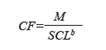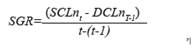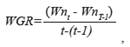Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.47 no.2 Santa Marta July/Dec. 2018
https://doi.org/10.25268/bimc.invemar.2018.47.2.751
Research Articles
Use of commercial foods in the headstarting of hawksbill turtles (Eretmochelys imbricata, Cheloniidae)
1 Grupo de investigación Dinámica y Manejo de Ecosistemas Marinos Costeros, Programa de Biología Marina, Facultad de Ciencias Naturales e Ingeniería, Universidad de Bogotá Jorge Tadeo Lozano, Carrera 2 # 11-68 Edificio Mundo Marino El Rodadero, Santa Marta, Colombia. sarmientoricardo20@yahoo.es. aminta.jauregui@utadeo.edu.co; adolfo.sanjuan@utadeo.edu.co
Headstarting is a recovery strategy for sea turtle populations. It requires captive handling of hatchlings, which are transferred from nesting beaches with low percentages of hatching success. Providing adequate nutritional resources for hatchlings is costly but important, as it influences growth rates of young turtles. Assessing the potential of commercial diets as option for promoting healthy growth and reducing the costs of maintenance for captive Hawksbill Turtles, we evaluated the viability of two commercial feeds on the growth rates of the Hawksbill Turtle (Eretmochelys imbricata). We fed turtles to satiation twice a day between the sixth and tenth month of age. Individuals fed with fish flour meal (n=20) exhibited average body mass and straight carapace length (SCL) growth rates of 2.45±1.39 g.day-1 and 0.04±0.02 cm.day-1, respectively. The turtles fed with squid flour meal (n =13) displayed growth rates of 3.35±1.11 g.day-1 and 0.04±0.01 cm.day-1. These differences, associated with the low avidity of the specimens for these pellets, may be due to the food characteristics, particularly the size, flotation capability and palatability of the food. However, the presence of amino acids and vitamins in these compounds, and their low cost, can make them viable as a supplementary item suggesting the use of commercial foods only as a dietary supplement.
KEYWORDS: Artificial feeding; Captivity; Commercial meals; Sea turtles; Size and body mass rates of growth
El levante o headstarting es una estrategia de recuperación para poblaciones de tortugas marinas donde los neonatos son mantenidos en cautiverio por períodos de unos pocos meses hasta alrededor de un año de edad. Para ello, los nidos son transferidos desde playas de anidación, con porcentajes bajos de éxito de eclosión, hasta viveros controlados; o los neonatos son colectados al momento de la eclosión para su traslado a acuarios. Abastecer a las tortuguillas de dietas nutricionalmente adecuadas es fundamental para asegurar su óptimo desarrollo en cautiverio. Sin embargo, conlleva costos económicos muy elevados. En este estudio se evaluó el efecto de dos alimentos de uso común en acuicultura en el crecimiento de juveniles de tortuga carey (Eretmochelys imbricata), alimentadas a saciedad dos veces al día, entre el sexto y décimo mes de edad. Los individuos alimentados con un concentrado con base en harina de pescado (n = 20), exhibieron una masa corporal promedio y tasas de crecimiento en talla (largo recto del caparazón recto, SCL) de 2.45 ± 1.39 g.día-1 y 0.04 ± 0.02 cm.día-1, respectivamente. Las tortugas alimentadas con un pellet a base de harina de calamar (n = 13), mostraron tasas de crecimiento de 3.35 ± 1.11 g. día-1 y 0.04 ± 0,01 cm.día-1. Las diferencias observadas son relacionadas con la poca avidez de los especímenes por estos alimentos, a su vez, relacionadas con sus características físicas, en particular, el tamaño, la capacidad de flotación y la palatabilidad de los granos. Sin embargo, la presencia de aminoácidos esenciales y vitaminas en estos compuestos, junto a su bajo costo, permiten sugerir el uso de alimentos comerciales como un suplemento complementario en la dieta de tortugas marinas en cautiverio.
PALABRAS CLAVE: Alimentación artificial; Cautiverio; Comidas comerciales; Tortugas marinas; Tasas de crecimiento en talla y peso corporal
INTRODUCTION
Hawksbill Turtles (Eretmochelys imbricata) are listed as a critically endangered species by the International Union for Conservation of Nature (IUCN). Reviews of the trends on the nesting beaches of the Caribbean region in previous decades showed the small size of the population (>100 nesting females per country) and the overall reduction trends in nesting activity (Meylan, 1999). Estimates based on information crosschecks of trade in turtle shell products and reproductive activity on the nesting beaches show that the Caribbean populations continue to decline (McClenachan et al., 2006; Wyneken et al., 2013). Multiple anthropogenic factors (e.g., lighting) also continue to impact the turtles’ reproductive success (Harewood and Horrocks, 2008). The disappearance of Hawksbill Turtles in several ecosystems has caused deep changes in the structure and biodiversity of coral reefs (McClenachan et al., 2006).
Headstarting is an experimental conservation strategy aimed at promoting recruitment in populations of sea turtles by maintaining hatchlings in captivity during the first months or years, until they reach sizes that allow them to successfully face sources of natural mortality (Mortimer, 1995; Ross, 1999; Burke, 2015). In captivity, turtles have access to higher nutritional quality and bigger amounts of food; In addition, they spend less energy on facing predators, waves or temperature changes. This leads to faster growth rates and perhaps to an earlier age of sexual maturity (Bjorndal et al., 2013b). Once headstarted turtles are released into the marine environment, despite having been in constant contact with humans for months or years, they display the typical behavior of wild turtles (Nagelkerken et al., 2003; Monterrosa and Salazar, 2005). Head-started Hawksbills can deploy migration or resting behaviors that differ from those of wild turtles, although these abilities might be improved by some environmental enrichment during the period of captivity (Okuyama et al., 2010). Recent studies of telemetry monitoring have demonstrated that once released, head-started Hawksbills can migrate to typical feeding areas, using natural routes (Pabón-Aldana et al., 2012). In addition to promoting the turtles’ survival in early life stages, headstarting programs can encourage human awareness, especially among young people, about environmental issues and the importance of biodiversity.
In headstarting, feeding animals constitutes one of the major costs (Pelegrín and Fraga, 2002). The diet ideally should mimic the natural prey and offer the nutritional requirements for proper body development; for example, a diet should ensure proper physiological functioning, good health, and sufficient growth rates to provide an environmental advantage (Espinosa and Labarta, 1987). For Hawksbill Turtles, an opportunistic species with a preference for corals and sponges (León and Bjorndal, 2002), developing a complete diet represents a major challenge. As headstarting requires a large financial investment, the elements of the diet of a species in captivity must present acceptable costs and be locally available.
Headstarting farms and research centers that house sea turtles (E. imbricata, Lepidochelys olivacea, Caretta caretta, and Chelonia mydas) have fed turtles with diets made of fish muscle (Gutiérrez and Cabrera, 1996; Mann et al., 2000a), fresh fish, vegetables (Kanghae et al., 2014), creeping beach plants, macroalgae (Mann et al., 2000b), or squid (Moein et al., 2003). In captive breeding sites in Nagoya (Japan), C. caretta females are fed three times per week with fish flesh, shrimp, squid, and Chinese cabbage as well as macroalgae, in an amount equivalent to 0.5% of their body mass (Kakizoe et al., 2013).
The diet of hatchlings in captivity has also included artificial foods that are produced by mechanical agglomeration and the compaction of mixtures (Tacon, 1989) designed specifically for sea turtles or other reptiles (Endres et al., 2012; Kanghae et al., 2014). The use of artificial diets allows the targeting of specific nutritional requirements for turtles and the promotion of fast growth rates and healthy body condition. Commercial feed manufacturers mix several protein sources to create diets with a balanced amino acid spectrum and portions of protein of high nutritional value (Kellems and Church, 2002). Also, the use of processed foods facilitates the maintenance of water quality in filtered and recirculating pond systems, which leads to reduced logistic costs.
Several studies have attempted to establish optimal conditions for the formulation of artificial diets (Kanghae et al., 2014). With juvenile C. mydas, Wood and Wood (1981) found that the digestibility of the diet increases with protein concentration and they suggested that turtles should be fed with food containing a minimum of 40% protein, at least until reaching 14 weeks of age. Similarly, Harfush et al. (2000) recommended that diets for L. olivacea containing mixtures of fish meat, shrimp heads and enriched meat (dextrin). For C. caretta, Monterrosa and Salazar (2005) compared diets formulated with different concentrations of protein and obtained the highest values of growth rates using protein levels above 40%. Similar results were obtained in tests for E. imbricata where squid meal, compared with other sources of protein, showed the best effects on growth rates and development (Pelegrín et al., 2006).
Other studies have focused on testing the effects of enriching the artificial diets. Pelegrín and Fraga (2002) found that alternating the consumption of fresh protein with feed pellets improves the corporal development of the individuals; including plant material (e.g., Sargassum sp.) between meals also has benefits related to the increased appetite and to the opportunistic habits of the turtles (Mann et al., 2000a). Similarly, Joya and Molina (2006) concluded that a diet based on animal protein presents greater palatability than a diet based on vegetable protein (e.g., soy), despite having a similar nutritional composition. Improved palatability facilitates the consumption of the pellets by the turtles and also influences their assimilation.
Recent studies with juvenile sea turtles in captivity used commercial diets (e.g., Turtle Chow), designed specifically for turtles (see Endres et al., 2012). In some cases, artificial diets have been complemented by dietary supplements, shrimp meat, jellies, or homemade preparations in which vegetables (spinach and lettuce), liver (cooked) and/or fish oil are mixed with A, D, E and B vitamin complexes (Mann et al., 2000a). Commercial diets for turtles have high costs and limited availability for developing countries because of the cost of importation from producer countries but other processed foods are available. Although not specifically formulated for marine turtles, these artificial diets may fulfill the requirements established for the maintenance of these species.
We evaluated the effects on growth rates produced by feeding two commercial diets, originally designed for commercial species such as shrimp (Litopenaeus vannamei) and tilapia (Oreochromis sp.), as exclusive diet in the headstarting of E. imbricata hatchlings between their sixth and tenth month of life. The effects of the diets on condition factors and animal welfare were also analyzed. Our goal was to determine whether these commercial diets are a suitable option for promoting healthy growth and reducing the costs of maintenance for captive Hawksbill Turtles.
MATERIALS AND METHODS
Animals and Maintenance Systems
For the past several years, sea turtle nests at Tayrona National Natural Park (Colombian Caribbean) have been monitored and protected by volunteers or park rangers with the academic support of the Acuario Mundo Marino from the Universidad Jorge Tadeo Lozano (Santa Marta, Colombia). When there is imminent risk of nest loss due to flooding or other causes, the nests are moved to an artificial hatchery until hatching, using standard procedures (Wood and Wood, 1979; Mortimer, 1999). Then, the hatchlings are maintained in captivity for periods of approximately one year, which allows time for studies of the proper conditions for the handling of captive sea turtles and programs of environmental awareness for the local community.
During the 2006 nesting season, a Hawksbill nest on the main beach of the Arrecifes Sector (11º 20’ 15” N - 73º58’ 44” W) was relocated to the hatchery 45 days after oviposition. The turtle neonates (n = 92, hatchling success 85.7%, incubation 62 days) were kept in outdoor 500 L ponds at a density of 30-31 individuals per pond with daily changes (always that was possible) of water and exposed to 12 h light/12 h night, based on the local circadian cycle. Also, a covered trefoil fibe glass tank with an 8.94 m3 (8940 L) capacity, an area of 8.13 m2, and a physical- chemical-biological filtration mechanism was used for the maintenance water quality, subdivided into two or three sections by mobile plastic net barriers.
The turtles were maintained in water at a temperature of approximately 25 °C, salinity of 39.2-39.7, and average pH of 8.05 ± 0.33, conditions suitable for the maintenance of aquatic species in a closed system in a tropical area (Adey and Loveland, 2007). The water was collected from Gaira Bay (Santa Marta, Colombia), which presents a pattern of surface circulation that allows the waters inside the bay to be constantly changed (Franco- Herrera, 2005). The system (tank, surfaces, and accessory structures) was periodically disinfected with commercial sodium hypochlorite and abundant fresh water.
Starting on the sixth day after hatching, we fed turtles twice daily ad libitum with fish muscle from regional species such as: Opisthonema oglinum, Mugil incilis, Scomberomorus brasiliensis, Ocyurus chrysurus and Sicydium sp. and, occasionally, shrimp (Litopenaeus vanamei), squid (Loligo sp.) and crab (Callinectes sp.). These conditions were maintained until the fifth month of age (172 days after hatching), when the clutch (n = 92) was divided into two groups, with 46 individuals per group, to evaluate the effects of the two commercial feeds.
At the beginning of the experiment, individuals had a mean size of 12.5 ± 0.1 cm of straight carapace length (SCL) and a body mass of 305.14 ± 54.68 g. We did not record the clutch incubation temperature that would have allowed us to estimate the sex ratio of the turtles in our study; the sex of the individuals was not considered under the assumption that there are no morphometric differences between male and female juvenile sea turtles (Kilic and Candan, 2014).
Morphometrics
To estimate the growth of the specimens during the experiment, the straight carapace length (SCL; Bolten, 1999) was measured every 10-16 days with 0.1 cm precision, while each specimen weight was recorded using a digital semi-analytical scale (0.01 g precision and maximum capacity of 4000 g). Previously, at the fifth month of age, a two-digit number (00, 01, 02...92) was fixed on the second costal scute of each individual for identification purposes. Numbers were carved into the surface layers of the scute using a mini electric vibro-speed drill (110 V) with a carbide mill (Discover - China).
Experimental diets
For this study, we tested two commercial diets with similar compositions and concentrations of vitamins (Table1), introducing these pellets into the turtles’ diet at the fifth month of age (d 172 post-hatching). Food A is a diet widely used in rearing juvenile tilapia (Oreochromis sp.) at high density. It consisted of light brown spherical particles (2.3-mm diameter) that floated on the surface of the water and remained intact for about two hours. Food B is a supplement for marine shrimps. It consisted of thick, dark brown cylindrical grains (4.5 mm length and 1.5 mm diameter); once in the water, those grains fell directly to the bottom, where they remained intact for long periods. Food A and Food B differed in the presence of some essential amino acids and salts (Table 1) but especially with regard to the protein source. Food A is manufactured from fishmeal, while Food B consists of squid meal. Furthermore, Food A included wheat flour as a binder, while in Food B, rice flour was used to introduce pellets into the diet, the portion of fish muscle offered to the complete experimental group of specimens was gradually reduced from approximately 3 kg day-1 to 0 kg day-1, over five days, while the portion of experimental food offered was increased. After 105 more days (i.e., 110 days total), the turtles were fed pellets twice daily, morning and afternoon. Veterinary management of dermatological and systemic diseases, without any relation to the essay, led to the removal of several individuals from the experiment. During the experiment, several individuals suffered dermic bacterial and fungal infections caused by aggressions that resulted in traumatic injuries (see Bailey, 2008). This kind of infections apparently are common in captive sea turtles, and are promoted by overcrowding in the closed systems, that increase biting incidents, and low water quality conditions (Sison et al., 1990; Bailey, 2008; Chuen- Im et al., 2010). In this case, these injuries were treated daily with topical antibiotic or antiseptic solutions (e.g., rifampin, povidone, iodine tincture 1:2 dilution, oroxytetracycline) and “sunbaths”, resulting in a progressive regeneration of the wounds. In other cases, these diseases resulted in stomatitis with a secondary bacterial infection, which led to the intravenous application of broad-spectrum antibiotics (e.g., enrofloxacin 5 mg/kg i. m. every 48 hours).
Table 1 Composition, nutrients, and characteristics of commercial pellets evaluated in this study as a complete diet for Hawksbill Turtle hatchlings. The ingredients that differentiate them in their composition are highlighted in gray.
| Elemento / Item | Alimento A / Food A | Alimento B / Food B |
|---|---|---|
| Ceniza máxima (%) / Maximum ash (%) | 10 | 10 |
| Fibra máxima (%) / Maximum Fiber (%) | 6 | 4 |
| Bajo en grasa (%) / Low fat (%) | 4 | 6 |
| Humedad máxima (%) / Maximum Humidity (%) | 12 | 12 |
| Bajo en proteínas (%) / Low protein (%) | 38 | 35 |
| Aceite de soya y/o aceite de maíz / Soybean oil and/or corn oil | X | |
| Aceite de pescado / Fish oil | X | X |
| Aceite de calamar / Squid oil | X | |
| Ácido ascórbico (antimicrobiano) / Ascorbic acid (antimicrobial) | X | X |
| Ácido fólico / Folic acid | X | X |
| Arroz / Rice | X | X |
| Biotina / Biotin | X | |
| Antioxidantes BHT (2,6-di-tert-butil) -4-metifenol) oretoxiquina (6-etoxi-, 1,2-dihidro-2, 2,4-trimetil-quinolina) / BHT (2,6-di- tert-butyl) -4-metifenol) orethoxyquin (6-Etoxy-, 1,2-dihydro-2, , 2,4-trimethyl-quinoline)antioxidants | X | X |
| Carbonato de calcio / Calcium carbonate | X | |
| Cloruro de colina / Choline chloride | X | X |
| Cloruro de sodio / Sodium chloride | X | |
| Colesterol / Cholesterol | X | |
| Fosfato bicálcico desfluorado / Calcium phosphate bi de fluorinated | X | |
| Fosfato monosódico / Monosodium phosphate | X | |
| Harina de calamar / Squid meal | X | |
| Harina de pescado / Fish meal | X | |
| Harina de carne / Meat meal | X | |
| Lecitina / Lecitin | X | |
| Lisina / Lisina | X | X |
| Trigo/maíz molido/sorgo / Wheat/ground corn/sorghum | X | X |
| Metionina / Methionine | X | X |
| Niacina / Niacin | X | X |
| Sulfato u óxido de zinc / Zinc sulfate or oxide | X | X |
| Sulfato u óxido de manganeso / Manganese sulfate or oxide | X | X |
| Sulfato u óxido ferroso / Ferrous sulfate or oxide | X | X |
| Pantotenato de calcio / Calcium Pantothenate | X | X |
| Piridoxina / Piridoxin | X | X |
| Riboflavina / Riboflavin | X | X |
| Sulfato de cobre / Copper sulfate | X | X |
| Sulfato de magnesio / Magnesium sulfate | X | |
| Tiamina / Thiamine | X | X |
| Harina de soya / Soy bean meal | X | X |
| Pastel de algodón / Cotton cake | X | |
| Treonina / Threonine | X | |
| Vitaminas A, B12, E, / Vitamin A, B12, E, | X | X |
| Vitamina C / Vitamin C | X | |
| Vitamina D / Vitamin D | X | |
| Vitamina D3 / Vitamin D3 | X | |
| Vitamina K / Vitamin K | X | |
| Yodato de calcio y/o dihidroioduro de etilendiamina (EDDI) / Calcium iodate and/or EDDI | X | X |
| Costo (CO pesos/kg en febrero de 2007) / Cost (CO pesos/kg in February 2007) | $ 1997.5 | $ 2300.0 |
Then, those individuals given the intravenous antibiotics were removed from the experiment because of the potential effects of the veterinary treatment on the individual weight of the turtles. Thus, the estimates presented here are based on measurements made with 33 turtles divided into two groups: Food A (n = 13) and Food B (n = 20). We present estimates for the rest of the turtles fed fresh protein, here called group Fish (n = 43), which were not directly compared with the experimental groups after they had received veterinary treatments that included antibiotic and anti-inflammatory medicines that could influence their body condition.
On d 287, we switched all the turtles to a diet of fish muscle, some dietary supplements, squid and shrimp. Further, before being released into the ocean from the nesting site11 months post hatching, the turtles were fed algae, shrimps and crabs as live foods.
Condition factor
Based on every measurement, we calculated each individual condition factor (CF) as follows:
Where M is the individual mass, SCL is the straight carapace length, and b is the regression coefficient for the regression between height and mass (b = 2.6043, Labrada- Martagón et al., 2010).
Growth Rates
For this study, we define growth as the difference in size (SCL) or mass for each individual during a specific time interval. Based on Bjorndal et al. (2013a) and Casale et al. (2009), the Size Growth Rate (SGR) is estimated as
Where n refers to a specific individual, SCL nt is the size of that individual (n) at a given time (t), and SCLnt-1 is the size of the same individual in a previous measurement made t days prior. There was a period of 10 to 16 days between each SCL measurement. Using a similar approach to that used by Sampson et al. (2015) and Pelegrín et al. (2003a), we estimated weight growth rate (WGR) as the increase in mass of the individual over time, according to the following equation:
Where Wnt is the weight (grams) of the individual at a given time (t), and Wnt-1is the mass of the same individual at the time of the previous measurement (t-1). Then, the effect of the diet on turtle WGR and SGR was estimated by a Repeated Measured ANOVA [Food: A and B; Age (days)], while normality was tested by the Shapiro-Wilk analysis, using statistics software STATISTICA 8.0 by Stat. Soft. Inc.
RESULTS
Acceptance and consumption
Once the experimental foods were introduced to the diet, the behavioral response varied among individuals. For group Food A, when the particles were first laid on the surface of the water, the post-hatchlings had no immediate response. Progressively (minutes), they approached and ate some grains. As the size of the fish muscle portions decreased, their interest in and ingestion of the pellets increased. For Food B, the grains immediately caught the attention of the specimens as they fell by the water column to the bottom of the pond. In response, from the first time they were given this concentrate, the turtles moved to the point where the grains fell, held them in their mouths several times and finally bit and swallowed them. In this case, the grain size made it easy for bits of the feed to escape through the jaw edges.
Five days after the study started, when the fish muscle supply was completely suspended and the offer of experimental foods increased, individuals overall showed inappetence. Then, they showed an increased avidity, especially for Food A. These grains, floating on the water surface, seemed to get their attention and reduce the stress generated by the change of diet. Forty- five days after the start of the study, grain consumption increased from 30 g (approx.0.7 g ind-1day-1) to over 120 g per group. Subsequently, the supply of food was doubled even if the amount of food eaten did not seem to change. This measure was implemented to reduce anxiety and stress among individuals that attacked each other. This behavior is common in captive Hawksbill sea turtle post-hatchlings, probably due to space constraints in the tank (pers. obs.), although density conditions for this essay (6.4 - 11.2 ind. m-2), were close or below those used in similar studies with captive sea turtles (See table 2).
Reduction in Sample Sizes.
During experiments, 16 (17.4%) hatchlings died. Further, 43 became infected with ailments and were treated with full recovery. All these were removed from experimental analyses. Of the initial sample of 46 per trial, we had only 13 (28.3%) in group A and 20 (43.5%) in group B remaining for comparisons.
Assimilation of Food
Body Condition and Growth Rates
For this study, the condition factor presented values between 8.56 and 10.43 x10-4. For this variable, the group fed with Food B had the highest oscillation range, between 9.55 x 10-4(d 233) and 8.56 x 10-4 (d 286).
Size and Weight Growth Rates
The size growth rate (SGR) presented values between 0.01 and 0.21 cm.day-1, normally distributed (Shapiro-Wilk W = 0.96, P = <0.001), with an average of 0.03 ± 0.04 cm day-1. The SGR values did not show a consistent trend but increased or decreased between each measurement (Figure 1).
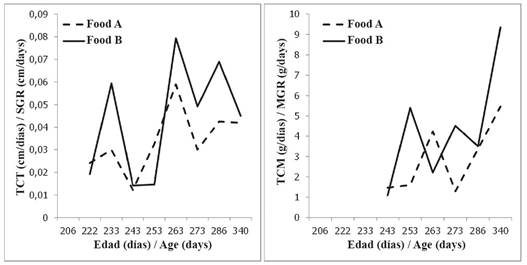
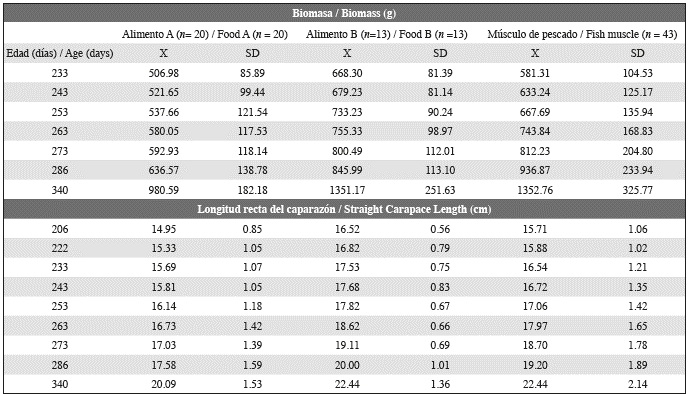
Figure 1 Average growth rates in size (SGR) and body mass (MGR) for two experimental groups (Food A, n: 30 individuals; Food B, n: 13 individuals) of Hawksbill Turtle juveniles fed with commercial diets.
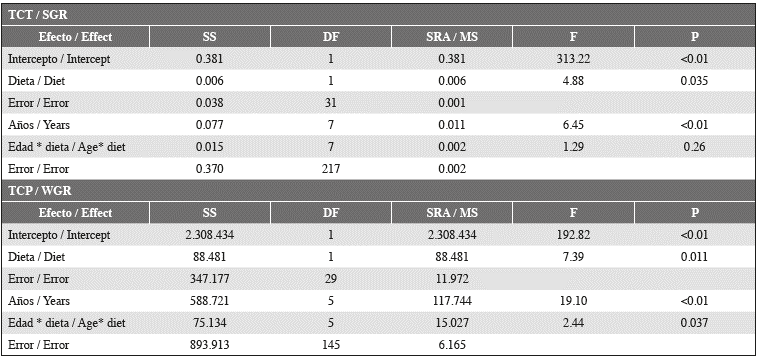
Further, the SGR decreased for all individuals between the second and the last measurement, as evidence of a general slowdown trend of growth when the post- hatchlings approached 11 months of age (Figure 2). A Repeated Measures ANOVA test evidenced (Table 3) significant differences related to both the experimental diet and the date (age) of measuring. However, only in the case of the experimental diets did clear difference appear between the experimental groups, with those individuals fed Food B deploying a higher SGR.
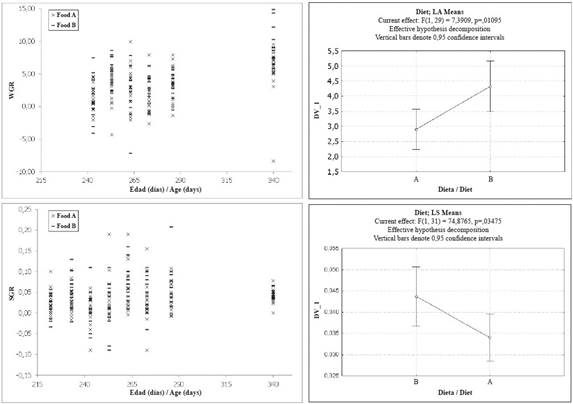
Figure 2 Growth rates in size (SGR) and weight (WGR) for two experimental groups (Food A, n: 30 individuals; Food B, n: 13 individuals) of Hawksbill Turtle juveniles fed with commercial diets.
Weight growth rates for the experimental groups presented values between -8.37 and 14.90 g day-1, normally distributed (Shapiro-Wilk W = 0.987, P = 0.086) with an average value of 3.4 ± 3.4 g day-1. Mean values presented significant differences between groups (t-Test, t-value = 2.42, P = 0.016, df = 193), being higher in the group fed Food B (4.13 ± 3.079 g day-1) than in the one fed Food A (2.92 ± 3.079 g day-1). A Repeated Measures ANOVA (Table 3) evidenced that both diet and age (i.e., date of measure) of the individuals had an effect on the WGR values, although the effect related to the diet was lower. The WGR values did not present a general trend except for an overall increase from the moment the experiment end and the complete group of turtles was fed with fresh protein.
Table 3 Repeated measures analysis of variance for SGR and WGR values for two experimental groups of Hawksbill Turtles fed two different diets (A and B), measured six different times (age) during almost three months of experiment.
Individual weight was compared, considering as explanatory factors the period of experimentation and the different diets tested. This allowed us to establish significant differences between individuals(ANOVA, df= 5, P< 0.001) conditioned by the diet and measuring time (initial vs. final).The proposed ANOVA model explained 87% of the variation (R2). Estimation of the variance components shows that 83% of the variance is due to the age (day) at which individuals were measured rather than to the experimental groups.
Meanwhile, a contrast analysis of the weight differences between individuals showed that the mass for Food A specimens differed significantly from that of both those fed fish muscle and those fed Food B. From this point of view, the weight increase observed here was also influence by differences in development due to individuals. At the end of the captivity period, the individuals presented an average size (SCLmax) and weight of 21.8 ± 2.1 cm and 1255.61 ± 323.72 g, respectively (Table 4).
DISCUSSION
Hawksbill Turtles have a narrow and serrated beak that provides easy access to crevices, and are described as an opportunistic species that feeds on the epibiota of sponges and corals (Witzell, 1983). Although they have a strong preference for invertebrates (León and Bjorndal, 2002; Carrión et al., 2013), several studies based on stomach content analysis show an abundance of macroalgae (Bell, 2013). Because of that, the Hawksbills are currently described primarily as a consumer of macroalgae and second as an omnivore. From this point of view, to replicate an optimal diet for captive post hatchlings-juveniles represents a challenge. In these stages, sea turtles are characterized by high protein and carbohydrate requirements (Pelegrín et al., 2003a; Bell, 2013).
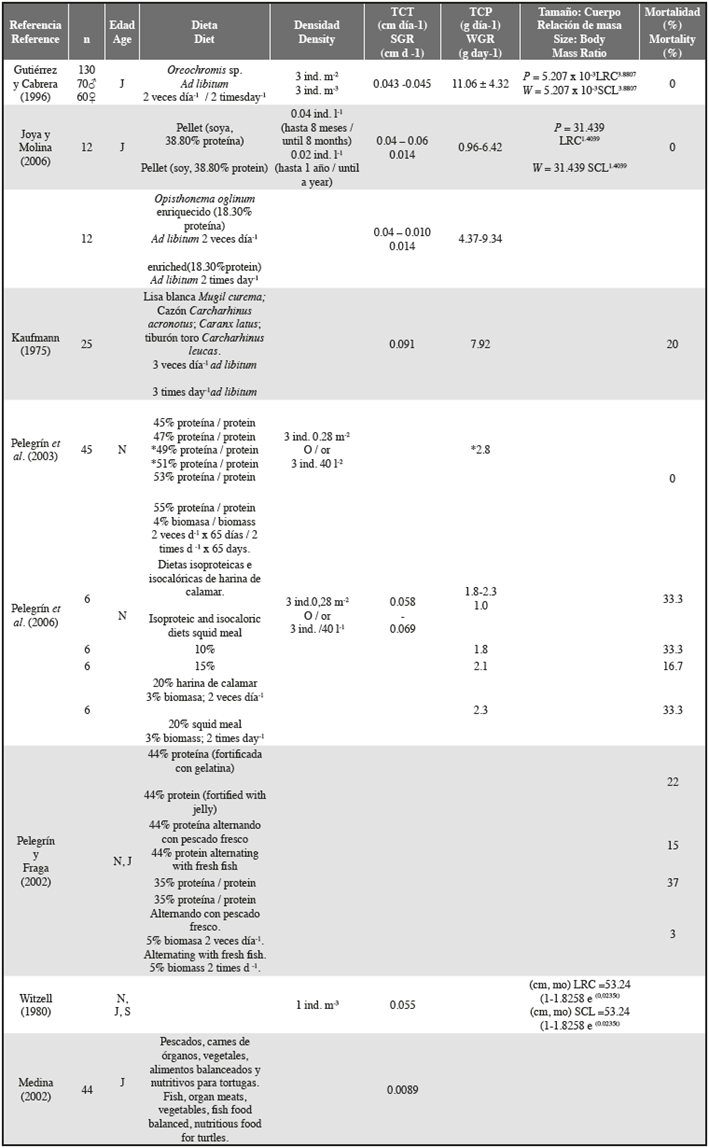
Despite these constraints, the values of body size (16.3-26.8 cm SCL) and body mass (648.29-2221.95 g) obtained at the end of this study are similar to those obtained in previous studies for the maintenance of Hawksbill turtle post- hatchlings. For example, Joya and Molina (2006) reported values of SCL between 22.5 and 24.9 cm and mass between 943.44 g and 1318.08 g for eleven-month-old individuals. Our results for SCL and mass extend beyond these ranges. This may be related to the greater number of individuals used in this study, which all originated from the same nest. Both elements could increase the heterogeneity in the sizes and mass of the specimens.
Gutierrez and Cabrera (1996) reported an average body mass of 1521 ± 280 g and 1534 ± 253g and an SCL of 23.60 ± 1.88 cm and 20.20 ± 1.57cm for two groups of 130 captive Hawksbill Turtle post-hatchlings (Table 2) at 11 months of age. SCL values are similar to those we obtained, but the mass is >250 g higher. Gutierrez and Cabrera (1996) used a diet of fish muscle (Oreochromis sp.) throughout the head-start process.
The use of diets based only on artificial feed, as in this study, led to a lesser body development (weight) and also had an effect on size growth rates. The range of SGR (0.01 and 0.21 cm day-1) was lower than those obtained by Joya and Molina (2006) or Gutiérrez and Cabrera (1996). Our results are consistent with the observations made by Joya and Molina (2006), who concluded that a pellet made of animal protein can promote differences in growth rates and body condition. The values of WGR reported here are below those reported in other studies where individuals were fed with fresh protein and formulated supplements (See Table 2). Besides the lower growth rates obtained by the experimental diets we tested, significantly higher values were evident for both the SGR and the WGR obtained for individuals fed a pellet based on squid meal (Food B). This finding agrees with those of Pelegrín et al. (2006), who found that diets based on this source of protein promoted higher WGR values.
Further, we found that the maximum values of WGR (11.35 g day-1) correspond to the values for individuals fed fresh protein. This finding agrees with the results reported by Gutiérrez and Cabrera (1996), who gave evidence that feeding with fresh protein increased the body development (mass) of captive turtles. When individuals are fed with fresh protein after a long period of artificial meals, they respond by over increasing their consumption, which promotes higher and faster growth rates.
The quantity of Food A and Food B consumed was influenced by the visibility in the water column, which depended on the frequency of the maintenance of the tanks. Eventually, when the visibility inside the tank was reduced after two or three days of not changing the water caused by logistical limitations or maintenance of the reservoir system, the turtles did not eat and showed increased stress and aggressiveness.
The level of food consumption is also influence by each individual’s access to food; this in turn depends on how much it competes with others or the risks it faces to acquire it. In the case of sea turtles, individual size apparently influences the growth of individuals and indirectly impacts the age at which they reach reproductive maturity (Bjorndal et al., 2013a). The smallest individuals in a group grow at a slower rate not only because they are genetically predisposed to do so but also because their body condition limits their ability to access food. Bjorndal et al. (2013a) described this situation by comparing growth rate and size at maturity in captive C. mydas in Cayman Islands. This could explain why the body mass differences obtained between the experimental groups in this study were better explained by the specimens than by the provided diet. From this point of view, new approaches that assess individual assimilation are required, but they should also consider the competition among individuals for food and the energy costs associated with the process of eating.
Shaw et al. (1989) drew attention to the need for caution in the exclusive use of commercial foods in the maintenance of sea turtles. They argued that the use of products derived from soybeans as a protein source in many of these formulations poses risks to the health of reptiles due to high concentrations of phytoestrogens, genistein and diadzein. These compounds present progressive accumulation, and the activity in the bloodstream can cause liver disorders, depression of the pituitary gland, or alterations in sexual development. Joya and Molina (2006) reported lower efficiency using a pellet based on such an oleaginous compound; their results showed lower food palatability and digestibility difficulties for some of its components. Here, it should be noted that although both tested foods have ingredients derived from soy, they are not the basis of its composition. Thus, it is presumed that soy does not represent a risk. Then, there is no evidence linking the results obtained with the presence of soy flour in the composition of foods we tested. However, there are studies that give evidence of a positive effect of soy use for the maintenance of reptiles. Zhou et al. (2016) reported that the replacement of 30% of fresh meal by soybean protein concentrate is feasible if always accompanied by the adhesion of phosphatase enzyme (phytase).
The selection of a food should consider the potential palatability of the diet for the species of interest, which depends not only on its composition but also on the assimilation of nutrients by individuals both individually and in combination with other foods (Espinosa y Labarta, 1987). For artificial foods used for marine turtles, there are no estimations regarding the factors that might influence the palatability of artificial foods, which makes palatability a relevant topic to consider in future studies. Based on our results, conservation programs that might consider captive maintenance of juveniles or post hatchlings sea turtles, must evaluate the response of turtles to artificial foods at earlier life stages, and before including items of high preference for these species, as shrimp or fish muscle, in their diet.
Diets based exclusively on processed foods should consider the composition of such mixtures (protein concentrations should be above 40%) and the associated logistics and economic costs. In economic terms, in March 2007, Food A cost COP $1,997.5 (US$ 0.79) per kilogram, while Food B cost COP $2,300 (US$ 0.91) per kilogram. Maintaining a weekly consumption of 250 g/individual for a group of 100 six-month-old turtles would cost less than COP $500/week (US$ 0.2/week). Approximately 18 kg of fish muscle would be needed to feed 100 six-month-old turtles for a week, which would cost COP $25,000/week (US$ 9.94/week), far more than the cost of the pellets. Also, the palatability for and avidity of the species of interest must be evaluated at the time that the food is offered to the individuals. Based on our results, artificial foods we tested are not adequate to be exclusive diets for E. imbricata hatchlings. However, the presence of amino acids and vitamins in these compounds, and their low cost, can make them viable as a supplementary item for turtles, especially if the foods are based on over 40% of animal protein and include squid meal in their composition. Therefore, there is a need for the execution of new projects measuring the effects of diets composed of different mixtures of fresh meals and artificial foods, or applied at different ages, on the growth and physiology of captive sea turtles.
ACKNOWLEDGMENTS
This study was part of the project “Conservación de Tortugas Marinas Parque Tayrona”, supported by PETROBRAS Unlimited Colombia, Acuario Mundo Marino, Universidad de Bogotá Jorge Tadeo Lozano (Santa Marta Campus), and the Unidad Administrativa Especial de Parques Nacionales Naturales of the Colombian government. The study was part of the activities of Programa de Conservación de Tortugas Marinas (ProCTM), Grupo de Investigación Dinámica y Manejo de Ecosistemas Marino-Costeros (DIMARCO) -UJTL during 2006-2007. We acknowledge the support and participation of all the technical staff and volunteers involved, especially María P. Molina, Catalina Ospina, Juan Manuel Rodriguez Baron, and Paola Saenz, whose efforts made this possible. We also would like to several anonymous reviewers for their comments and suggestions, which improved the presentation of this manuscript. All procedures of animal manipulation were submitted and approved by the Director Committee of the project “Caracterización de las Playas de Anidamiento del Parque Nacional Natural Tayrona - Caribe Colombiano, e Implementación de Estrategias de Manejo y Conservación para las Tortugas Marinas” conducted by Universidad de Bogotá Jorge Tadeo Lozano and Parques Nacionales Naturales de Colombia during 2006-2008.
REFERENCES
Adey, W.H. and K. Loveland. 2007. Dynamic aquaria: building living ecosystems. Academic Press. Washington, D.C. 528 p. [ Links ]
Bailey, T. 2008. Mortality at a Hawksbill Turtle (Eretmochelys Imbricata) rearing center. Wildlife Middle East News, 3:2 [ Links ]
Bell, I. 2013. Algivory in Hawksbill Turtles: Eretmochelys imbricata food selection within a foraging area on the Northern Great Barrier Reef. Mar. Ecol., 34:43-55. [ Links ]
Bjorndal, K., J. Parsons, W. Mustin and A. Bolten. 2013a. Threshold to maturity in a long-lived reptile: interactions of age, size, and growth. Mar. Biol., 160: 607-616. [ Links ]
Bjorndal, K ., B. Schroeder, A. Foley, B. Witherington, M. Bresette, D. Clark, R. Herren, M. Arendt, J. Schmid, A. Meylan, P. Meylan, J. Provancha, K. Hart, M. Lamont, R. Carthy and A. Bolten . 2013b. Temporal, spatial, and body size effects on growth rates of Loggerhead Sea Turtles (Caretta caretta) in the Northwest Atlantic. Mar. Biol., 160(10): 2711-2721. [ Links ]
Bolten, A. 1999.Techniques for measuring sea turtles. 1-10. In: Eckert, K.L., K. Bjorndal, A. Abreu-Grobois and M. Donnelly (Eds.). Research and Management Techniques for the Conservation of Sea Turtles. IUCN/SSC Marine Turtle Specialist Group Publ., Washington. 248 p. [ Links ]
Burke, R.L. 2015. Head-starting turtles: learning from experience. Herpetol. Conserv. Biol., 10 (Symposium): 299-308. [ Links ]
Carrión, J., C. Canales-Cerro, R. Arauz and R. Riosmena-Rodríguez. 2013. Habitat use and diet of juvenile Eastern Pacific Hawksbill Turtles (Eretmochelys imbricata) in the North Pacific coast of Costa Rica. Chelonian Conserv. Biol., 12: 235-245. [ Links ]
Casale, P., A.D. Mazaris, D. Freggi, C. Vallini and R. Argano. 2009. Growth rates and age at adult size of Loggerhead Sea Turtles (Caretta caretta) in the Mediterranean Sea, estimated through capture-mark-recapture records. Sci. Mar., 73: 589-595. [ Links ]
Chuen-Im, T., M. Areekijseree, S. Chongthammakun and S.V. Graham. 2010. Aerobic Bacterial Infections in Captive Juvenile Green Turtles (Chelonia mydas) and Hawksbill Turtles (Eretmochelys imbricata) from Thailand. Chel. Conserv. Biol., 9:135-142. [ Links ]
Endres, C. S. and K. J. Lohmann. 2012. Perception of dimethyl sulfide (DMS) by loggerhead sea turtles: a possible mechanism for locating high-productivity oceanic regions for foraging. J. Exp. Biol., 215: 3535-3538. [ Links ]
Espinosa de los Monteros, J. y U. Labarta. 1987. Alimentación en acuicultura. Plan de Formación de Técnicos Superiores en Acuicultura. Comisión Asesora de Investigación Técnica y Científica, Madrid. 325 p. [ Links ]
Franco-Herrera, A. 2005. El Rodadero, más que un centro turístico en el Caribe colombiano. Una aproximación a la oceanografía de la Ensenada de Gaira. Universidad de Bogotá Jorge Tadeo Lozano, Santa Marta. 58 p. [ Links ]
Gutiérrez, W. and J.C. Cabrera. 1996. Growth, food conversion and mortality of Eretmochelys imbricata (Reptilia: Chelonidae) in artificial ponds (Costa Rica). Rev. Biol. Trop., 44: 847-851. [ Links ]
Harewood, A. and J. Horrocks. 2008. Impacts of coastal development on Hawksbill hatchling survival and swimming success during the initial offshore migration. Biol. Conserv., 141: 394-401. [ Links ]
Harfush, M., C. Martínez, E. López and C. Rojas. 2000. Advances in the determination of dietary protein requirements for ad libitum fed Lepidochelys olivacea hatchlings. 313. In: Abreu-Grobois F.A., R. Briseño-Dueñas, R. Márquez-Millán and L. Sarti-Martínez (Eds.). 18th. Annual Symposium on Sea Turtle Biology and Conservation, Mazatlán, Sinaloa, México. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Southeast Fisheries Science Center. [ Links ]
Joya, M. y M.P. Molina. 2006. Levante de neonatos de tortuga carey Eretmochelys imbricata (Linnaeus, 1766) mediante la implementación de dos tipos de dietas en el Acuario Mundo Marino, Santa Marta. B.Sc. Thesis. Universidad Jorge Tadeo Lozano, Bogotá. 111 p. [ Links ]
Kakizoe, Y., K. Sakaoka, Y. Akune, Y. Kanou, T. Saito and I. Uchida. 2013. Change of plasma chemistry values in captive breeding turtles (Caretta caretta). ISRN Zoology. 1-7. [ Links ]
Kanghae, H., K. Thongprajukaew, A. Madlee and K. Kittiwattanawong. 2014. Is artificial feed suitable for juvenile Green Turtles (Chelonia mydas)? Aquaculture, 428-429: 97-103. [ Links ]
Kaufmann, R. 1975. Observaciones sobre el crecimiento de tortugas marinas en cautividad. Caldasia, 11:139-150. [ Links ]
Kellems, R.O. and D.C. Church. 2002. Livestock feeds and feeding. 5th ed. Prentice Hall, New Jersey. 705 p. [ Links ]
Kilic, C. and O. Candan. 2014. Hatchling sex ratio, body weight and nest parameters for Chelonia mydas nesting on Sugozu beaches (Turkey). Anim. Biodiv. Conserv., 37: 177-182. [ Links ]
Labrada-Martagón, V., L.C. Méndez-Rodríguez, S.C. Gardner, V.H. Cruz-Escalona and T. Zenteno-Savín. 2010. Health indices of the Green Turtle (Chelonia mydas) along the Pacific coast of Baja California Sur, Mexico: II. Body condition index. Chelonian Conserv. Biol., 9: 173-183. [ Links ]
León, Y.M. and K.A. Bjorndal. 2002. Selective feeding in the Hawksbill Turtle, an important predator in coral reef ecosystems. Mar. Ecol. Prog. Ser., 245: 249-258. [ Links ]
Mann, M., R. Mellgreen, A. Arenas and A. Amiano. 2000a. Growth and behavior during the first year of life in two species of sea turtles. 227-229. In: Abreu-Grobois, F.A., R. Briceño-Dueñas, R. Márquez and L. Sarti (Eds.). Proc. 18th An. Symp. Sea Turtle Biol. Cons. NOAA Tech. Mem., NMFS-SEFSC-436. Miami. [ Links ]
Mann, M ., R. Mellgren, R. Figuero, C. Aguilar-Cardozo, J. Martínez-Aguilar and M. Coba-Ríos. 2000b. Diet dependent growth in Green sea turtle hatchlings. 291. In: Kalb H.J. and T. Wibbels (Eds.). Proc. 19th An. Symp. Sea Turtle Biol. Cons. NOAA Tech. Memo., NMFS-SEFSC-443. Miami. [ Links ]
McClenachan, L., J.B. Jackson and M. Newman. 2006. Conservation implications of historic sea turtle nesting beach loss. Front. Ecol. Environ., 4: 290-296. [ Links ]
Meylan, A.B. 1999. Status of the Hawksbill Turtle (Eretmochelys imbricata) in the Caribbean Region. Chelonian Conserv. Biol ., 3: 177-184. [ Links ]
Moein, S., R. Mellgren and J.A. Musick. 2003. Visual acuity of juvenile Loggerhead Sea Turtles (Caretta caretta): A behavioral approach. Int. J. Comp. Psych., 16: 143-155. [ Links ]
Monterrosa, M.C. and M.F. Salazar. 2005. Levante de neonatos de Caretta caretta (Linnaeus, 1758) y su proceso de adaptación al medio natural, Santa Marta, Colombia. B.Sc. Thesis, Universidad de Bogotá Jorge Tadeo Lozano. Bogotá. 196 p. [ Links ]
Mortimer, J.A. 1995a. Headstarting as a management tool. 613-615. In: Bjorndal, K.A. (Ed.). Biology and conservation of sea turtles. Smithsonian Institution Press, Washington. 615 p. [ Links ]
Mortimer, J.A. 1995b. Feeding ecology of sea turtles. 103-109 In: Bjorndal, K.A. (Ed.). Biology and conservation of sea turtles. Smithsonian Institution Press, Washington. 615 p. [ Links ]
Mortimer, J.A. 1999. Reducing threats to eggs and hatchlings: hatcheries. 175-178. In: Eckert, K.L ., K. Bjorndal , A. Abreu-Grobois and M. Donnelly (Eds.).Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publ., 4. Washington. 248 p. [ Links ]
Mortimer, J.A. and M. Donnelly 2008. Eretmochelys imbricate. IUCN SSC Marine Turtle Specialist Group. The IUCN Red List of Threatened Species 2008: e.T8005A12881238. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T8005A12881238.en. 16/07/2016. [ Links ]
Nagelkerken, I., L. Pors and P. Hoetjes. 2003. Swimming behavior and dispersal patterns of headstarted loggerhead turtles Caretta caretta. Aquat. Ecol., 37: 183-190. [ Links ]
Okuyama, J., T. Shimizu, O. Abe, K. Yoseda and N. Arai. 2010. Wild versus head-started Hawksbill Turtles Eretmochelys imbricata: post-release behavior and feeding adaptions. Endanger. Species Res., 10: 181-190. [ Links ]
Pabón-Aldana, K., C.L. Noriega-Hoyos and G.A. Jaúregui. 2012. First satellite track of a head-started juvenile Hawksbill in the Colombian Caribbean. Mar. Turt. Newsl., 133: 4-7. [ Links ]
Pelegrín, E. and I. Fraga. 2002. Breeding of Hawksbill Turtle Eretmochelys imbricata with artificial food. 185-187. In: Mosier, A., A. Foley and B. Brost (Eds.). Proc. 20th An. Symp. Sea Turtle Biol. Cons. NOAA Techn. Mem. NMFS-SEFSC-477. Miami. [ Links ]
Pelegrín, E ., S. Álvarez, I. Fraga y J. Galindo. 2003a. Requerimientos de proteína en sub juveniles de Tortuga Carey (Eretmochelys imbricata). II Congr. Iberoam. Virtual Acuic., 978-985. [ Links ]
Pelegrín, E ., I. Fraga , J. Galindo, S. Álvarez, G. Nodarse and Y. Cruz. 2003b. Lipids requirements of Hawksbill Turtle hatchlings (Eretmochelys imbricata): 283. In Proc. 23rd An. Symp. Sea Turtle Biol. Cons. Pilcher, N. (Ed.). NOAA Techn. Mem. NMFS-SEFSC-536. Miami. [ Links ]
Pelegrín, E ., S. Álvarez , J. Galindo y E. Regueira. 2006. Evaluación de la harina de calamar en dietas para juveniles de tortuga carey (Eretmochelys imbricata): 9. IV Congr. Iberoam. Virtual Acuic. 189-197 [ Links ]
Ross, J.P. 1999. Ranching and captive breeding sea turtles: Evaluation as a conservation strategy. 197-199. In: Eckert, K.L ., K. Bjorndal , A. Abreu-Grobois and M. Donnelly (Eds.). Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publ., 4. Washington. 248p. [ Links ]
Sampson, L., A. Giraldo, L. Payán, D. Amorocho, T. Eguchi and J. Seminoff. 2015. Somatic growth of juvenile Green Turtle (Chelonia mydas) morphotypes in the Colombian Pacific. Mar. Biol ., 162: 1559-1566. [ Links ]
Shaw, S., R. Willlit, P. Lutz and G. Bossart. 1989. Possible effects of artificial foods on sea turtle health: 232-306. In Eckert S.A., K.L. Eckert and T.H. Richardson (Eds). Proc. 9th An. Symp. Sea Turtle Biol. Cons. NOAA Tech. Mem., NMFS-SEFC-232. Miami. [ Links ]
Sison, T., M. Padilla, M. Vizmanos and M. Follosco. 1990. Isolation and identification of fungi found in necrotic skin lesions of captive marine turtles (Eretmochelys imbricata). Philippine J. Vet. Medic., 27(2): 35-36. [ Links ]
Tacon, A.G. 1989. Nutrición y alimentación de peces y camarones cultivados. Manual de capacitación. [ Links ]
Witzell, W.N. (Ed). 1983. Synopsis of biological data on the Hawksbill Turtle, Eretmochelys imbricata (Linnaeus, 1766). 137. FAO. Miami. 86 p. [ Links ]
Wood, J.R. and F.E. Wood. 1979. Artificial incubation of green sea turtle eggs (Chelonia mydas). Proc. World Mar. Soc., 10: 215-221. [ Links ]
Wood, J.R . and F.E. Wood . 1981. Growth and digestibility for the Green Turtle (Chelonia mydas) fed diets containing varying protein levels. Aquaculture, 25: 269-274. [ Links ]
Wyneken, J., K.J. Lohmann and J.A. Musick (Eds). 2013. The biology of sea turtles. 3. CRCMar. Biol. Ser. Taylor and Francis. 457 p. [ Links ]
Zhou, F., Y.Q. Wang, X.Y. Ding, W.K. Ng, F. He and H-L.Xue. 2016. Partial replacement of fish meal by soy protein concentrates in diets for a new Japanese strain of juvenile soft-shelled turtle, Pelodiscus sinensis. Aquac. Res., 47: 875-886. [ Links ]
Received: March 12, 2018; Accepted: September 20, 2018











 text in
text in