Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Boletín de Investigaciones Marinas y Costeras - INVEMAR
versión impresa ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.47 no.2 Santa Marta jul./dic. 2018
https://doi.org/10.25268/bimc.invemar.2018.47.2.745
Research Articles
Preliminary studies of sperm traits and cryopreservation of the Caribbean reef building coral Orbicella Faveolata
1 Instituto de Investigaciones Marinas y Costeras Jose Benito Vives de “Andrés”- INVEMAR. For correspondence: majuli_19@hotmail.com
2 Fundación para la Investigación y Conservación Biológica Marina - Ecomares, Colombia. valeria.pizarro@ecomares.org
Cryopreservation has been recently applied to coral gametes and tissue with successful results that can be applied for different purposes on coral conservation and restoration. In this study, we decided to determine the sperm morphology of the coral Orbicella faveolata and assess the feasibility of sperm cryopreservation using a combination of intracellular (1,2-Propadiol) and extracellular (milk) cryoprotectants, and two frozen treatments for 24 h. Mature spermatozoa had a triangular-like head shape measuring 4.10 ± 0.69 µm (mean ± SD) and long flagellum (43.24 ± 7.99 µm). Fresh sperm remained viable and mobile for more than five hours after being released from the gamete bundles. After cryopreservation, all post-thaw sperm components assessed (morphology, motility and viability) showed no difference in contrast to fresh sperm. This study is the first report of cryopreservation of O. faveolata sperm, however further research is needed to increase the success of the cryopreservation protocol for broad-scale application.
KEYWORDS: Orbicella faveolata; Sexual reproduction; Coral gametes; Morphology; Tayrona National Natural Park
La criopreservación ha sido recientemente aplicada a gametos y tejidos coralinos obteniendo resultados exitosos que pueden ser utilizados para la conservación y la restauración de arrecifes de coral. En este artículo, se determinó la morfología espermática del coral Orbicella faveolata y la viabilidad de la criopreservación del esperma utilizando una combinación de crioprotectores intracelulares (1,2-Propadiol) y extracelulares (leche) en dos tratamientos de congelación durante 24 h. Los espermatozoides maduros tienen una cabeza con forma triangular de 4.10 ± 0.69 µm (promedio ± DS) y un flagelo largo ( 43.24 ± 7 .99 µ m). El esperma fresco permanece viable y motil por más de cinco horas después de ser liberado de las bolsas gaméticas. Todos los componentes evaluados después de descongelado el esperma (morfología, motilidad y viabilidad) no mostraron diferencias significativas con el esperma fresco. Este estudio es el primer registro de criopreservación para el esperma de O. faveolata; sin embargo, es necesario realizar más investigaciones con el fin de incrementar el éxito del protocolo de criopreservación para que este pueda ser aplicado a mayor escala.
PALABRAS CLAVE: Orbicella faveolata; Parque Nacional Natural Tayrona; Reproducción sexual; Gametos de coral; Morfología
INTRODUCTION
Coral reefs are the most complex and productive of all marine ecosystems (Grigg et al., 1984; Díaz et al., 1996). Their biodiversity has been compared to that found in tropical rain forests (Connell, 1978), and have been called ‘oases of the sea’ due to their high productivity (Hoegh-Guldberg, 1999). Unfortunately, during recent decades, coral reefs have undergone major changes worldwide. Several nature- and human-based threats such as global climate change, coral bleaching and diseases contribute to their degradation and destruction (Goldberg and Wilkinson, 2004). Although reef vulnerability and resilience vary in relation to many factors including different species and population-specific responses (Rowan et al., 1977), most coral reefs show changes on community composition (Reaser et al., 2000), and even in some cases both reef structures and marine communities have collapsed (Graham et al., 2006). Consequently, human communities living off these ecosystems have been affected (Wilkinson, 1999).
Different coral conservation strategies have been developed, including in situ conservation practices, which comprise the design and implementation of effective Marine Protected Areas (Carr, 2000), governance, and regulatory and economic policies (White and Courtney, 2004), as well as ecological restoration methods (Edwards, 2010). However, the interaction of global and local threats calls for ex situ conservation practices to maintain and secure the future of coral reefs. Ex situ practices includes different approaches such as the use of on land nurseries for asexual rearing of corals, rearing coral larvae and recruits to juveniles (Rinkevich, 2005; Edwards, 2010) and the preservation of biological samples in frozen banks by cryopreservation (Hagedorn et al., 2010). The latter is an effective conservation tool where cells are frozen in sugar-like compounds called cryoprotectans (Hagedorn and Spindler, 2014). These substances preserve the cell structures intact during the freezing process (Pegg, 2007), while the freezing temperatures stabilize conditions preserving living tissues for decades (Hagedorn and Spindler, 2014).
Gamete cryopreservation has already acted as an effective insurance policy to maintain the genetic diversity of many wildlife species including ferrets, antelopes (Wolf et al., 2001; Wildt et al., 2010) and also marine invertebrates as oysters and sea urchins (Gakhova et al., 1988; Paniagua-Chaves and Tiersch, 2001). Cells that are cryopreserved and banked properly can retain viability for years without DNA damage (Hagedorn et al., 2012). However, the use of these frozen banks is new for coral species (Hagedorn et al., 2012), and is expected to secure existing species and genetic diversity as part of rehabilitation processes. Sperm cryopreservation has been successfully applied in some species of corals, including Acropora palmata (from the Caribbean), A. millepora, A. tenuis, A. loripes, Fungia scutaria, Platygyra lamolina, P. daedalea and Goniastrea aspera (Hagedorn et al., 2006; Hagedorn and Spindler, 2014). No coral larvae and eggs have yet been successfully cryopreserved (Hagedorn and Spindler, 2014).
As most Caribbean coral reef areas, Colombian coral reefs health and ecosystem services are decreasing. Between 2010 and 2013, three Colombian institutions (Universidad Jorge Tadeo Lozano, Calipso Dive Center and Tayrona National Natural Park Authority) joined their work towards coral reef restoration projects. This study was part of this effort, and aim to describe the spermatozoa morphology on the Caribbean reef building coral Orbicella faveolata and to assess gamete performance in terms of motility and vitality after two different cryopreservation treatments.
MATERIALS AND METHODS
Collection of sperm
Coral sperm was collected during the spawning event of September 6th, 2012 at Gayraca Bay (Tayrona National Natural Park - Colombia), from five colonies of the reef builder coral O. faveolata. This species is hermaphroditic and has external fertilization, releasing one night per year gamete bundles that contain both eggs and sperm (Levitan et al., 2011). These bundles are highly buoyant and therefore their collection was done using modified net traps (Vermeij et al., 2003). Gamete bundles were obtained from net traps, transported to land where they were gently agitated until separate sperm from eggs using sterilized seawater and a 60 mm plankton mesh.
Assessment of sperm morphology and motility
The morphology and quality of the sperm were characterized following methods by Rurangwa et al. (2004) and Gaitán-Espitia et al. (2013). To describe sperm morphology one drop of sperm was placed on a microscope slide and covered with a glass coverslip. Digital images (n=10) were obtained using a digital camera (Nikon digital sight DS-Fi1) attached to a microscope (Nikon eclipse E200). These images were analyzed using software-free Image J (Abramoff et al., 2004) to measure the length of the head and the flagellum of the spermatozoa. Sperm was observed under the microscope (100x magnification) and spermatozoa motility duration and viability were assessed. Motility duration was defined as the time period leading to cessation of any movement. Sperm viability, defined as the percentage of living sperm cells, was determined using the eosin-nigrosin method (Rose and Heath, 1978), all live and dead sperm cells were quantified. A smear was prepared by mixing one drop of coral sperm with one drop of eosin:nigrosin (1:1) in each of five slides. After being air-dried, these slides were examined under x100 magnification (Nikon eclipse E200). Alive spermatozoa did not stain (uncolored), while dead cells stained red. Sperm motility and viability were assessed every two hours. After 6 hours sperm stop moving but still alive, and the last assessment were made 8 hours after sperm released from gamete bundles considering that it no longer fulfilled the viability conditions.
Cryopreservation assays
The cryoprotectant used in the assays consisted of a solution of 1,2-propadiol, powdered milk and ringer-C (modified from Gaitán-Espitia et al., 2013). This cryoprotectant was mixed with coral sperm in a 1:10 proportion respectively and allowed to equilibrate for 20 minutes approximately (Gaitán-Espitia et al., 2013). A total of 20 French straws of 0.5 ml were filled with the mix and divided in two groups for the cryopreservation essays: the first group was stored at 4° C (slow cryopreservation treatment) and the second group in liquid nitrogen at -196°C (fast cryopreservation treatment), both for 24 h. To determinate the success of these assays and the differences between them, all straws were thawed in a water bath at 37° C until the liquid medium was visible (González and Díaz, 2001; Gaitán- Espitia et al., 2013). Immediately after thawing, part of the cryopreserved sperm (about 5 mL) was removed, placed on a microscope slide and observed under 100x magnification. Both sperm motility and vitality were measured. To find if the cryopreservation had any effect on the motility and the vitality of the coral sperm, a contingency table was built, and a Chi-square-test of independence was performed. These analyses allowed the comparison between cryopreservation essays. All statistical analysis were performed with the software STATGRAPHICS 5.0 (Moreno-Gil, 1998).
RESULTS
Sperm morphology, motility and vitality
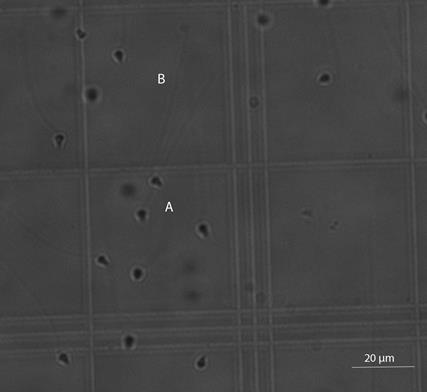
Orbicella faveolata sperm have spade shape heads and long tails (Figure 1). Mature spermatozoa had a mean flagellum length of 43.24 ± 7.99 µm (mean ± SD), and a mean head length of 4.10 ± 0.69 µm (N= 40 spermatozoa were measured). Sperm density was 8.58 x106 spermatozoa ml-1.
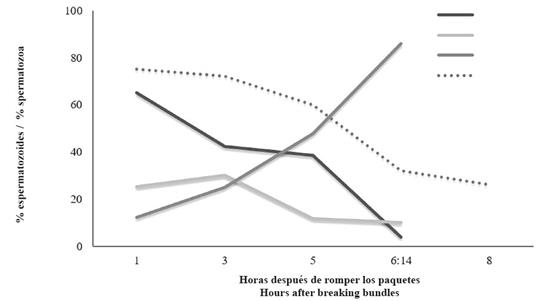
After being released from the gamete bundles, 90% of the sperm had motility; as expected, sperm motility decreased over time and the last movement was observed on less of 15% of sperm after 6 h and 14 min of breaking the gamete bundles. Fresh sperm motility varied from swimming (65.0 ± 1.26 %) to vibrating (whip-like motion; 23.5 ± 1.29 %) and, 12.3 ± 1.43 % was inactive (no motile). Three hours after breaking the gamete bundles 42.3 ± 1.01 % were swimming, 30.1 ± 0.91 % vibrating, and 25.1 ± 1.26 % inactive. After five hours 38.6 ± 1.29 % were vibrating, 11.8 ± 0.34 % swimming, and 47.9 ± 2.80 % inactive. Although sperm viability decreased with time as well, its reduction was less pronounced than its motility. Alive spermatozoa in fresh sperm were 75%, and after five hours around 60%, declining to less than 30% after eight hours (Figure 2).
Cryopreservation assays
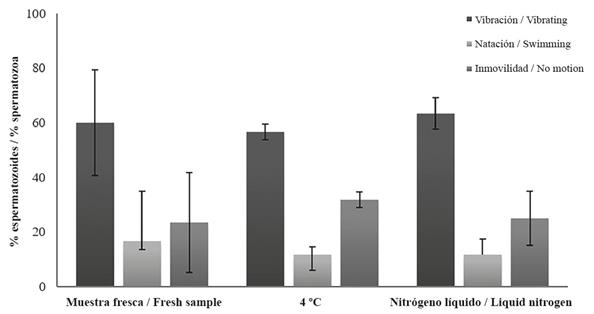
Although there was a slight decrease in the sperm swimming abilities, no statistical differences were found between fresh sperm and both treatments for cryopreserved sperm motility and vitality (X2=2.92, p=0.572; X2=1.69, p=0.428 respectively. Figure 3). After 24 h, the sperm from the slow cryopreservation treatment (4 °C) was 70% viable and moving, while the sperm exposed to the quick cryopreservation (liquid nitrogen) treatment had a vitality average of 55% and motility average of 75% after 24 h. No changes in the sperm morphology were observed within the treatments.
DISCUSSION
In this study, we describe the spermatozoa morphology of the coral Orbicella faveolata as well as two cryopreservation methods (at 4 °C and in liquid nitrogen) used to successfully preserve sperm for 24 h, maintaining at least 70% of sperm motility and vitality after thawing. This is the first study on O. faveolata sperm cryopreservation.
The observed triangular-like head shape sperm observed for O. faveolata is common in many marine invertebrates such as sea urchins (Landry et al., 2003). Specifically for corals, two different spermatozoa head shapes have been previously described: pear-shaped and elongated conical head (Harrison and Wallace, 1990). O. faveolata spermatozoa shape resembles the second one. As described by Harrison and Wallace (1990), the anterior process located on the spermatozoa head could have an acrosomal function; however, this has never been studied. Although differences in head length could be a species- specific taxonomic trait and be used to identify genetic clades (Briones et al., 2012), there is no data for O. faveolata or other Orbicellids for comparisons. Available data from Albright (2011), who measured the O. faveolata head area using scanning electron micrographs, could not be used for comparison due to the difference on the resolution between images.
Sperm limitation, defined as the dilution of sperm in seawater (Yund, 2000), is one of the key factors limiting reproductive success of sessile broadcast- spawning invertebrates (Levitan et al., 1992; Oliver and Babcock, 1992). To maximize the success of fertilization and hence reproduction, multiple strategies have evolved (Yund, 2000). Some of these strategies in broadcasting corals are spawning synchrony (Harrison, 2011), floating gametes (Oliver and Babcock, 1992), and specific sperm morphology and longevity (Benzie and Dixon, 1994). O. faveolata colonies have simultaneous spawning events, and both egg and sperm bundles are positively buoyant. Gamete interactions are controlled by attributes like egg size, sperm swimming ability, and how likely a sperm-egg collision is to result in fertilization (Levitan and Petersen, 1995). Long spermatozoa flagella are associated to higher swimming speed and longer distances traveled, which is highly important for fertilization success (Fitzpatrick et al., 2010). In other species of marine invertebrates as the black sea urchin, Diadema antillarum, has been measured that sperm availability and proximity between male and female during the spawn events affects the fertilization success (Levitan, 1991).
Sperm longevity in free-spawning marine invertebrates varies from few hours to days and, besides being species specific depends on concentration, temperature and egg contact (Jhonson and Yun, 2004). Our results shown than more than 50% of the spermatozoa can stay alive and mobile for six hours after being released. This factor could play an important part in the reproductive success of O. faveolata despite ocean dilution (Jhonson and Yun, 2004). It has been generally assumed that dilution of sperm in the water reduced sperm longevity in tens of minutes (Levitan, 1991); however, our results show that it is possible that O. faveolata sperm remains viable for long times despite seawater dilution, increasing the chances of fertilizing eggs from isolated colonies.
For several marine invertebrates, including some coral species of the genera Acropora, sperm motility initiates in response to specific chemical cues secreted by the eggs (Morisawa, 1994; Yoshida et al., 2002; Morita et al., 2006). Specifically, for three species of Acropora from the Pacific, sperm movement was initiated in the presence of eggs at 150-300 mm distance when pH was increased by adding choline chloride (Na-free artificial seawater containing NH4Cl) to the artificial seawater (Morita et al., 2006). These authors argue that sperm regulatory mechanisms are modulated by egg secretions which play an important role in the fertilization success. As Acropora spp., the Orbicella annularis species complex has similar spawning times (Fukami et al., 2004): nevertheless, in this study sperm motility in O. faveolata was observed from the beginning when gametes were separated and eggs were absent in the samples. Thus, as sperm regulatory mechanisms are modulated by egg secretions, it is possible that some of the egg secretions were washed with the sperm during separation or that the chemical inducing sperm movement for this species is present in the seawater. This aspect must be further studied.
Orbicella faveolata sperm cryopreservation assays were successful; the sperm exposed to the slow cryopreservation treatment had a post thaw average motility of 70%, and the one exposed to quick cryopreservation treatment had a motility of 75%. In both treatments, sperm morphology did not chanced. In comparison, Hagedorn et al. (2006) obtained a post-thaw sperm motility of >95% after storage but noticed a change in the morphology of the sperm in both the head diameter and tail length. It seems that these changes were a result of the cryoprotectant used. All cryoprotectants are toxic or lethal to sperm cells, especially at high concentrations. In our study, the absence of sperm morphology changes could be the result of combining intracellular (1,2-Propadiol) and extracellular (milk) cryoprotectant. The extracellular could provide an initial protection against the formation of intracellular ice (Gaitán-Espitia et al., 2013) and the milk’s lipid content may stabilize cell membranes during freeze-thaw processes (Odintsova and Boroda, 2012).
Although we assessed sperm performance in terms of motility and vitality, the next challenge is determining fertilization success of the sperm after cryogenic preservation. Frozen-thawed sperm from different species of corals have been used to fertilize conspecific egg released in the same spawn and from successive spawns, reaching fertilization success of 60%, and resulting in functioning larvae (Hagedorn et al., 2012). Moreover, in preliminary experiments on A. clathrata, larvae produced from frozen-thawed sperm developed, settled and assimilated symbionts creating new corals in vitro (Hagedorn and Spindler, 2014).
All cryopreservation methods in coral have been successfully applied to sperm, however coral oocytes have not been successfully cryopreserved yet. One of the main reasons is eggs sensitivity to chilling (Hagedorn and Spindler, 2014) which results on affectations on the cell membrane properties, function and integrity (Lin and Tsai, 2012). We obtained similar results when tested cryopreservation of O. faveolata oocytes, after the freeze-thaw processes all oocytes disintegrated.
This work supports Hagedorn et al. (2006; 2012) calls for the development of cryopreservation protocols that can be used to create a cryobiology bank to secure existing coral species and their genetic diversity. Today, there are three long-term cryobanks in the world where coral cells are stored, two of them located in the U.S. at the Smithsonian Institution at the Hawaii Institute of Marine Biology and the U.S. department of Agriculture´s Animal Germplasm Program, and one in Australia at the Taronga Western Plains Zoo (Hagedorn and Spindler, 2014). Cryopreservation of coral gametes could play an important role in near-future coral restoration and may also contribute to expand coral nurseries projects by including sexually reproductive corals (Hagedorn and Spindler, 2014), that like O. faveolata, reproduces sexually only once a year.
More studies on coral cryopreservation techniques, fertilization success and development of larvae and recruits after cryopreservation are in need. The use of sperm cryopreservation techniques, such as the one tested in this study, can potentially help to preserve gene diversity, prevent coral extinctions, and create opportunities for diversifying shrinking populations by avoiding natural loss in heterozygosity due to genetic drift (Hagedorn and Spindler, 2014). In addition, it can allows us to advance in research on coral developmental biology, genetics, systematics and molecular biology.
ACKNOWLEDGEMENTS
Special thanks for the institutions that were part of the project and support us all the way: Universidad Jorge Tadeo Lozano (UJTL), Calipso Dive Center and Tayrona National Natural Park Authority; and to all that were part of the project. Special thanks to M.A. Martínez-Silva for the advice and assistance in the laboratory, Vanessa Carrillo from the Universidad Jorge Tadeo Lozano (UJTL) and Cesar García (TNNP) for their invaluable collaboration, and to all the volunteers that helped us in the field. Financial support was provided by UJTL, TNNP and the Fundación para la Promoción de la Investigación y la Tecnología, Banco de la República (Grant # 3193). Finally, we want to thank the two reviewers of the manuscript, their comments improved the quality of the manuscript.
REFERENCES
Abrámoff, M.D., P.J. Magalhaes and S.J. Ram. 2004. Image processing with Image J. Imaging software. 7. [ Links ]
Albright, A. 2011. Effects of ocean acidification on early life history stages of Caribbean scleractinian corals. Ph.D. Thesis, University of Miami/Rosenstiel School of Marine and Atmospheric Science, Miami. 137 p. [ Links ]
Benzie, J. and P. Dixon. 1994. The effects of sperm concentration, sperm: egg ratio, and gamete age on fertilization success in crown-of-thorns starfish (Acanthaster planci) in the laboratory. Biol. Bull., 186: 139-152. [ Links ]
Briones, C., R. Guiñez, O. Garrido, P.A. Oyarzún, J.E. Toro and M. Pérez. 2012. Sperm polymorphism and genetic divergence in the mussel Perumytilus purpuratus. Mar. Biol., 159: 1865-1870. [ Links ]
Carr, M.H. 2000. Marine Protected Areas: challenges and opportunities for understanding and conserving coastal marine ecosystems. Environ. Conserv., 27: 106-109. [ Links ]
Connell, J.H. 1978. Diversity in tropical rain forest and coral reefs. Science, 199: 1302-1310. [ Links ]
Edwards, A. 2010. Reef rehabilitation manual. Coral Reef Targeted Research and Capacity Building for Management. Santa Lucía, Australia. 166 p. [ Links ]
Fitzpatrick, J.L., F. García-Gonzalez and J.P. Evans. 2010. Linking sperm length and velocity: The importance of intramale variation. Biol. Lett., 6: 797-799. [ Links ]
Fukami, H., A.F. Budd, D.R. Levitan, J. Jara, R. Kersanach and N. Knowlton. 2004. Geographic differences in species boundaries among members of the Montastraea annularis complex based on molecular and morphological markers. Evolution, 58: 324-337. [ Links ]
Gaitán-Espitia, J.D., M.A. Martínez-Silva, C.E. Borrero, L. Ramírez and J.P. Valencia. 2013. Cryogenic preservation of sperm from lane snapper (Lutjanus synagris): Testing the effects of extenders and freezing rates on sperm quality. Aquaculture, 384-387: 6-12. [ Links ]
Gakhova, E.N., I.V. Krasts, T. Naidenko, N.A. Savel and B.I. Bessonov. 1988. Embryonic development of sea urchin after low temperature preservation. Ontogenez, 19: 175-180. [ Links ]
Goldberg, J. and C. Wilkinson. 2004. Global threats to coral reefs: coral bleaching, global climate change, disease, predator plagues, and invasive species. 67-92. In: Wilkinson, C. (Ed.) Status of coral reefs of the world: 2004. Global Coral Reef Monitoring Network, Sidney, Australia. 572 p. [ Links ]
González, E. y J. Díaz. 2001. Principios básicos de la criopreservación de esperma en peces. 253-264. In: Rodríguez, H., P. Victoria and M. Carrillo (Eds.). Fundamentos de acuicultura continental. INPA-Serie Fundamentos No 1, Bogotá. 286 p. [ Links ]
Graham, N.A.J., S. Wilson, S. Jennings, N.V.C. Polunin and J.P. Bijoux. 2006. Dynamic fragility of oceanic coral reef ecosystems. Proc. Natl. Acad. Sci., 103: 8425-8429. [ Links ]
Hagedorn, M. and R. Spindler. 2014. The reality, use and potential for cryopreservation of coral reefs. 317-329. In: Holt, W., J.L. Brown and P. Comizzoli (Eds.). Reproductive sciences in animal Conservation. Adv. Exp. Med. Biol., Springer, New York. 753 p. [ Links ]
Hagedorn, M ., V.L. Carter, R.A. Steyn, D. Krupp, J.C. Leong, R.P. Lang and T.R. Tiersch. 2006. Preliminary studies of sperm cryopreservation in the mushroom coral, Fungia scutaria. Cryobiology, 52: 454-458. [ Links ]
Hagedorn, M ., V.L. Carter , J.C. Leong and F.W. Kleinhans. 2010. Physiology and cryosensitivity of coral endosymbiotic algae (Symbiodinium). Cryobiology, 60: 147-158. [ Links ]
Hagedorn, M ., M.J.H. Van Oppen, V. Carter, M. Henley, D. Abrego, E. Puill-Stephan, A. Negri, A. Heyward, D. MacFarlane and R. Spindler. 2012. First frozen repository for the Great Barrier Reef coral created. Cryobiology, 65: 157-158. [ Links ]
Harrison, P.L. 2011. Sexual reproduction of scleractinian corals. 59-85. In: Dubinsky, Z. and N. Stambler (Eds.). Coral reefs: An ecosystem in transition. Springer, Netherlands. 552 p. [ Links ]
Harrison, P.L. and C.C. Wallace. 1990. Reproduction, dispersal and recruitment of scleractinian corals. 133-207. In: Dubinsky, Z (Ed.). Coral Reefs. Ecosystems of the World. Elsevier, Amsterdam. 550 p. [ Links ]
Hoegh-Guldberg, O. 1999. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Fresh. Res., 50: 839-866. [ Links ]
Johnson, S.L. and P.O., Yun. 2004. Remarkable longevity of dilute sperm in a free-spawning colonial ascidian. Biol. Bull ., 39: 144-151. [ Links ]
Landry, C., L.B. Geyer, Y. Arakaki, T. Uehara and S.R. Palumbi. 2003. Recent speciation in the Indo-West Pacific: Rapid evolution of gamete recognition and sperm morphology in cryptic species of sea urchin. Proc. Biol. Sci., 270: 1839-1847. [ Links ]
Levitan, D.R. 1991. Influence of body size and population density on fertilization success and reproductive output in a free-spawning invertebrate. Biol. Bull ., 181: 261-268. [ Links ]
Levitan, D.R. and C. Petersen. 1993. Sperm limitation in the sea. TREE reviews, 10(6): 228-231. [ Links ]
Levitan, D.R ., M.A. Sewell and F.S. Chia. 1992. How distribution and abundance influences fertilization success in the sea urchin Strongylocentrotus franciscanus. Ecology, 73: 248-254. [ Links ]
Levitan, D.R ., N.D. Fogarty, J. Jara , K.E. Lotterhos and N. Knowlton . 2011. Genetic, spatial, and temporal components of precise spawning synchrony in reef building corals of the Montastraea annularis species complex. Evolution, 65: 1254-1270. [ Links ]
Lin, C. and S. Tsai. 2012. The effect of chilling and cryoprotectants on hard coral (Echinopora spp.) oocytes during short-term low temperature preservation. Theriogenology, 77: 1257-1261. [ Links ]
Moreno-Gil, J.S. 1998. Procedimientos estadísticos con Statgraphics. Esic editorial, Madrid, España. 528 p. [ Links ]
Morisawa, M. 1994. Cell signaling mechanism for sperm motility. Zool. Sci., 11: 647-662. [ Links ]
Morita, M., A. Nishikawa, A. Nakajima, A. Iguchi, K. Sakai, A. Takemura and M. Okuno. 2006. Eggs regulate sperm flagellar motility initiation, chemotaxis and inhibition in the coral Acropora digitifera, A. gemmifera and A. tenuis. J. Exp. Biol., 209: 4574-4579. [ Links ]
Odintsova, N.A. and A.V. Boroda. 2012. Cryopreservation of the cells and larvae of marine organisms. Russ. J. Mar. Biol ., 38(2): 101-111. [ Links ]
Oliver, J. and R. Babcock. 1992. Aspects of the fertilization ecology of broadcast spawning corals: Sperm dilution effects and in situ measurements of fertilization. Biol Bull., 183: 409-417. [ Links ]
Paniagua-Chávez, C.G. and T.R. Tiersch . 2001. Laboratory studies of cryopreservation of sperm and trochophore larvae of the eastern oyster. Cryobiology, 43: 211-223. [ Links ]
Pegg, D. E. 2007. Principles of cryopreservation. Meth. Mol. Biol., 368: 39-57. [ Links ]
Rearser, J.K., R. Pomerance and P.O. Thomas. 2000. Coral bleaching and global climate change: Scientific findings and policy recommendations. Cons. Biol., 14: 1500-1511. [ Links ]
Rinkevich, B. 2005. Conservation of coral reefs through active restoration measures: recent approaches and last decade progress. Environ. Sci. Technol., 39: 4333-4342. [ Links ]
Rose, C.D. and E. Heath. 1978. Viability of American oyster, Crassotrea virginica, spermatozoa exposed to stress. Estuaries, 1: 245-251. [ Links ]
Rurangwa, E., D. Kime, F. Ollevier and J. Nash. 2004. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture, 243: 1-28. [ Links ]
Vermeij, M.J.A., E. Sampayo, K. Bröker and R.P.M. Bak. 2003. Variation in planulae release of closely related coral species. Mar. Ecol. Prog. Ser., 247: 75-84. [ Links ]
White, A.T. and C.A. Courtney. 2004. Policy instruments for coral reef management and their effectivenes: 128-148. In: Ahmed, M., C.K. Chong and H. Cesar (Eds.). Economic valuation and policy priorities for sustainable management of coral reefs. WorldFish Center Conf. Proc. 70: 222 p. [ Links ]
Wildt, D.E., P. Comizzoli , B. Pukazhenthi and N. Songsasen. 2010. Lessons from biodiversity-The value of nontraditional species to advance reproductive science, conservation, and human health. Mol. Reprod. Dev., 77: 397-409. [ Links ]
Wilkinson, C.R. 1999 Global and local threats to coral reef functioning and existence: Review and predictions. Mar. Fresh. Res ., 50: 867-878. [ Links ]
Wolf, K.N., D.E. Wildt, A. Vargasm P.E. Marinari, M.A. Ottinger and J.G. Howard. 2001. Reproductive inefficiency in male black-footed ferrets (Mustela nigripes). Zoo. Biol., 19: 517-528. [ Links ]
Yoshida, M., K. Inaba, K. Ishida and M. Morisawa. 2002. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc. Natl. Acad. Sci ., 99: 14831-14836. [ Links ]
Yund, P.O. 2000. How severe is sperm limitation in natural populations of marine free-spawners? Trends. Ecol. Evol., 15: 10-13. [ Links ]
Received: November 29, 2017; Accepted: June 25, 2018











 texto en
texto en