded in November (July: 1.2 to 19.0 mg/m3, November: 16.4 to 148.2 mg/m3) (Table 2, Figure 2).
Relationship between fish larvae and environmental variables
The first two ordination axes of the CCA explained 73.24% of total variance in the fish larval assemblage of Bahía Portete. The concentration of dissolved oxygen was inversely related to temperature, salinity, pH, and chlorophyll-a on axis 2 (Figure 7). In addition, the total abundance of fish larvae was directly related to chlorophyll-a (r = 0.87; p =-0.035) and inversely related to zooplankton biomass (r= -0.31; p = 0.046).
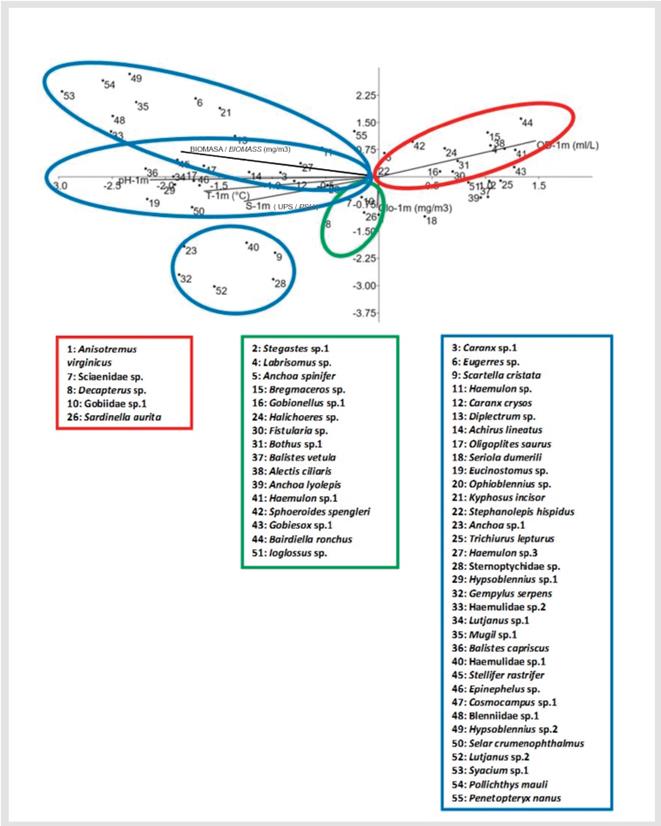
Figure 7 Canonical correspondence analysis of species with > 5% abundance. The first two canonical axes explain 73.24% of the total variance.
Specifically, the following species were directly correlated with the concentration of dissolved oxygen: Labrisomus sp., A. spinifer, Bregmaceros sp., Gobionellus sp., Halichoeres sp., Fistularia sp., Bothus sp.1, Alectis ciliaris, Haemulon sp.1, Sphoeroides spengleri, Gobiesox sp., Bairdiella ronchus, Ioglossus sp., Stegastes sp., Balistes vetula, and Anchoa lyolepis. Furthermore, the following species were directly correlated with the concentration of chlorophyll-a: A. virginicus, Sciaenidae sp., Decapterus sp., Gobiidae sp. 1, and Sardinella aurita. Lastly, most species identified in the two study periods were directly correlated with temperature, salinity, pH, and biomass, including Scartella cristata, C. crysos, Oligoplites saurus, Haemulon sp.1, and Stellifer rastrifer (Figure 7), among others.
DISCUSSION
According to Garzón-Ferreira (1989), the most diverse fish families in Bahía Portete are Haemulidae (12), Sciaenidae (12), and Carangidae (11), and those most frequently caught are Eucinostomus gula, Archosargus rhomboidalis, and Diapterus rhombeus. Based on the taxonomic analysis of ichthyoplankton in the present study, the family with the highest species richness was Carangidae (10) followed by Haemulidae (4), and the most frequently caught species were A. virginicus, Caranx sp. 1, and A. spinifer. Comparing these two studies, the most diverse families are similar, yet the most frequently caught adult fish species in the first study differed from the most frequently caught fish larvae in Bahía Portete.
In addition, the previous study by Garzón-Ferreira (1989) was based on an analysis of adult fish caught using a small fishing boat and gill nets in addition to a visual census and the use of an ichthycide. While it is the most complete bibliographic reference on the fish fauna of Bahía Portete to date, it did not account for the gonadal development state of the specimens. For this reason, the fish species are not necessarily using the catch locality for reproduction, so a direct comparison of the results obtained by Garzón-Ferreira (1989) and those of the present study is not possible.
Comparing the structure and composition of the fish larval assemblage of Bahía Portete in the present study with the results of similar studies in other marine ecosystems and the shallow estuaries of the Caribbean Sea and the Gulf of Mexico, the larval densities reported in other studies tend to be relatively low. Overall, small larvae are dominant with low species richness in comparison with the ichthyoplankton and ichthyofauna recorded for adjacent coastal environments (Houde and Lovdal, 1984; Ditty, 1986; Powell et al., 1989; Vásquez-Yeomans and Richards, 1999).
Previous studies along the northern coastal zone of the Colombian Caribbean found Gobiidae, Carangidae, Sciaenidae, and Synodontidae to be the most abundant families of fish larvae and Eucinostomus sp., Lutjanus synagris, and Upeneus parvus to be the most common species (Manjarrés et al., 2005a, 2005b). The authors of these latter studies identified larvae caught with a mesh opening of 500 /<m, whereas the fish larval composition in the present study was based on catches using 300-and 500-¿ím mesh, which substantially increased the probability of capturing larvae from a greater number of families. In this study, the ichthyoplankton of Bahía Portete was dominated by five fish families (Haemulidae, Carangidae, Engraulidae, Labrisomidae, and Pomacentridae) that together represented 72.12% of the total fish larvae identified.
In addition, the larvae of A. virginicus, Stegastes sp., Caranx sp. 1, and Labrisomus sp. were dominant in the study area. These fishes are widely distributed in the Caribbean Sea and have been previously recorded in the Colombian Caribbean (Manjarrés et al., 2005a, 2005b). These species were found during both study periods in Bahía Portete, suggesting that their presence in a larval stage is not seasonal. In general, hemulids are tropical and subtropical fish that form shoals in shallow coastal waters in areas with reefs and seagrass (Nelson, 1994), but little is known about the reproductive season of this family, particularly A. virginicus. Thus, the results of the present study represent a starting point from which the reproductive periods of A. virginicus may be examined in greater depth, as this species is of commercial interest in the region (Correa and Manjarrés, 2004; Viloria-Maestre et al, 2016).
Over the last several decades, the highly dynamic spatial and temporal behavior of marine fish larvae assemblages has been described, and variations in composition and abundance are presented both horizontally and vertically and at temporal scales that range from several hours to seasons (Leis, 1991). Generally, temporal variation modulates the simultaneous effects of multiple hydrographic and environmental factors that can determine the survival of larvae (Robertson, 1991; Leis and McCormick, 2002; Lo et al., 2010; Sánchez et al., 2017), so in pelagic environments, temporal variation in environmental conditions modulates the distribution, abundance, and overall structure of fish larval assemblages (Franco et al., 2002; Funes, 2002; Aceves et al., 2003, 2008; Hsieh et al., 2016).
According to the obtained results, the community attributes of the fish larval assemblage in the study area differed between the study periods; the richness and diversity of fish larvae were greater in November than in July. In this region of Alta Guajira, the typical annual climate and its variability have been described as a dry season in July and August characterized by strong winds and a rainy season from September to November with less intense winds (Andrade, 1993; Guzmán-Alvis et al., 2006). During the dry period (strong winds), coastal upwelling intensifies in front of Bahía Portete, whose center is located 100 km from the coastline, so the primary productivity of the pelagic coastal system increases (Andrade and Barton, 2005; Rueda-Roa and Muller-Karger, 2013), thus enhancing food availability for fish larvae and increasing their probability of survival (Castonguay et al, 2008; Ottersen et al., 2010; Coelho-Souza et al., 2012).
Furthermore, the circulation pattern is one of the most important physical factors influencing the spatial distribution of the abundance of fish larvae (Reiss et al., 2000; Feng et al., 2010); it originates advective or retention processes that tend to increase or decrease the abundance of fish larvae in a locality (Sánchez-Velasco et al., 2006; Olivar et al., 2010). In Bahía Portete, the dominant surface circulation pattern during both study periods was characterized by the circulation of water toward the western portion and the mouth of the bay, which probably favors the surface mobility of fish larvae from the western sector, where mangroves, coral formations, and rock subtidal zones are found, throughout the bay. Water circulation at medium water depths or near the bottom of the mouth of the bay was not analyzed, but the surface circulation pattern toward the mouth of the bay suggests that Bahía Portete is contributing fish larvae to the surrounding coastal area throughout the year. This process is fundamental to the maintenance of the fishing resources of the region (Pineda et al., 2007, Planes et al., 2009, Harrison et al., 2012).
Furthermore, considering that fish larvae mainly feed on planktonic organisms, the survival and growth of fish larvae largely depend on the availability of food sources (Somaraskis et al., 2002). Generally, the density of fish larvae is closely related to zooplankton biomass and coincides with increases in the local food availability (Kaunda-Arara et al, 2009; Sponaugle et al, 2009). These findings were also confirmed in the present study, as most larval species found in the bay were directly related to zooplankton biomass.
Overall, seasonal fluctuations in larval production are strongly associated with the reproductive events of species, which appear to be synchronized with the requirements for increasing successful larval recruitment, so these events define the spatial distribution patterns of species (Johannes, 1978; Robertson, 1991; Pineda et al., 2010; China and Holzman, 2012). In heterogeneous systems, there is a high diversity of fish species with different spawning requirements and patterns; some species may present one or two reproductive peaks during the year while others may reproduce continuously (Pittman et al., 2004). Similarly, the temporal variability in the Bahía Portete assemblage is likely reflective of the different reproductive patterns of the species inhabiting the system as adults, so it is possible that species such as A. virginicus, which has a greater than 60% occurrence frequency in Bahía Portete, reproduce several times per year. In contrast, larval stages that were only recorded in one of the sampling periods possibly correspond to species with a single annual reproductive period or that were accidentally transported to the study area by local advective processes (Gray, 1993).
CONCLUSIONS
In Bahía Portete, the assemblage of fish larvae was represented by five abundant families: Haemulidae, Pomacentridae, Labrisomidae, Carangidae, and Engraulidae. In addition, of the 66 species identified, 38% were of commercial interest in the Caribbean. Notably, A. virginicus was recorded in high abundance during the two study periods, and its presence supports the importance of Bahía Portete for fishing as this species is of interest for fisheries in the Colombian Caribbean.
The fish larval assemblages encountered during two study periods in Bahía Portete significantly differed in abundance and richness, and this variation may be influenced by several factors including the surface water circulation pattern, food availability, and the reproductive events of the species in the area. In addition, habitat heterogeneity is likely influential; the area contains seagrasses, rocky reefs, mangrove areas, and muddy bottoms that may favor high species richness.
The results of the present study increase the existing knowledge of the fish larval assemblage in PNN Bahía Portete-Kaurele and are relevant to the management and conservation of this protected area. However, considering the temporal scope of the present study, it was not possible to establish long-term trends in the spatial variation of fish species, which limits the capacity of this study to predict spawning zones or reproductive periods.











 text in
text in 


