Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Boletín de Investigaciones Marinas y Costeras - INVEMAR
versión impresa ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.47 no.2 Santa Marta jul./dic. 2018
https://doi.org/10.25268/bimc.invemar.2018.47.2.744
Research Articles
Corallivory of the gastropod Jenneria pustulata (Ovulidae: Pediculariinae) in two coral reefs at Gorgona Island NNP
1 Universidad del Valle, Departamento de Biología, Grupo de Investigación en Ecosistemas Rocosos Intermareales y Submareales Someros-LITHOS, Apartado aéreo 25360, Cali, Colombia. mauro.zucconi@correounivalle.edu.co
2 Universidad del Valle, Departamento de Biología, Grupo de Investigación en Ecosistemas Rocosos Intermareales y Submareales Someros-LITHOS, Apartado aéreo 25360, Cali, Colombia. levy.obonaga@correounivalle.edu.co
3 Universidad del Valle, Departamento de Biología, Grupo de Investigación en Ecosistemas Rocosos Intermareales y Submareales Someros-LITHOS, Apartado aéreo 25360, Cali, Colombia. edgardo.londono@correounivalle.edu.co
Coral reefs are very important and highly biodiverse ecosystems that are exposed to various stressors, including biological ones, such as parasitism and corallivory - the direct consumption of coral tissue by a predator. Knowledge on the effects of corallivory on the coral reefs in the Colombian Pacific is poor. Therefore a study was set up to quantify the abundance of and the corallivory rate by the snail Jenneria pustulata in La Azufrada and Playa Blanca coral reefs (Gorgona Island, Colombia). Snails were manually sampled from the underside of Pocillopora sp. colonies and measured in situ to determine their size structure for each reef. To measure possible damage caused by corallivory, several snails were kept under controlled laboratory conditions for 24 h. Snail sizes and corallivory varied significantly between reefs (P=0.0001; P«0.001). Snails from Playa Blanca were larger than snails from La Azufrada, while corallivory was higher in La Azufrada than in Playa Blanca. Although corallivory rates by J. pustulata are smaller than rates reported for other predators in different coral species, it is recommended to continue this kind of investigations in order to increase the knowledge on biological dynamics of this species and to understand how they affect the reefs at Gorgona Island.
KEYWORDS: Colombian Pacific; Coral reef; Gastropod; Coral tissue consumption; Jenneria pustulata
Los arrecifes coralinos son ecosistemas importantes y altamente biodiversos; estos son afectados por diversos procesos, entre ellos la coralivoría, proceso que consiste en el consumo directo de tejido coralino por parte de un depredador. Es poco el conocimiento existente sobre los efectos de los invertebrados sobre los arrecifes del Pacífico colombiano, es por esto que el objetivo de la presente investigación fue determinar la densidad y tasa de coralivoría de Jenneria pustulata en los arrecifes La Azufrada y Playa Blanca (Isla Gorgona, Colombia). Los caracoles se buscaron y colectaron activamente bajo colonias coralinas, siendo medidos para determinar su estructura de tallas para cada arrecife. Adicionalmente, se realizó una fase de laboratorio en la cual se midió el consumo de tejido coralino de J. pustulata durante 24 h, para ambos arrecifes. La talla promedio de J. pustulata fue de de 19.56 ± 4.71 mm en La Azufrada y 20.53±3.40 mm en Playa Blanca. La talla promedio y la tasa de consumo de tejido coralino fueron estadísticamente diferentes (P=0.0001; P«0.001) entre los arrecifes. Aunque las tasas de coralivoría se encuentran por debajo de las reportadas para otras especies de moluscos coralívoros, se sugiere continuar con los estudios para comprender la biología de J. pustulata; las dinámicas bióticas y abióticas que le afectan y además, entender su efecto sobre los arrecifes de la Isla Gorgona.
PALABRAS CLAVE: Pacífico colombiano; Arrecife de coral; Gastrópoda; Consumo de tejido coralino; Jenneria pustulata
INTRODUCTION
Coral reefs are bioconstructions (i.e. structures constructed by organisms) that provide a great variety of microhabitats to different species of fish and marine invertebrates (Cumming, 1999; Alvarez-Filip and Gil, 2006). Thus, coral reefs are among the ecosystems with the greatest biological diversity worldwide (Rotjan and Lewis, 2008), a feature that confers ecosystems high resilience (Valiente et al., 2015). Despite de above, these ecosystems are vulnerable to a guild of threats, including biotic (bioerosive effects of fishes, annelids, crustaceans, echinoderms and mollusks among others - Glynn and Enochs, 2008), and abiotic factors (e.g. temperature changes, extreme low tides and storms). In addition, coral reefs are affected by human activities, such as overfishing, water pollution and manual extraction of corals (White et al., 2000; Huges et al., 2003), which can accelerate and exacerbate the natural hazards. Recently, the role of biotic stressors on corals has been highlighted because these factors directly and negatively affect the fitness of corals (Rotjan and Lewis, 2008), thereby affecting the entire ecosystem. One of these biotic factors is corallivory: the direct consumption of coral polyps by predators. This can cause the total or partial death of the coral colony or restrict the ability of the corals to recover from the wounds inflicted (Baums et al., 2003).
Organisms from different animal groups have specialized as corallivorous, being mollusks one the most representative. Currently, 20 species of this group have been identified as corallivorous, all of them belonging to the class Gasteropoda (Rotjan and Lewis, 2008), including Jenneria pustulata (Lightfoot, 1786), a sea snail that belongs to the Ovulidae family. This snail is found from the northern Gulf of California to Peru and feeds mainly on scleractinian corals (Lorenz and Feshe 2009).
In Colombia, this species has been registered in the coral reefs of Gorgona Island and the Utria inlet (Navas- Camacho et al., 2010); however, no research has been conducted to elucidate the effects of this snail on the coral reefs of these localities. Thus, the present research aims to determine the size distribution and quantify the corallivory rate of this species in La Azufrada and Playa Blanca reefs of the Gorgona Natural National Park.
STUDY AREA
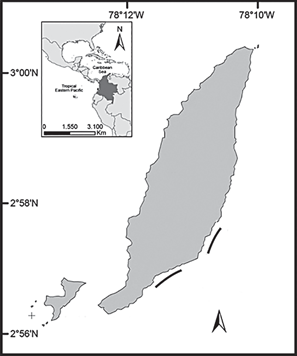
Gorgona Island (2°58' N, 78°11' W; Figure 1) is located approximately 30 km from the mainland (Guapi, Cauca) and represents the most extensive island territory (13.2 km2) on the continental shelf of the Colombian Pacific coast. The island is influenced by the Intertropical Convergence Zone, which determines its bimodal climatic regime (Zapata et al., 2010). The sea surface temperature varies between 26º and 29ºC, although it can occasionally descend below 19ºC during upwelling events at the beginning of the year (Díaz et al., 2001; Zapata, 2001). The main coral reef formations (La Azufrada and Playa Blanca) are located on the eastern side of the island. La Azufrada (LA) measures ~780 m long by ~80-180 m wide and covers approximately 0.094 km2. Playa Blanca (PB) is composed of two large coralline patches separated by a channel ~60 m wide: the smaller to the north is ~240 m long by ~40 m wide, and the largest to the south is ~930 m long and a width that varies between ~60 m and 230 m. Together, these patches cover an approximate area of 0.098 km2. The main reef-building coral species is the branched coral Pocillopora damicornis (family Pocilloporidae) (Parada and Nikolaevna, 1990; Zapata, 2001; Zapata and Vargas-Ángel, 2003; Palacios et al., 2014)
MATERIALS AND METHODS
Field and laboratory work
Field data collection was carried out during April 2015 in LA and August 2015 in PB reefs. Because J. pustulata normally dwells the underside of coral colonies when inactive (daytime, Figure 2), loose colonies from the reef framework were surveyed, using SCUBA diving (twice a day). All individuals were counted and measured (shell length in mm) in order to determine the size structure of the species in each reef. In addition, 15 individuals from each reef (and an identical number of coral fragments) were collected and used for the coral consumption experiment performed under controlled conditions in the Gorgona NNP laboratories.
Each pair, composed of one specimen of J. pustulata and one coral fragment (n =15 pairs) were individually introduced into 16 L capacity aquaria over a period of 24 h. The coral fragment assigned to each snail was obtained from the colony where the snail was originally found and captured. During the experiment, the aquaria were continuously monitored, and water was replenished and oxygenated (with ventilation motors). The injuries caused to the fragments by the snails (Figure 3) were photographed using a Canon D30 camera. Using the free software ImageJ 64 (Abramoff et al., 2004), the area (mm2) of the injuries was calculated from the photographs.
Statistical analysis
After confirming the assumptions of the parametric tests, snails size was compared between reefs (2 levels fixed) using one-way analysis of variance. The size structure for each reef was compared using an Anderson-Darling test for two samples (Engmann et al., 2011). In addition, a one- way ANOVA was used to evaluate coral tissue consumption between reefs. Finally, an ANCOVA was performed on coral tissue consumption with reef as the explanatory variable and shell size as the covariate. All the statistical analyses were carried out using the free software R (R Development Core Team, 2014) and following the statistical assumptions of Zar (Zar, 1999).
RESULTS
Size and size structure
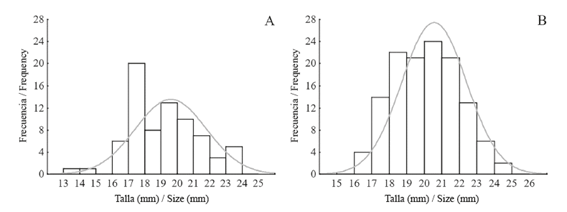
A total of 201 individuals of J. pustulata were examined and measured, with 74 (36.8%) collected from LA and 127 (63.2%) from PB. The mean (±DE) shell length was slightly higher for specimens from PB (20.5±3.40 mm) than for those from LA (19.6±4.71 mm) (Figure 4). Although the previous values appear similar, statistical analysis showed differences (F (0,05(2)1;199) =11.24; P < 0.001). In addition, PB snails’ size ranged from 16.5 to 25.0 mm, whereas in LA the size variation was wider and ranged from 14.0 to 24.0 mm.
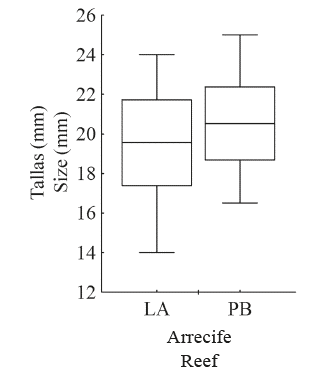
Figure 4 Mean size (box: SD, whiskers: minimum and maximum) of Jenneria pustulata in La Azufrada and Playa Blanca reefs.
The size distribution showed that most (60.8%) of the snails from LA ranged between 17 and 21 mm long; this size distribution differed from the Normal distribution (A=0.92; P=0.019) (Figure 5A). The same analysis for the snails of PB showed that most (78.4%) clumped between 18 and 22 mm; this size distribution also differed significantly from the Normal distribution (A=1.44; P<0.001) (Figure 5B). Finally, a comparison of the size structure between both reefs showed that they depicted different structures (A=5.48; P=0.002).
Consumption of coral tissue
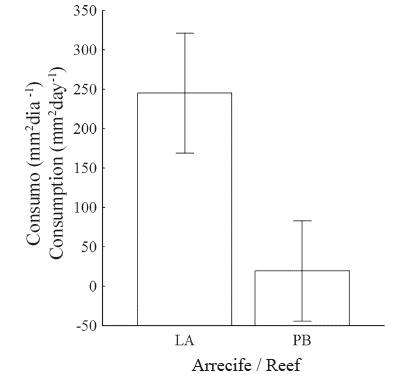
The corallivory experiment with LA snails had a success rate of 44.4%; that is, of the initial 18 snails, only 8 consumed tissue. However, among the PB snails, 66.7% (10 of 15) consumed coral tissue. A positive relationship between snails’ size and the amount of coral tissue consumed in LA (R2=0.81) and PB (R2=0.53) was observed (Figure 6). The average consumption of coral tissue (mm2day-1) per snail ±DE was 105.9±77.01 in LA and 130.5±105.50 in PB. The consumption range varied between (31.1 - 242.2) and (21.0 - 298.0) mm2day-1 for LA and PB respectively.
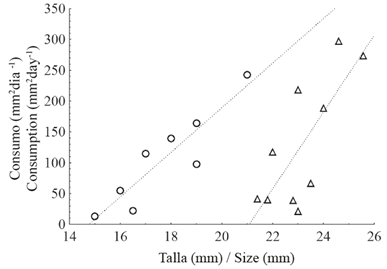
Figure 6 Relation between size (shell length in mm) and coral tissue consumed (mm2) per day by Jenneria pustulata in La Azufrada (P=0.002; circles) and Playa Blanca (P=0.01; triangles) reefs.
Without considering the size of the snails, the average consumption of coral tissue was higher in PB than in LA (130.5 vs. 105.9); however, the ANCOVA showed that snail size has a very significant effect (P), with a snail of a given size consuming more coral tissue in LA than in PB (Figures 6 and 7).
DISCUSSION
The coral reefs located on the eastern side of Gorgona Island show a high degree of similarity, both in their size and structure as well as in the dominance of the genus Pocillopora (Prahl, 1986; Palacios et al., 2014). In addition, these reefs are geographically close; thus, a high degree of communication could be inferred. However, in the present study, the number of J. pustulata individuals was 1.7-times greater in PB than in LA despite the similar sampling effort in the two reefs (24 h in each reef). While the two reefs are very similar, the amount of sediment they receive by runoff varies significantly and is higher in PB (293 g/m2 day-1) than in LA (95 g/m2 day-1) (Blanco, 2009). Sediment load entering the reef has an important effect on thereefhealthandcouldeventuallyincreaseitsvulnerability to disease and reduce the ability of the coral to compete for space with other organisms (e.g., algae) (Granja and López, 2008; Erftenmeijer et al., 2012). Observations in the field suggest that J. pustulata has a marked preference for colonies with some degree of deterioration, which may be related to the ability of the snail to mimic this type of colony, thereby avoiding predation by fish, such as Arothron meleagris, Sufflamen verres and Pseudobalistes naufragium (Guzman 1988; Glynn, 2004). The greater number of individuals of J. pustulata found in the PB reef means a higher predation rate by the snail in this reef when compared to the LA reef; therefore, the synergy of the effects resulting from the influx of sediment carried from the rivers on the island, the consumption of coral tissue by J. pustulata and the subsequent colonization of the available substrate by algae, could significantly affect the health status of the PB reef. However, to corroborate these statements, additional research into the physiological state of the corals in the PB reef is required; because according to Tiliyanov and Tiliyanova (2005), the coral ability to recover from an injury on the living tissue depends on several factors, including the type of injury suffered, the growth form (branched, massive or submassive) and the current physiological state of the colony.
Size and size structure
The length of a gastropod is a characteristic that can be affected by internal factors, such as the animal’s stage of development and age, and by external factors, such as environmental conditions (Wilbur and Owen, 1964). According to Bashevkin and Pechenik (2015), temperature has an important effect on the growth of mollusks, with high temperatures leading to faster growth rates than low temperatures. The reefs were visited during two different seasons: LA was visited during April 2015, while PB was visited during August of the same year, and these two seasons have contrasting climatic conditions. Between January and April, the waters cool due to upwelling in the central part of the Gulf of Panama that generates a cold front from the north and influences the waters of the Colombian Tropical Eastern Pacific, whereas from May to December, the water temperatures are higher (Giraldo et al., 2008). Seasonality may have been partly responsible for the observed differences in shell length (larger snails on average in August in PB [warm waters] and smaller in April in LA [cold waters]) between the snails in both reefs; however, this hypothesis should be tested via simultaneous sampling. Moreover, it has been shown that there is a directly proportional relationship between sample size and population density (Connor et al., 2000), which could also be interpreted as samples with higher abundances could sample a wider range of population characteristics. In PB, more individuals were captured than in LA; therefore, the size range of PB individuals would likely be broader, which would be reflected in the larger average sizes from this reef.
Consumption of coral tissue
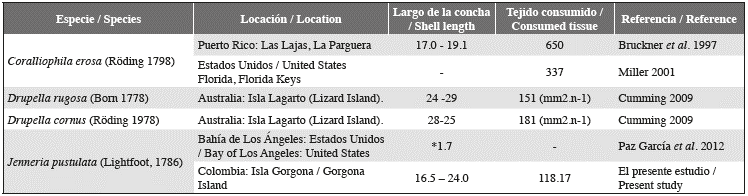
The ecological role of corallivores has been considered by some authors as a driver of the structural dynamics of prey populations, although this role can be affected by various factors, such as the relative abundance of the prey and the size and density of the predator in the reef (Bruckner et al., 1997). Jenneria pustulata is a highly specialized predator, almost exclusively feeds on scleractinian corals and shows a marked preference for the genus Pocillopora (Bertsch, 1984; Glynn, 2004; Figure 2). Considering that the two reefs are relatively similar in species composition and total size, the snails from each of these reefs were not expected to present significant variations in the amount of coral consumed in the experiments. However, according to the results of the ANCOVA, snails that were comparable in size would consume on average more coral tissue from LA than from PB. Since the snails from LA are on average smaller, they may be fulfilling the same function at LA as the large snails at PB in terms of coral consumption. Although this represents one possible explanation, whether interference competition occurs in this species should be investigated to help explain why the smaller snails from LA have the same consumption rate as the larger snails from PB. Studies on other corallivores, such as Coralliophila erosa, Drupella cornus and D. rugosa (Bruckner et al., 1997; Miller, 2001; Cumming 2009), show higher rates than those found for J. pustulata in the Gorgona reefs (Table 1). This could indicate that although this species is larger on average than C. erosa (Del Mónaco et al., 2008) and of similar size to Drupella sp, it has a less significant effect on the coral ecosystem relative to the previously mentioned species. In addition, the J. pustulata radula has two pairs of lateral teeth with a rectangular base and strong hook-shaped rigid cusps (Simone, 2004), and this provision may allow it to feed without causing permanent damage to the corallites, thus allowing for more rapid and less energetically costly coral recovery.
Table 1 Shell lengths (mm) and coral tissue consumed (mm2.day-1) for different mollusk species (mm2.n-1): Recorded over 12 h. * Measured in cm.
Studies focused on the ability of branched corals of the genus Pocillopora to recover from tissue injuries (where the calcareous skeleton is not damaged) show that although this process is energetically less expensive than recovering from a lesson in the calcareous skeleton, these wounds tend to be colonized by algae 4 or 5 days after the injury appears in the tissue. Moreover, although the coral tends to recover almost completely from these wounds, this recovery involves a significant energy expenditure since the coral must not only generate new polyps to cover the injured area again but also regenerate the polyps that have been partially damaged by the corallivore (Titliyanov et al., 2005; Traylor- Knowles, 2016). Therefore, although the damage caused by the feeding of J. pustulata is less than that of other corallivorous mollusks, this species may have a strong effect on the condition of the coral due to the important energy investment required for the corals to recover from the damage and to prevent the concomitant colonization of the calcareous skeleton by algae. These complications support the need for additional research to broaden our knowledge about the structure and population dynamics of this snail and corroborate the significance of its effects on the coral reefs of the Gorgona National Park.
ACKNOWLEDGEMENTS
The authors thank Universidad del Valle for funding this research, which was carried out under the Internal Research Project (C.I 7970). We also thank the Unidad de Parques Nacionales Naturales (National Park Unit) and especially the Gorgona PNN staff (X. Zorrilla and L. Payán) for their help in the logistics and organization of the field phase of this project. Thanks are also extended to Juan Felipe Lázarus, Jaime R. Cantera, Óscar Murillo, José Tavera, Andrés Carmona, Wilmar Torres, Fernando Zapata and Laksmy Gallego for their support during the different phases of the project. Finally, we would like to thank the members of the Research Group on Intertidal and Shallow Subtidal Rocky Ecosystems - LITHOS.
REFERENCES
Alvarez-Filip L. and I. Gil. 2006. Effects of hurricanes Emily and Wilma on coral reefs in Cozumel, Mexico. Coral Reefs, 25: 583-583. [ Links ]
Abramoff, M.D., P.J. Magalhaes and S.J. Ram. 2004. Image Processing with ImageJ. Biophotonics Internat., 11:36-42. [ Links ]
Baums, I., M. Miller and A. Szmant. 2003. Ecology of a corallivorous gastropod Coralliophila abbreviata, on two scleractinian hosts. II. Feeding, respiration and growth. Marine Biology, 142:1093-1101. [ Links ]
Bashevkin, S. and J. Pechenik. 2015. The interactive influence of temperature and salinity on larval and juvenile growth in the gastropod Crepidula fornicata(L.). J. Exp. Mar. Biol. Ecol., 470:78-91. [ Links ]
Bertsch, H. 1984. Jenneria pustulata, the pustulate cowrie. Opisthobranch, 16:9-10. [ Links ]
Blanco, J. 2009. Características físico-químicas de las quebradas del Parque Nacional Natural Gorgona, Pacífico Colombiano. Actual. Biol., 32:123-140. [ Links ]
Bruckner, R.J., A.W. Bruckner and E.H. Williams. 1997. Life history strategies of Coralliophila abbreviata Lamarck (Gasteropoda, Coralliophilidae) on the Southwest coast of Puerto Rico. Proc. 8th Int. Coral Reef Sym., 1: 627-632. [ Links ]
Connor, E., A. Courtney and A. Yoder. 2000. Individuals-Area relationship: The relationship between animal population density and area. Ecology, 3: 734-748. [ Links ]
Cumming, R. 1999. Predation on reef-building corals: multiscale variation in the density of three corallivorous gastropods, Drupella spp. Coral Reefs , 18: 147- 157. [ Links ]
Cumming, R. 2009. Case study: Impact of Drupella spp. on reef building corals of the Great Barrier Reef. Great Barrier Reef Marine Park Authority. Australia. 51 p. [ Links ]
Del Mónaco, C., E. Villamizar y S. Narcizo, S. 2008. Tasa de depredación Coralliophila abbreviata (Neogastropoda: Coralliophilidae) sobre algunas especies coralinas del Parque Nacional Morrocoy, Venezuela. Rev. Biol. Trop., 56:235-246. [ Links ]
Díaz, J., J. Pinzón, A. Perdomo, L. Barrios y M. López-Victoria. 2001. Generalidades. 17-26. En: López-Victoria M. y L.M. Barrios (Eds.). Gorgona marina: contribución al conocimiento de una isla única. INVEMAR, Santa Marta. 160 p. [ Links ]
Engmann, S. and D. Cousineau. 2011. Comparing distributions: The Two-Sample Anderson-Darling test as an alternate to the Kolmogorov-Smirnoff test. Journal of Applied Quantitative Methods, 6:1-17 [ Links ]
Erftemeijer, P.L.A., B. Riegl, B.W. Hoeksema and P.A. Todd. 2012. Environmental impacts of dredging and other sediment disturbances on corals: A review. Marine Pollution Bulletin, 64:1737-1765. [ Links ]
Giraldo, A., E. Rodríguez y F. Zapata. 2008. Condiciones oceanográficas en Isla Gorgona, Pacífico oriental tropical de Colombia. Lat. Am. J. Aquat. Res., 36:121-128. [ Links ]
Glynn, P. 2004. High complexity food webs in low diversity Eastern Pacific Reef-Coral Communities. Ecosystems, 7:358-367. [ Links ]
Glynn, P., H. v. Prahl and F. Guhl. 1982. Coral reefs of Gorgona Island, with special reference to corallivores and their influence on community structure and reef development. An. Inst. Inv. Mar. Punta de Betín, 12:185-214. [ Links ]
Glynn, P . and C. Enochs. 2011. Invertebrates and their roles in coral reefs ecosystems. 273-325 in: Dubynsky, Z and N. Stambler. (Eds.). Coral reefs: An ecosystem in transition. Springer Science, New York. 540 p. [ Links ]
Granja, M. y R. López. 2008. Sedimentación en comunidades arrecifales de Huatulco, Oxaca, México. Rev. Biol. Trop ., 56:1179-1187. [ Links ]
Guzmán, H. 1988. Distribución y abundancia de los organismos coralívoros de los arrecifes coralinos de la Isla del Caño, Costa Rica. Revista Biología Tropical, 36: 191-207. [ Links ]
Hughes, T.P., A.H. Baird, D.R. Bellwood, M. Card, S.R. Connolly, C. Folke, R. Grosberg, O. Hoegh-Guldberg, J.B.C. Jackson, J. Kleypas, J.M. Lough, P. Marshall, M. Nyström, S.R. Palumbi, J.M. Pandolfi, B. Rosen and J. Roughgarden. 2003. Climate change, human impacts, and the resilience of coral reefs. Science, 301:929-933. [ Links ]
Lorenz, F. and D. Feshe. 2009. The living Ovuliidae: a manual of the families of allied: Ovulidae, Pediculariidae and Eocypraeidae. ConchBooks, Hackenheinm, Germany. 651 p. [ Links ]
Miller, M. 2001. Corallivorous snail removal: evaluation of impact on Acropora palmata. Coral Reefs , 19:293-295. [ Links ]
Navas-Camacho, R., A. Rodríguez-Ramírez and M. Reyes-Nivia. 2010. Agents of coral mortality on reef formations of the Colombian Pacific. Rev. Biol. Trop ., 58:133-138. [ Links ]
Palacios, M., C. Muñoz andF. Zapata. 2014. Fish corallivory on a pocilloporid reef and experimental coral responses to predation. Coral Reefs , 33: 625 - 636. [ Links ]
Parada, C. y N. Nikolaevna. 1990. Foraminíferos y sedimendos de Playa Blanca, Isla Gorgona. Geol. Col., 17:227-327. [ Links ]
Paz-García, D., A. Aldana-Moreno, R. Cabral-Tena and E. Balart. 2012. High predation by the corallivore sea snail Jenneria pustulata in a high-latitude reef in the Gulf of California. Mar. Bio. Rec., 5: 1-2. [ Links ]
Prahl, H. 1986. Corales y arrecifes coralinos. Isla de Gorgona. Biblioteca Banco Popular, Bogotá. 252 p. [ Links ]
R Development Core Team. 2014. R: A language and environment for statistical computing. http://www.Rproject.org. 07/07/2014. [ Links ]
Rotjan, R. and S. Lewis. 2008. Impact of coral predators on tropical reefs. Mar. Ecol. Prog. Ser., 367: 73-91. [ Links ]
Sánchez, J., P. Fuentes-Pardo, Í. Ní Almhain, E. Ardila-Espitia, J. Cantera-Kintz and M. Forero-Shelton. 2016. The masquerade game: marine mimicry adaptation between egg-cowries and octocorals. PeerJ 4:e2051 https://doi.org/10.7717/peerj.2051. [ Links ]
Simone, L. 2004. Morphology and Phylogeny of the Cypraeoidea (Mollusca, Caenogastropoda). Papel Virtual Editora, Río de Janeiro. 186 p. [ Links ]
Starrett, A. 1993. Adaptative resemblance: A unifying concept for mimicry and crypsis. Biol. J. Lin. Soc., 48: 299-317. [ Links ]
Titlyanov, E. and T. Titlyanova. 2009. The Dynamics of the Restoration of Mechanical Damage to Colonies of the Scleractinian Coral Porites lutea under Conditions of Competition with Algal Settlers for Substratum. Rus. J. Mar. Biol., 3: 230-235. [ Links ]
Titlyanov, E ., T. Titlyanova , M. Yakovleva, Y. Nakano and R. Bhagooli. 2005. Regeneration of artificial injuries on scleractinian corals and coral/algal competition for newly formed substrate. J. Exp. Mar. Bio. Ecol., 323: 27-42. [ Links ]
Traylor-Knowles, N. 2016. Distinctive wound-healing characteristics in the corals Pocillopora damicornis and Acropora hyacinthus found in two different temperature regimes. Mar. Biol., 163: 231. [ Links ]
Valiente-Banuet, A., M. Aizen, J. Alcántara, J. Arroyo, A. Cocucci, et al. 2015. Beyond species loss: the extinction of ecological interactions in a changing world. Functional Ecology, 29: 299-307. [ Links ]
White, A., P. Helge and A. Tijen. 2000. Philippine coral reefs under threat: the economic losses caused by reef destruction. Mar. Pollut. Bull., 40:598-605. [ Links ]
Wilbur, K. and G. Owen. 1964. Growth. Physiology of Mollusca. Vol I. Academic Press, New York. 473 p. [ Links ]
Zapata, F. 2001. Formaciones coralinas de isla Gorgona. 27-40. En: López-Victoria, m. y L.M. Barrios. (Eds.). Gorgona marina: contribución al conocimiento de una isla única. INVEMAR, Santa Marta. 160 p. [ Links ]
Zapata, F. and B. Vargas-Ángel. 2003. Corals and coral reefs of the Pacific coast of Colombia. 419-447. En: Cortés, J. (Ed.). Coral Reefs of Latin America. Elsevier, Amsterdam. 497 p. [ Links ]
Zapata, F ., A. Rodríguez-Ramírez , C. Caro-Zambrano and J. Garzón-Ferreira. 2010. Mid-term coral-algal dynamics and conservation status of a Gorgona Island (Tropical Eastern Pacific) coral reef. Rev. Biol. Trop ., 58: 81-94. [ Links ]
Zar, J. H. 1999. Biostatistical Analysis. Prentice Hall. 3rd Edition, New Jersey. 663 p. [ Links ]
Received: July 25, 2017; Accepted: May 01, 2018











 texto en
texto en