Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.48 no.1 Santa Marta Jan./June 2019 Epub Aug 30, 2019
https://doi.org/10.25268/bimc.invemar.2019.48.1.761
Research Articles
Revision and update of the checklist of copepods (Crustacea: Hexanauplia) of the Colombian Caribbean
1 University of Vienna, Dep. of Limnology and Bio-Oceanography and "Technisches Büro für Biologie Dr. Gaviria-Melo", Fred-Raymond-Gasse 19/2/4, Vienna, Austria. santiago.gaviria@gmx.at.
2 Universidad Jorge Tadeo Lozano, Laboratorio de Limnología, Cra 4 No 22-61, Módulo 5 piso 8, Bogotá, Colombia. johnh.dorador@utadeo.edu.co, michael.ahrens@utadeo.edu.co.
The aim of the study was to obtain a revised and updated checklist of the species of copepods of the Colombian Caribbean. Methods for updating included a critical compilation of records in published and unpublished articles, and our own results of a study of zooplankton in a project on bioinvasions of coastal waters, conducted in 2010. Twenty taxa reported solely in undergraduate thesis need taxonomical comprobation and were excluded from the inventory. As a baseline, we used the most recent inventory published by Medellín-Mora and Navas (2010). Ten species recorded in the already mentioned project are new records. Thirteen species reported by other researchers in publications after 2010 increased the species list. The inventory totals 214 species of copepods (158 Calanoida, 38 Cyclopoida, 15 Harpacticoida, 2 Mormonilloida and 1 Monstrilloida). Most species are planktonic (201), while only a few are benthic (10) or epibenthic (3). Nomenclature of the taxa was revised and updated as well. The highest copepod diversity corresponds to the ecoregion Colombian Oceanic (127 species), followed by Tayrona (94), Magdalena (82) and Morrosquillo (78). The lowest number corresponds to San Andrés and Providencia Archipelago (49). The limited information existing about benthic and parasitic copepods warrants an increment on the study these groups. Most of them belong to orders Harpacticoida and Siphonostomatoida.
KEYWORDS: Coastal and oceanic waters; Biodiversity; Geographical distribution; Meiobenthos; Zooplankton
El objeto del estudio fue adelantar la revisión y actualización de la lista de especies de copépodos del Caribe colombiano. El método utilizado se basó en la recopilación crítica de registros en artículos publicados y no publicados, y en resultados propios obtenidos en el estudio del zooplancton recolectado durante el desarrollo de un proyecto en bioinvasiones de aguas costeras en 2010. Veinte especies registradas únicamente en tesis de pregrado requieren comprobación taxonómica y fueron excluidas del inventario. El modelo base de la lista de especies fué aquel publicado por Medellín-Mora y Navas (2010). Diez especies recolectadas durante el proyecto mencionado constituyen nuevos registros. Trece especies registradas en publicaciones de otros investigadores después de 2010 enriquecieron el inventario. Un total de 214 especies de copépodos (158 Calanoida, 38 Cyclopoida, 15 Harpacticoida, 2 Mormonilloida y 1 Monstrilloida) conforman actualmente la lista de especies. La mayoría de especies son de hábitos planctónicos (201), pocas son bentónicas (10) o epibentónicas (3). Se actualizó la nomenclatura de los taxones. La mayor diversidad corresponde a la ecorregión Colombia Oceánica (127 especies), seguida por Tayrona (94), Magdalena (82) y Morrosquillo (78). El menor número corresponde al Archipiélago de San Andrés y Providencia (49). La baja información existente sobre copépodos bentónicos y parásitos sugiere incrementar el estudio de esos grupos, la mayoría de ellos pertenecientes a los órdenes Harpacticoida y Siphonostomatoida.
PALABRAS CLAVE: Aguas costeras y oceánicas; Biodiversidad; Distribución geográfica; Meiobentos; Zooplancton
INTRODUCTION
Copepods are a widely distributed group of small crustaceans, present in almost all aquatic environments. They reach very high abundances and, together with nematodes, are considered the most abundant metazoan group on Earth (Boxshall and Halsey, 2004). In the sea, copepods constitute between 60 and 80 % of the zooplankton biomass (Morales and Suárez-Morales, 2009), also reaching enormous abundances in benthic habitats. Their density varies between 100.000 and 1.000.000 harpacticoid copepods per square meter in intertidal sediments. Such densities decrease with depth, reaching 10.000 individuals per square meter in the deep sea (Boxshall and Halsey, 2004). Symbiotic and parasitic forms of copepods also number more than 1.500 species: they are also very diverse and have been found in almost all major metazoan phylums (Huys and Boxshall, 1991). Currently, the subclass Copepoda includes more than 11.300 accepted species, and this number is permanently growing. In the last decade more than 1.000 species were described (Walter and Boxshall, 2018). Copepods play an important role in the pelagic food chain as consumers of phytoplankton (Paffenhöfer, 1971; Mayzaud et al., 2002; Franco-Herrera, 2006), and as main prey for others zooplankton, including the larvae of important commercial fish species (Brown and Marcotte, 1987; Uye and Yamaoka, 1990) and whales (Hardy, 1970).
In Colombia, the first species of copepods recorded at the Caribbean were those reported by Park (1970) in the ecoregions Colombian Oceanic and San Andrés and Providencia Archipelago. Some years later, Gómez (1975) recorded planktonic copepods in Cartagena Bay. Monsalve (1976) published first records from the Pacific and Renteria (1977) new records from the Caribbean. Michel and Foyo (1977), in a faunal survey of the zooplankton of the Caribbean Sea, included copepod records of the Colombian Oceanic ecoregion. During their ecology studies, some authors mentioned copepod species from the neritic zone (Bernal, 1994, 2000; Bernal and Zea, 2000). Additional records of planktonic copepods from San Andrés and Providencia (Giraldo and Villalobos, 1983), Santa Marta region (Campos and Plata, 1990), Providencia and Santa Catalina (Martínez-Barragán et al., 2009) appeared subsequently. Among publications related to non-native species and the effects of marine traffic on the composition of zooplankton, several species of copepods were recorded (Rendón et al., 2003; Ahrens et al., 2011). A study on copepod feeding focussing on the species Eucalanus subtenuis in the Colombian Caribbean was contributed by Franco-Herrera (2006). Recently, articles on distribution of epipelagic copepods from the Colombian Pacific were published (López and Mojica, 2015; Jérez-Guerrero et al., 2017).
Several undergraduate theses on zooplankton include copepod records (Samper, 1970; Alvarado, 1978; López and Mesa, 1983; Lozano, 1986; Marino and Merchán, 1993; Fisco, 2006; Uribe and Calero, 2006; Barón, 2007; Dorado-Roncancio, 2015). Unfortunately, those documents were not formally published and species identifications were probably not confirmed by taxonomists. For instance, the report of Eucalanus finmarchicus in Santa Marta region (Samper, 1970) or of Microsetella norvegica in Cispatá Bay (Fisco, 2006) strongly point toward misidentifications. The existing information on Colombian Caribbean copepod diversity known until 2008, containing mainly planktonic species, was published by Medellín-Mora and Navas (2010).
Over the last decade, copepods inhabiting phytal habitats of Gaira Bay have been intensively studied (Fuentes-Reinés and Suárez-Morales, 2017c; Fuentes-Reinés et al., 2017; Gómez and Fuentes-Reinés, 2017a, 2017b; Suárez-Morales and Fuentes-Reinés, 2018), culminating in the description of seven new species and the addition of two species to the Caribbean species inventory. Moreover, planktonic species of Kelleria, Lubbockia, Farranula, and Oncaea (Fuentes-Reinés and Suárez-Morales, 2017b) and one species of Neocyclops (Fuentes-Reines and Suárez-Morales, 2017a) have been recorded. The discovery of the first Monstrilloid copepod found in Colombia (Dorado-Roncancio E.F. and J. Dorado-Roncancio, 2018) was recently published.
During the development of a project on bioinvasions related to marine traffic, we studied copepods in coastal waters of three main Caribbean ports: Coveñas, Cartagena, Santa Marta, in contrast to adjacent reference areas characterized by less international shipping activity. Additionally, zooplankton contained in ballast water of ships anchored in the Morrosquillo Gulf was studied. Several new records and non-native species were found (Ahrens et al, 2011).
The aim of the present article is to publish an update of the species list of planktonic and benthic copepods of the Colombian Caribbean, based on a critical revision of published and unpublished literature. Additionally, we describe the ecoregions inhabited by the known species and the new records, and call attention to the less studied groups and habitats.
METHODS
The present article is the result of a revision and update of the species registered hitherto in plankton and benthos of the Colombian Caribbean. The location of the geographical area analysed is shown in Figure 1.
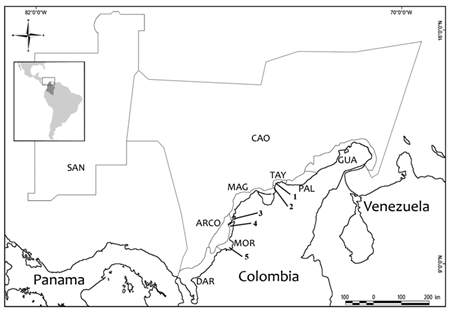
Figure 1: Marine and coastal ecoregions of the Colombian Caribbean: GUA, Guajira; PAL, Palomino, TAY, Tayrona; MAG, Magdalena; MOR, Morrosquillo; ARCO, Coralline Archipelago; DAR, Darién, SAN, San Andrés and Providencia Archipelago; CAO, Oceanic Caribbean. Numbers indicate localities with records after 2010 (Tables 1 and 2): 1, Santa Marta Bay and Gaira Bay; 2, Pozos Colorados; 3, Cartagena Bay; 4, Barú-Barbacoas; 5, Coveñas and Cispatá. Map redrawn from Díaz and Acero (2003).
The checklist includes: 1) Copepod records of the project on bioinvasions in coastal waters of the Colombian Caribbean (Ahrens et al, 2011, 2012) (Table 1); 2) Records from articles published until 2010 and compiled by Medellin-Mora and Navas (2010); 3) Records from unpublished references until 2010. These species were marked as "not confirmed" records if they appeared solely there; 4) Species described or recorded after 2010 by several authors, from coastal waters of Gaira and Cartagena Bays. Genera mentioned in the references or as the result of the mentioned project without species determination, were not considered in the present checklist.
Table 1 Taxonomic list of copepods registered in the project "Evaluation of marine bioinvasions in coastal wetlands and their relationship with sea traffic in three major harbor zones of the Colombian Caribbean: Cartagena, Santa Marta and Coveñas" (Ahrens et al., 2012): sampling locality (see Figure 1), date and inventory number of voucher material (UJTL-LL, collection Universidad Jorge Tadeo Lozano, Laboratory of Limnology, Bogotá; SG-COP, copepod collection of S. Gaviria). ? Identification not confirmed, * taxa identified in samples of the collection after the project end by SG (S. Gaviria) or JD (J. Dorado); ** no voucher material available. ▲first record for the Colombian Caribbean. 1, Santa Marta and Rodadero; 2, Pozos Colorados; 3, Cartagena Bay; 4, Barú-Barbacoas; 5, Coveñas and Cispatá Bay; ballast water of ships Chemtrans Sky (I),
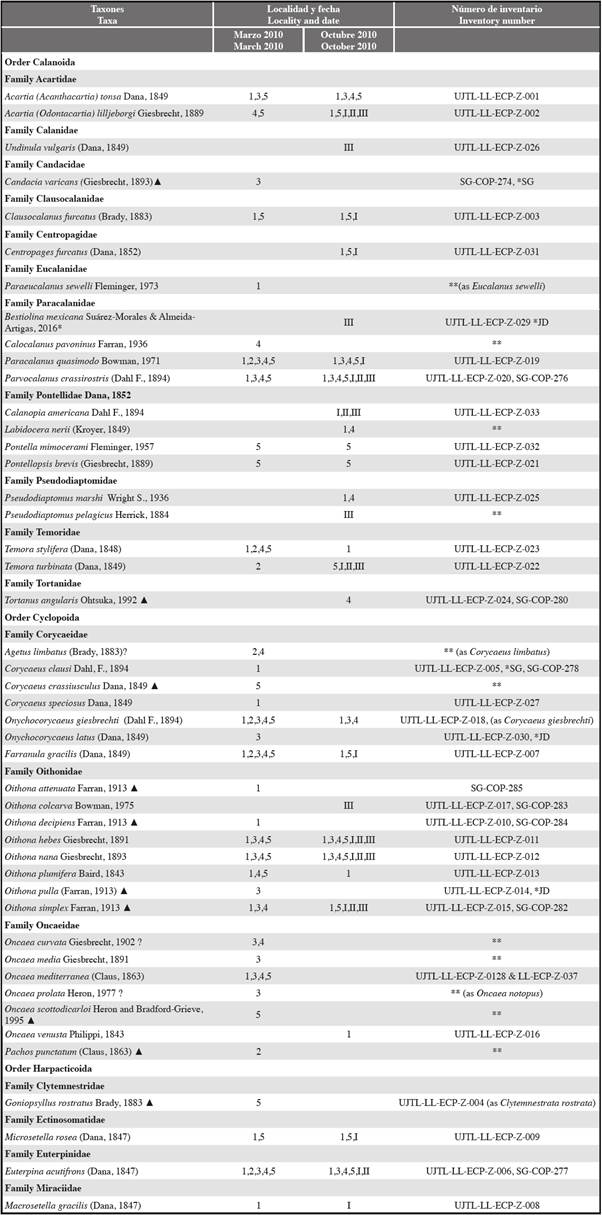
Table 1 shows the species of copepods recorded during the project. Samples of zooplankton containing copepods were collected in surface waters of the neritic zone using a standard plankton net with 130 mesh size. Collection of samples was done in March and October 2010 at the following areas: 1) adjacent waters of Santa Marta and Gaira (El Rodadero) Bays, 2) Pozos Colorados, 3) Cartagena Bay, 4) Barú-Barbacoas, and 5) Coveñas and Cispatá Bay. In October 2010 samples of zooplankton were taken from ballast waters of four ships anchored several kilometers off Coveñas. Samples of the water tanks were collected via vertical trawls with the same plankton net. Sampling locations are enumerated in Figure 1. The list also includes inventory numbers of the specimens of the reference collection of most of the species registered.
The checklist (Table 2) was elaborated based on the model published nine years ago by Medellín-Mora and Navas (2010), with some modifications. The inventory was completed using published and unpublished records. In the present inventory, we considered taxa reported exclusively in unpublished references as "not confirmed records" (Table 2). With exception of two epibenthic species of Pseudodiaptomus, the inventory of 2010 only mentioned planktonic species.
Table 2: Taxonomic list of copepod taxa reported in the Colombian Caribbean. Recently added marine ecoregions of already known taxa are indicated in bold. For abbreviations of ecoregions, see Figure 1. * unpublish references; ** species reported after the 2010 inventory; pl, plankton, epi, epibenthos, ben; benthos; par, parasite; ?, record not confirmed; ü, identification considered reliable. Acronyms collections: CBUMAG: MEI, Centro de Colecciones Biológicas Universidad del Magdalena (Santa Marta); ICML-EMUCOP, Instituto de Ciencias del Mar y Limnología (Mazatlán); SG-COP, copepod collection of S. Gaviria (Viena); UARC, Colecciones Biológicas Universidad del Atlántico (Barranquilla); UJTL-LL, Universidad Jorge Tadeo Lozano, Limnological Laboratory (Bogotá)
Taking into account that over the last decade the copepod fauna of benthic environments has started to be surveyed, we also include in the updated checklist benthic copepods (Fuentes-Reinés and Suárez-Morales, 2017c, 2018; Fuentes-Reinés et al., 2017; Gómez and Fuentes-Reinés, 2017a, 2017b), collected in phytal habitats of coastal waters of Gaira Bay. Two of those publications also reported planktonic cyclopoids (Fuentes-Reinés and Suárez-Morales, 2017a, 2017b) for the same study area.
The checklist includes the report of the first monstrilloid copepod found in Colombia (Dorado-Roncancio and Dorado-Roncancio, 2018) collected in an inner branch of the Cartagena Bay. Additionally, a parasitic species of Siphonostomatoida is also indicated (Dorado-Roncancio, 2014), but its taxonomy needs to be confirmed.
The inventory also summarizes the distribution of each species for the different ecoregions of the Colombian Caribbean, based on the division proposed by Díaz and Acero (2007): Guajira (GUA), Palomino (PAL), Tayrona (TAY), Magdalena (MAG), Morrosquillo (MOR), Coralline Archipielagos (ARCO), Darién (DAR), San Andrés and Providencia Archipelago (SAN), and the Oceanic Caribbean (CAO) (Figure 1). Ecoregions with reported presence of species lacking confirmed identification were also included in Table 2, but indicated as “not confirmed”, pending confirmation of the species. The species list includes habitat, the respective reference collection of the species and bibliographic references. Nomenclature follows WoRMS Editorial Board (2019), based on Walter and Boxshall (2019) and Boxshall & Halsey (2004).
RESULTS
A total of 43 species of planktonic copepods (18 calanoids, 21 cyclopoids, 4 harpacticoids) were reported based on samples collected during the project on bioinvasions of the Colombian Caribbean (Ahrens, 2012). Five additional species (Candacia varicans, Bestiolina mexicana Corycaeus clausi, Onychocorycaeus latus and Oithona pulla) were found during later examination of the zooplankton collection (Table 1). Fourteen of those 48 species were also found in ballast waters of the four ships examined. Three of the species reported in ballast water (Bestiolina mexicana, Pseudodiaptomus pelagicus and Oithona colcarva) were found exclusively in the tanks of ships and hence cannot be considered part of the Colombian copepod fauna, as they have not been found yet in natural waters. Two species found only in ballast waters (Undinula vulgaris and Calanopia americana) are common inhabitants of the plankton of the Colombian Caribbean (Park, 1970; Michel and Foyo, 1976; Giraldo and Villalobos, 1983; Bernal, 2000; Bernal and Zea, 2000; Martínez-Barragán et al., 2009) and, thus, form part of the Colombian copepod fauna.
Ten species identified during the project in natural waters were new records for the Colombian territory: Candacia varicans, Tortanus angularis (Calanoida), Corycaeus crassiusculus, Oithona attenuata, Oithona decipiens, Oithona pulla, Oithona simplex, Oncaea scottodicarloi, Pachos puntatum (Cyclopoida) and Goniopsyllus rostratus (Harpacticoida). Records of three species (Oithona attenuata, Oithona decipiens and Oithona simplex) were found during the first phase of the project (Ahrens et al, 2011). Three cyclopoid species identified as Agetus limbatus?, Oncaea curvata? and Oncaea prolata?, should be considered as taxa without confirmed identification.
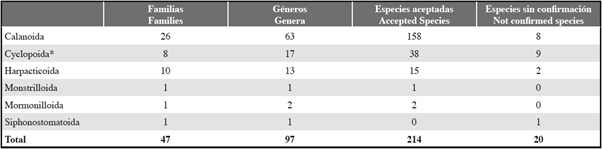
A total of 214 species belonging to the order Calanoida (158), Cyclopoida (38), Harpacticoida (15), Mormonilloida (2) and Monstrilloida (1) are now known from the Colombian Caribbean (Table 2) and are summarized at Table 3. Seventeen additional species (eight calanoids, six cyclopoids, two harpacticoids, one siphostomatoid) reported merely in unpublished theses are considered yet not confirmed. Calanoid species without confirmed identification are Acartia spinata, Acartia longiremis, Calanus finmarchicus, Candacia bipinnata, Xanthocalanus marlyae, Labidocera fluviatilis, Pontellopsis perspicax and Pseudodiaptomus acutus. Cyclopoid species considered not confirmed are Ditrichocorycaeus amazonicus, Dioithona oculata, Oithona atlantica, Oithona robusta, Oithona setigera and Sapphirina auronitens. Harpacticoid species Microsetella norvegica and Miracia efferata are also considered as taxa without confirmed identification. That is also true for the only siphonostomatoid copepod reported, namely, Caligus curtus.
Table 3: Number of families, genera and species of the copepod orders of the Colombian Caribbean, (for taxonomic details and distribution ranges, see Table 2). *Including family Clausiidae without genus and species determination.
Of the 214 species listed in Tables 2 and 3, twentyfour species (3 calanoids, 11 cyclopoids, 9 harpacticoids and 1 monstrilloid) correspond to records obtained after the last inventory of marine copepods from the Colombian Caribbean (Medellín-Mora and Navas, 2010). Besides the 10 new records of the mentioned project, 11 new species or new records were found at Gaira Bay (Fuentes-Reinés and Suárez-Morales, 2017a, 2017b, 2017c; Fuentes-Reinés et al., 2017; Gómez and Fuentes-Reinés, 2017a, 2017b; Suárez-Morales and Fuentes-Reinés, 2018). The cyclopoid Oncaea scottodicarloi was reported in both studies, one near Coveñas (Morrosquillo ecoregion, Ahrens et al., 2012) and Gaira (Tayrona ecoregion, Fuentes-Reinés and Suárez-Morales, 2017b). The register of the monstrilloid copepod corresponds to Cartagena Bay (Dorado-Roncancio E.F. and J. Dorado-Roncancio, 2018). Most of the species are planktonic (201), 10 are benthic and 3 epibenthic.
Table 2 includes information on the presence of each species in the Caribbean ecoregions. The Colombian Oceanic ecoregion, with 127 known species, shows the highest species richness, followed by Tayrona (94 species), Magdalena (82) and Morrosquillo (78). Moreover, 65 species were recorded at Darien and Guajira and 64 species at Palomino and Coralline Archipielago. The lowest number (49 species) corresponds to San Andrés and Providencia.
The updated anlaysis of the distribution of species in the afore mentioned ecoregions yielded 28 records of confirmed species previously unreported. These correspond to Tayrona, Magdalena and Morrosquillo, where new faunal surveys have been done after 2010.
Calanoida, with 26 families, 63 genera and 158 species with reliable identification, is the order with the highest number of records. The most speciose genera of calanoids currently known in the epipelagic zone continue to be the same as in 2010: Lucicutia (12 species), Haloptilus (8 species) and Candacia (7 species) (Table 1). The family Tortanidae with Tortanus angularis, found in the Magdalena ecoregion, is new for the inventory. The family Pseudodiaptomidae shows additional species, i.e. Pseudodiaptomus cokeri, collected in coastal waters of Santa Marta (Fuentes-Reinés et al., 2017) and Pseudiaptomus pelagicus, found in the ballast water of a ship in the harbor of Coveñas (Dorado-Roncancio, 2012). The latter cannot be considered as part of the Colombian inventory because it has not been found in natural waters. Eucalanus elongatus, found in ballast water in the Magdalena ecoregion (Rendón et al., 2003; Medellín-Mora and Navas, 2010), is apparently also present in natural waters of the Oceanic Caribbean (Park, 1970).
Because the order Poecilostomatoida is now considered to be part of the Cyclopoida (Khodami et al., 2017), the order now includes eight families in the Colombian Caribbean: Clausiidae, Corycaeidae, Cyclopidae, Kelleridae, Lubbockiidae, Oithonidae, Oncaeidae and Sapphirinidae, with 13 genera and 38 species with reliable identification. Eleven species are new for the region: Corycaeus crassisculus and Oncaea curvata collected at the Morrosquillo ecoregion, Neocyclops ferrari, Kelleria reducta, Oithona attenuata, Oithona decipiens, Conaea rapax, Oncaea prolata and Pachos punctatum from the Magdalena ecoregion, and Oithona simplex and Oncaea scottodicarloi in both ecoregions (Dorado-Roncancio, 2012, 2014). Oithona colcarva was found only in ballast water of a ship in the Gulf of Morrosquillo. Oncaea conifera is now placed in the genus Triconia (Boettger-Schnack et al., 2011).
The order Harpacticoida is now represented by 10 families, 13 genera and 15 species with reliable identification (Table 2 and 3). The families Cletodidae, Dactylopusidae, Darcythompsonidae, Laophontidae and Tisbidae comprise benthic species, while Aethidae, Clytemnestridae, Ectinosomatidae and Euterpinidae contain exclusively planktonic species. One of the harpacticoid families (Miracidae), comprise planktonic and benthic genera. New species records after the 2010's inventory are the meiobenthic harpacticoids Geehydrosoma brevipodium, Goniopsyllus rostratus, Diarthrodes bodini, Diarthrodes gomezi, Darcythompsonia inopinata, Leptocaris colombiana, Leptocaris vicina, Echinolaophonte armiger, Echinolaophonte villabonae and Tisbrintra monroyi (Fuentes-Reinés and Suárez-Morales, 2017a, 2017b, 2017c; Fuentes-Reinés et al., 2017; Gómez and Fuentes-Reinés, 2017a, 2017b; Suárez-Morales and Fuentes-Reinés, 2018). All originated from recent samplings in coastal waters of Gaira Bay at the Tayrona ecoregion.
The order Monstrilloida is represented by one species Cymbasoma chelemense. This record corresponds to a planktonic adult female found in estuarine waters of Cartagena (Dorado-Roncancio and Dorado-Roncancio, 2018). Within the order Mormonilloida, only two species, Mormonilla phasma and Neomormonilla minor, are known in the studied area (Michel and Foyo, 1976).
The order Siphonostomatoida was only known through genus Caligus (Carmona, 1979). Larvae of Caligus curtus were identified in a planktonic sample from Cartagena Bay (Dorado-Roncancio, 2015), but its identification still needs confirmation.
DISCUSSION
Nomenclature notes
The nomenclature of the different taxa was updated according to WoRMS Editorial Board (2019), based on Boxshall and Halsey (2004) and Walter and Boxshall (2019). The bibliographic references in Tables 1 and 2 show the names of species as they were written in the original publication. Several changes in the nomenclature of orders, families, genera, subgenera and species have taken place over the last years. Within Calanoida, the genus Farrania was transferred from Clausocalanidae to Aetideidae. Eucalanus sewelli now belongs to Pareucalanus (family Eucalanidae). Four species of Eucalanus (E. subtenuis, E. crassus, E. monachus and E. mucronatus) are now considered belonging to Subeucalanus (family Eucalanidae). The latter three species were reported earlier in both genera, Eucalanus and Subeucalnus (Medellín-Mora and Navas, 2010). In the family Scolecithrichidae, Scolecithricella tenuiserrata was transferred to Amallothrix.
As already mentioned, the order Poecilostomatoida is now recognised as part of Cyclopoida. Therefore, Cyclopoida now includes the families that belonged before to Poecilostomatoida. Within the family Corycaeidae, Corycaeus flaccus was transferred to the genus Agetus. Two subgenera of the genus Corycaeus (Onychocorycaeus and Urocorycaeus) were elevated to genus and include the species Onychocorycaeus giesbrechtii, O. latus, and Urocorycaeus lautus. One species (Corycaeus subulatus Herrick, 1887) is considered taxon inquirenda (Giesbrecht, 1893; Walter and Boxshall, 2019). Within the family Oithonidae, Oithona oculata now belongs to the genus Dioithona. In the family Oncaeidae, the genus Oncaea was split into two genera (Oncaea and Triconia) (Boettger-Schnack et al., 2011). Oncaea conifera is called now Triconia conifera. The species Oncaea notopus is not accepted anymore and was synonymised with Oncaea prolata. Oncaea gracilis was known in the past as Conaea gracilis (Michel and Foyo, 1976).
Within Harpacticoida, Distioculus minor originally belonged to the family Diosaccidae, but all genera of that family were transferred to the family Miraciidae (Willen, 2002). Such transfer was recognised by other authors like Vives and Shmeleva (2010). In the family Clytemnestridae, Clytemnestra rostrata is now called Goniopsyllus rostratus. In the order Mormonilloida, Mormonilla minor was transferred to the genus Neomormonilla (Walter and Boxshall, 2019).
Biodiversity and distribution
The updated checklist (Table 2) totals 214 copepod species with reliable identification belonging to 5 orders, 45 families and 97 genera (Table 3). The order Siphonostomatoida, represented by the genus Caligus, constitutes a new record. Nevertheles, its species Caligus curtus should be confirmed and the adult stage should be searched on appropriate invertebrate or fish hosts (Huys and Boxshall, 1991). A group of 16 species of copepods of the last checklist (Medellín-Mora and Navas, 2010) does not have a confirmed identification status because their records are solely mentioned in undergraduate theses. Likewise, we considered as "not confirmed" a further three species reported in the project on marine bioinvasions, as well as the siphostomatoid copepod.
Coastal lagoons with narrow openings to the sea, such as the Ciénaga Grande de Santa Marta or Laguna Navío Quebrado in Guajira, may be considered continental waterbodies. That is also true for the temporal ponds isolated from the sea and investigated by other researchers (Gómez et al., 2017) in Pozos Colorados and in Pueblo Viejo (Fuentes-Reines et al, 2015) in Magdalena. Copepod species of these ecosystems were recently compiled and included in an updated inventory of the continental copepods of Colombia (Gaviria and Aranguren-Riaño, in press).
In the Mexican Caribbean, Suárez-Morales and Gasca (1998) reported 154 species, a number that was revised to 193 species only a few years later (Hernández-Trujillo and Esqueda-Escárcega, 2001). Suárez-Morales et al. (2006) added another 30 species of harpacticoids to the inventory, thereby bringing copepod diversity in the Mexican Caribbean to 223 species. That number is slightly higher than the species number known in the Colombian Caribbean (214). Other nearby countries report lower diversities: Costa Rica (164 species, Morales-Ramírez and Suárez-Morales, 2009) with only 20 species from the Caribbean area (Morales-Ramírez et al, 2014) and 115 species from the Cuban neritic waters (Campos, 1982).
The number of harpacticoid copepods listed for the Caribbean Sea currently totals 178 species (Suárez-Morales et al., 2006). The 15 species of harpacticoids registered in the Colombian inventory thus represent only 8 %, suggesting a great deficit in the knowledgement of this suborder in that region.
The 214 species recorded in the Colombian Caribbean (202 planktonic) constitute less than 50% of the records known for the Caribbean Sea. Michel and Foyo (1976) mentioned 450 planktonic species for the Caribbean and adjacent areas, and Reid (1990) listed 430 species from Central America, Mexico and the entire Caribbean Sea. Another species list registers 723 species of planktonic copepods for the Caribbean Sea (Venezuela, Antilles, Gulf of Mexico, Caribbean) but includes neighboring areas like Florida and the Sargasso Sea (Razouls et al., 2019).
In the Colombian Caribbean, the calanoid copepods with 158 species show the highest diversity. This updated number includes a new family (Tortanidae: Tortanus angularis) and an additional species of Pseudodiaptomidae (Pseudodiaptomus cokeri) for the region.
The order with the second highest number of species (38) is the Cyclopoida (including Poecilostomatoida). The most specious genera are Oithona (eight species), Sapphirina (seven) and Oncaea (five). Two families: Cyclopidae (with one species of Halicyclops) and Kelleridae (with one species of Kelleria), constitute new records for the Colombian Caribbean. Three species of Oithona from plankton samples of the Santa Marta region (Oithona attenuata and Oithona decipiens), and Santa Marta and Cartagena (Oithona simplex) were previously recorded (Ahrens et al., 2011). The relatively higher species diversity of Harpacticoida (2010: 8 species, present inventory: 15) can be explained by the report of species of new benthic families associated with coastal waters, like Cletodidae, Dactylopusiidae, Darcythompsoniidae, Laophontidae and Tisbidae, as well as with the report of a Clytemnestridae (Goniopsyllus rostratus) as new for the Colombian Caribbean.
New records of species in the Colombian Caribbean
Ten species (two calanoids, seven cyclopoids and one harpacticoid) collected during the project in natural waters (Candacia varicans, Tortanus angularis, Corycaeus crassiusculus, Oithona attenuata, Oithona decipiens, Oithona pulla, Oithona simplex, Oncaea scottodicarloi, Pachos puntatum and Goniopsyllus rostratus) were new records for the Colombian Caribbean Sea. Within calanoids, Pseudodiaptomus pelagicus and Bestiolina mexicana cannot be considered as part of the Colombian inventory, as they have been found in ship tanks in the Gulf of Morrosquillo, and still not in natural waters. On the contrary, Eucalanus elongatus collected in ship ballast water in the Magdalena ecoregion (Rendón et al., 2003; Medellín-Mora and Navas, 2010) seems to be also present in natural waters of the Oceanic Caribbean, as reported by Park (1970) and was, therefore, included on the inventory.
As already mentioned, three species of Cyclopoida (Oithona attenuata, Oithona concarva and Oithona simplex) were new records for the Colombian Caribbean (Ahrens et al, 2011). Additionally, seven species (Candacia varicans, Tortanus angularis, Corycaeus crassiusculus, Oithona pulla, Oncaea scottodicarloi, Pachos punctatum and Goniopsyllus rostratus), not reported before, were found in the second phase of the project. Both calanoids, C. varicans and T. angularis, one cyclopoid, P. punctatum, and one harpacticoid, Goniopsyllus rostratus, are common species widespred in the Caribbean Sea (Suárez-Morales and Gasca, 1998). Oncaea scottodicarloi was found near Coveñas (Ahrens et al., 2012) and at Gaira Bay (Fuentes-Reinés and Suárez-Morales, 2017b), but was not known before 2012 for the Caribbean Sea. Corycaeus crassiusculus was not known for the Caribbean Sea before, while it is distributed in the tropical Pacific, the California coast and the Gulf of California (Suárez-Morales and Gasca, 1998). The presence of Oithona pulla in Cartagena Bay (Table 1) constitutes a new record for coastal waters of the Americas. It is distributed in the Mediterranean and Red Sea, Indian Ocean and Western Pacific (Japan, South Corea, and Taiwan, Razouls et al., 2019).
The family Clausidae was mentioned already in 2010 as part of the Colombian inventory, based on the report of Sapphirella tropica in San Andrés and Providencia (Martínez-Barragán et al., 2009). However, this species is not accepted anymore and is considered as a larval stage of another species of Clausiidae (Walter and Boxshall, 2019). Therefore, the family can be considered present in Colombia without knowledgement of genus and species.
Most representatives of the order Harpacticoida are inhabitants of the benthic realm. For the present checklist 10 benthic harpacticoid species were added, thanks to intensive faunal surveys at Gaira Bay. One planktonic species, Goniopsyllus rostratus, found at Morrosquillo Gulf, is also new for the inventory (Ahrens et al., 2012; Table 1). This is a small number compared to the known number of benthic harpacticoids of marine environments. For example, Gómez and Morales-Serna (2014) listed 71 species of marine harpacticoid from Mexico, and Suárez-Morales et al. (2006) recorded 178 species in the Caribbean Sea, which strongly suggests that this group is understudied in Colombia.
Some typical benthic families of harpacticoid copepods that inhabit coarse sandy sediments, like Harpacticidae, Thompsonidae, Danielssenidae and some Canthocamptidae like Heteropsyllus (Boxshall and Halsey, 2000), should be found in Colombia, but have not been so far. The same applies to inhabitants of medium sandy sediments and muddy substrates. The typical epibenthic family Thalestridae should also be present. An increase in the number of species of already known harpacticoid families is also expected, particularly for Ectinosomatidae, Laophontidae, Miraciidae and Tisbidae.
Monstrilloids have endoparasitic naupliar and copepodid stages on polychaetes and molluscs, and free-swimming, non-feeding adults. The entire Colombian record corresponds to one planktonic adult female (Dorado-Roncancio E.F. and J. Dorado-Roncancio, 2018). Monstrilloida are represented in the Caribbean and Gulf of Mexico by 24 species (Suárez-Morales, 2015). Thus, an increase in the number of species in the Colombian Caribbean is expected for the future.
The order Mormonilloida comprises two species (Mormonilla phasma and Neomormonilla minor = Mormonilla minor in the Colombian Oceanic Caribbean (Michel and Foyo, 1976; Medellín-Mora and Navas, 2010). No species additions are considered likely, since no more species seem to exist (Boxshall and Halsey, 2004). Thus, only an increase of their distribution in other ecoregions may be expected.
The order Siphonostomatoida, represented by Caligus minor was reported in an unpublished study (Dorado-Roncancio, 2015) and the species needs confirmation. Siphonostomatoids are symbiotic species and comprise more than 1500 species worldwide (Huys and Boxshall, 1991). Symbiotic copepods have still not been studied extensively in Colombian marine waters. Applying adequate methods of collecting and extracting copepods from their invertebrate hosts and fish is likely to yield a rich treasure trove of new species of this unknown order in Colombia.
There are no records of the orders Misophrioidea and Platycopioidea in the Colombian Caribbean. The order Misophrioidea occurs elsewhere in shallow coastal waters, deep-water plankton, hyperbenthic communities of the deep sea, and in anchialine caves. Platycopioidea are also hyperbenthic and known from anchialine caves but are mainly distributed in temperate regions with records in the Bahamas and Bermuda (Boxshall and Halsey, 2004).
CONCLUSIONS
Considering only species with realiable identification, the copepod fauna of the Colombian Caribbean currently comprises 214 species (201 planktonic, 10 benthic, 3 epibenthic). Twenty additional species, while mentioned in the checklist, are considered as "not confirmed", due to their only record being unpublished in undergraduated theses or doubtful identification. Recent studies of benthic harpacticoids associated with coastal habitats, a record of an monstrilloid at Cartagena Bay, as well as new surveys of plankton of three harbour zones corresponding to the Tayrona (Santa Marta and Gaira Bays), Magdalena (Pozos Colorados and Cartagena), and Morrosquillo (Barú-Barbacoas and Coveñas) ecoregions, are responsible for the increased species number in the current inventory compared to the previous one. Twenty-four species (3 calanoids, 11 cyclopoids, 9 harpacticoids, and 1 monstrilloid) constitute new records.
The total number of 214 species in Colombia represents less than 50 % of the diversity of copepods known in the Great Caribbean. Compared to Mexico, with 223 species, the richness is slightly lower.
The benthic representatives of harpacticoids, which constitutes most of the species of the order, have been poorly studied. No species of the parasite order Siphonostomatoida has been identified so far. Benthic substrates between intertidal and deep sea continue to be poorly known in Colombia. The highest diversity of copepods currently is found in the Oceanic Caribbean ecoregion, wheras the lowest is found in the San Andrés and Providencia Archipelago.
As already mentioned by Medellín-Mora and Navas (2010), the deep-sea layers of the Oceanic region (mesopelagic - 200 to 750 m -, batypelagic - 750 to 3000 m, and abyssal - below 3000 m) are still poorly investigated habitats. Thus, species numbers in the Colombian Caribbean should increase with the study of mesopelagic to abyssal layers, benthic habitats and parasitic copepods.
ACKNOWLEDGEMENTS
This article is the product of an oral presentation held 2018 at the "I Simposio Colombiano de Carcinología", during the "V Congreso Colombiano de Zoología", in Bogotá. We are grateful to J. Arias and E. Realpe (Universidad de Los Andes) for the invitation to the symposium. Further thanks go to E. Gaviria (Vienna) for editorial support, to M. Gaviria (Vienna) for drawing of the map, as well as to two anonymous reviewers for their valuable suggestions for improving the manuscript. Finally, we gratefully acknowledge the Colombian Petroleum Company (Ecopetrol) for previous funding of the research project "Bioinvasions of coastal wetlands of the Colombian Caribbean in relation to marine traffic" (DHS No. 134 2009).
REFERENCES
[References of taxonomic authors, see Razoul et al. (2015 - 2019) and Walter and Boxshall (2019)]. [ Links ]
Ahrens, M., J. Dorado-Roncancio, M. López-Sánchez, C.A. Rodriguez y L.A. Vidal. 2011. Biodiversidad exótica: presencia de especies marinas no-nativas introducidas por el tráfico marítimo en puertos colombianos. Biota Col., 12 (2): 3-14. [ Links ]
Ahrens, M ., M. López Sánchez y J. Dorado Roncancio. 2012. Evaluación de bioinvasiones marinas en humedales costeros y su relación con el tráfico marítimo en tres zonas portuarias mayores del Caribe colombiano: Cartagena, Santa Marta y Coveñas. Convenio Interadministrativo colaboración DHS No. 34, 2009. Inf. Final, Fund. Univ. Jorge Tadeo Lozano, Bogotá, 65 p + anexos A-S 215-725 p. [ Links ]
Alvarado, H. 1978. Contribución al conocimiento de los copépodos epiplanctónicos de la bahia de Santa Marta, Colombia. Tesis Biol. Mar., Univ. Jorge Tadeo Lozano, Bogotá. 72 p. [ Links ]
Barón, C. 2007. Caracterización de mesozooplancton superficial de las islas de Providencia y Santa Catalina, Caribe colombiano, para el mes de abril de 2005. Trabajo grado Biol. Mar., Univ. Jorge Tadeo Lozano, Santa Marta. 89 p. [ Links ]
Bernal, A. 1994. Aspectos ecológicos de la comunidad de zooplancton nerítico en el departamento del Magdalena, mar Caribe colombiano. Tesis Maestría, Univ. Nacional de Colombia, Bogotá. 54 p. [ Links ]
Bernal, A. 2000. Die Struktur der Zooplanktongemeinschaft im neritischen Bereich des kolumbiansichen karibischen Meeres. Dissertation, Univ. Justus Liebig-Giessen, Giessen, Germany. 142 p. [ Links ]
Bernal, A. y S. Zea. 2000. Estuctura taxonómica y trófica de la comunidad de zooplancton bajo un régimen alternante entre descarga continental y afloramiento costero en Santa Marta, Caribe colombiano. Bol. Inv. Mar. Cost., 29: 3-26. [ Links ]
Boettger-Schnack, R., J. Ryuj and J. Machida. 2011. Comparison of morphological and molecular traits for species identification and taxonomic grouping of oncaeid copepods. Hydrobiologia, 666: 111-125. [ Links ]
Boxshall, G.A. and S.H. Halsey. 2004. An introduction to copepod diversity. The Ray Society, London. 966 p. [ Links ]
Browman H.I. and B.M. Marcotte. 1987. The effect of zooplankton abundance on feeding behaviour and prey size selection in Atlanctic salmon, Salmo salar, alevins. Holarctic Ecol., 10: 163-170. [ Links ]
Campos, A. 1982. Lista de especies de copépodos planctónicos de aguas cubanas. Poeyana, 24: 1-27. [ Links ]
Campos, N. y J. Plata. 1990. Crustáceos epiplanctónicos de la región de Santa Marta, Caribe colombiano: 255-264. In: CVC-Colciencias (Eds.). Mem. VII Sem. Nal. Cienc. Tecn. Mar, Com. Col. Oceanogr., 540 p. [ Links ]
Díaz, J.M. and A. Acero P. 2003. Marine biodiversity in Colombia: Achievements, status of knowledge, and challenges. Gayana, 67 (2): 261-274. [ Links ]
Dorado-Roncancio, E.F. 2015. Estructura del zooplancton de la bahia de Cartagena (Caribe colombiano) en tres épocas climáticas de 2010. Trabajo grado Biol. Mar., Univ. Jorge Tadeo Lozano, Santa Marta. 89 p. [ Links ]
Dorado-Roncancio, E.F. y J. Dorado-Roncancio . 2018. Primer registro del copépodo Cymbasoma chelemense (Copepoda: Monstrilloida) en el mar Caribe colombiano. Bol. Inv. Mar. Cost ., 47 (2): 157-163. [ Links ]
Fisco, P. 2006. Contribución al conocimiento de la subclase Copepoda (Milne-Edwards, 1840) en un ecosistema estuarino del Caribe colombiano (bahía de Cispatá) durante los meses de agosto a diciembre de 2005. Trabajo grado Biol. Mar., Univ. Jorge Tadeo Lozano, Santa Marta. 99 p. [ Links ]
Franco-Herrera, A. 2006. Variación estacional del fitoplancton y mesozooplancton e impacto de herbivoría de Eucalanus subtenuis Giesbrecht, 1893 (Copepoda: Eucalanidae) en el Caribe colombiano. Tesis Doctorado, Univ. Concepción, Concepción, Chile. 125 p. [ Links ]
Fuentes-Reinés, J.M. and E. Suárez-Morales. 2017a. Complementary description and record of Neocyclops ferrari (Cyclopidae: Halicyclopidae) from northern Colombia. Acta Biol. Col.., 2 (1): 59-65. [ Links ]
Fuentes-Reinés, J.M . and E. Suárez-Morales . 2017b. New records of poecilostomatoid copepods (Crustacea) from a coastal system oft he Colombian Caribbean with notes on morphology. Check List, 13 (5): 513-523. [ Links ]
Fuentes-Reinés, J.M . and E. Suárez-Morales . 2017c. A new species of Echinolaophonte and record of E. armiger (Gurney, 1927) (Crustacea, Copepoda, Harpacticoida, Laophonticae) from the Caribbean with a key to species. Zookeys, 722: 19-36. [ Links ]
Fuentes-Reinés, J.M ., E. Suárez-Morales and C.E. Granados-Martínez. 2017. First record of Pseudodiaptomus cokeri González and Bowman, 1965 (Copepoda: Calanoida: Pseudodiaptomidae) from Colombia. Check List , 13 (1) (2057), 4 p. [ Links ]
Fuentes-Reinés, J.M ., E. Zoppi de Roa and R. Torres. 2015. A new species of Cletocamptus Schmankewitsch, 1875 (Crustacea, Copepoda, Harpacticoida) and the description of the male of C. nudus from Colombia. Panam. J. Aquat. Sci., 10 (1): 1-18. [ Links ]
Gaviria, S. and N. Aranguren-Riaño. 2019. Continental copepods (Crustacea: Hexanauplia) of Colombia: revision and additions to the inventory. Biota Col ., 20 (1): 50-74. doi:10.21068/c2019.v20n01a04. [ Links ]
Giesbrecht, W. 1893. Systematik und Faunistik der pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel und der Angrenzenden Meeres-Abschnitte, Herausgegeben von der Zoologischen Station zu Neapel, 19:1-831, pls. 1-54. [ Links ]
Giraldo, R. y S. Villalobos. 1983. Anotaciones sobre el zooplancton superficial de San Andrés y Preovidencia. Bol. Fac. Biol. Mar., 1: 6. [ Links ]
Gómez, S. 1975. Observaciones planctónicas en la bahía de Cartagena (10°20'N - 75°30'W), en febrero y marzo de 1974: 172-182. En: Pérez-Rodriguez, R. (Ed.). Mem. Simp. Latinoam. Oceanogr. Biol., México D.F. 382 p. [ Links ]
Gómez, S. and J.M. Fuentes-Reinés. 2017a. A new species of Tisbintra (Harpacticoida, Tisbidae), and range extension for Geehydrosoma brevipodum (Harpacticoida, Cletodidae) from northern Colombia. Caldasia 39, (1): 1-12. [ Links ]
Gómez, S . and J.M. Fuentes-Reinés . 2017b. New species of Leptocaris and a new record of Darcythompsonia inopinata (Harpacticoida: Darcythompsoniidae) from Colombia. Caldasia, 39 (2): 221-238. [ Links ]
Gómez, S ., R. Gerber and J.M. Fuentes-Reinés . 2017. Redescription of Cletocamptus albuiquerquensis and C. dominicanus (Harpacticoida: Canthocamptidae incerta sedis), and description of two new species from the US Virgin Islands and Bonaire. Zootaxa, 4272 (3): 301-359. [ Links ]
Hardy, A. 1970. The open sea world, the world of plankton, Collins, London. 335 p. [ Links ]
Hernández-Trujillo, S. y G.M. Esqueda-Escárcega. 2002. La diversidad de copépodos marinos en México. Oceanides, 17 (1): 57-68. [ Links ]
Humes A.G. 1994. How many copepods? Hydrobiologia, 292/293: 1-7. [ Links ]
Huys, R. and G. Boxshall. 1991. Copepod evolution. The Ray Society, London. 468 p. [ Links ]
Jerez-Guerrero, M., M.I. Criales-Hernández y A. Giraldo. 2017. Copépodos epipelágicos en bahía Cupica, Pacífico colombiano: composición de especies, distribución y variación temporal. Rev. Biol. Trop., 65(3): 1046-1061. [ Links ]
Khodami, S., J.V. McArthur, L. Blanco-Bercial, L and P Martínez Arbizu. 2017. Molecular phylogeny and revision of copepod orders (Crustacea: Copepoda). Scient. Rep., 7(1):1-11. [ Links ]
López, M.L. y D.N. Mesa. 1983. Distribución y abundancia del zooneuston en el Caribe Colombiano - Crucero Océano V. Area II y III. ARC Tesis Biol. Mar., Univ. Jorge Tadeo Lozano, Bogotá. 110 p. [ Links ]
López, R.H. and L.H. Mojica. 2015. Distribution and abundances of Oncaea media and O. venusta (Crustacea: Copepoda) in the Colombian Pacific Ocean during two periods in 2001. Rev. Univ. Cienc. Aplic. Ambient. Divulg. Cient. U.D.C.A., 18(1): 197-206. [ Links ]
Lozano, F. 1986. Determinación de biomasa y su variación especio-temporal de la comunidad zooplanctónica de la bahia de Santa Marta, Caribe colombiano, y contribución a la situación del zooplancton en las institutiones educativas colombianas. Tesis Biol., Pont. Univ. Javeriana, Bogotá. 89 p. [ Links ]
Marino, S. y J. Merchán. 1993. Estimación cualitativa y descripción cuantitativa del zooplancton del noreste de la Guajira (Puerto Estrella - Punta Espada) y su relación con parámetros físico-químicos del agua. Tesis Biol. Mar., Univ. Jorge Tadeo Lozano, Bogotá. 156 p. [ Links ]
Martínez-Barragán, M., J. Medina-Calderón, A. Franco-Herrera y A. Santos-Martínez. 2009. La comunidad de copépodos (Crustacea) en las islas de Providencia y Santa Catalina (Caribe colombiano) durante el período lluvioso de 2005. Bol. Inv. Mar. Cost . 38 (1): 85-103. [ Links ]
Mayzaud, P., S. Razouls, A. Errhif, V. Tirelli and J.P. Labat. 2002. Feeding, respiration and egg production rates of copepods during austral spring in the Indian sector of the Antartic Ocean: Role of the zooplankton community on the carbon transformation. Deep-Sea Res. Part 1, Oceanogr. Res. Pap., 49(6): 1027 - 1048. [ Links ]
Medellín-Mora, J. y G. R. Navas. 2010. Listado taxonómico de copépodos (Arthropoda: Crustacea) del mar Caribe colombiano. Bol. Inv. Mar. Cost ., 39(2): 265-306. [ Links ]
Michel, H.B. and M. Foyo. 1976. Caribbean zooplankton. Part 1. Siphonophora, Heteropoda, Copepoda, Euphasiacea, Chaetognatha and Salpidae. Office of Naval Research. Dep. of the Navy, U.S.A., 549 p. [ Links ]
Monsalve, B. 1976. Copépodos del Pacífico colombiano, crucero Pacífico V y VII. Div. Pesq., 18(3): 2-9. [ Links ]
Morales-Ramírez, A. and E. Suárez-Morales . 2009. Copepods: 291-306. In: Wehrtmann, I.S. and J. Cortés (eds.). Marine diversity of Costa Rica, Central America. Springer Science + Business Media B.V. [ Links ]
Morales-Ramírez, A ., E. Suárez-Morales , M. Corrales-Ugalde and O. Esquivel-Garrote. 2014. Diversity of the free-living marine and freshwater Copepoda (Crustacea) in Costa Rica: a review. ZooKeys, 457: 15-33. [ Links ]
Paffenhöfer, G.-A. 1971. Grazing and ingestion rates of nauplii copepodids and adults of marine planktonic copepod Calanus helgolandicus. Mar. Biol., 11: 286-298. [ Links ]
Park, T. 1970. Calanoid copepods from the Caribbean Sea and Gulf of Mexico 2. New species and new records from plankton samples. FAO Fish. Rep., 200: 275-289. [ Links ]
Razouls C., F. de Bovée, J. Kouwenberg and N. Desreumaux. 2005-2018. Diversity and geographic distribution of marine planktonic copepods. Sorbonne Université, CNRS. Available at Available at http://copepodes.obs-banyuls.fr/en [Accessed May 31, 2019]. [ Links ]
Reid, J.W. 1990. Continental and coastal free-living Copepoda (Crustacea) of México, Central America and the Caribbean region: 175-213. In: Navarro, D. y J.G. Robinson (eds.) Diversidad biológica en la Reserva de la Biósfera de Sian Ka'an, Quintana Roo. México, CIQRO/University of Florida, México. [ Links ]
Rendón, R., T. Vanegas y P Tigreros. 2003. Contaminación en la bahía de Cartagena por aguas de lastre de los buques de tráfico internacional. Bol. Cient. CIOH, 21: 91-100. [ Links ]
Rentería, B. 1977. Dinámica zooplanctónica e hidrografía de la bahía de Cartagena. Div. Pesq ., 10 (4,5): 1-41. [ Links ]
Samper, A. 1970. Contribución al conocimiento del zooplancton de las estaciones 87 a 118, según la operación Océano I. Tesis Biol. Mar., Univ. Jorge Tadeo Lozano, Bogotá. 82 p. [ Links ]
Suárez-Morales, E. 2015. Clase Maxillopoda: Subclase Copepoda: Orden Monstrilloida. Rev. IDE@ - SEA, 96: 1-12. [ Links ]
Suárez-Morales, E. and J.M. Fuentes-Reinés . 2018. Two new species of Diarthrodes (Copepoda, Harpacticoida: Dactylopusiidae) from the Caribbean coast of Colombia. Rev. Mex. Biodiv., 89: 365-374. [ Links ]
Suárez-Morales, E . and R. Gasca. 1998. Updated checklist of the free-living marine copepods (Crustacea) of Mexico. An. Inst. Biol., Univ. Nac. Autón. Méx., Ser. Zool., 69 (1): 105-119. [ Links ]
Suárez-Morales, E ., M. de Troch and F. Fiers. 2006. A checklist of the marine Harpacticoida (Copepoda) of the Caribbean Sea. Zootaxa, 1285: 1-19. [ Links ]
Uribe, C. y M. Calero. 2006. Evaluación de la composición zooplanctónica y variables fisicoquímicas en el agua de lastre de buques internacionales que arriban al puerto de Santa Marta (Caribe colombiano). Tesis Univ. Magdalena, Santa Marta. 130 p. [ Links ]
Uye, S.-I. and T. Yamaoka. 1990. Vertical and horizontal distribution of copepod nauplii as food for anchory larvae (Engraulis japonica) in Hiroshima Bay. Bull. Japan. Soc Fish. Oceanogr., 55: 341- 351. [ Links ]
Vives, F. y A.A. Shmeleva. 2010. Crustaceos, copépodos marinos II, Non Calanoida. Fauna Ibérica 33, 486 p. Mus. Nal. Cienc. Nat., Cons. Sup. Invest. Cient., Madrid. [ Links ]
Walter, T.C. and G. Boxshall . 2018. World of copepods database. Accessed at Accessed at http://www.marinespecies.org/copepoda on 2019-05-31. [ Links ]
Willen, E. 2002. Notes on the systematic position of the Stenheliinae (Copepoda, Harpacticoida) within the Thalestridimorpha and description of two new species from Motupore Island, Papua New Guinea. Cah. Biol. Mar., 43(1): 27-42. [ Links ]
WoRMS Editorial Board (2019). World register of marine species. Available from Available from http://www.marinespecies.org at VLIZ. Accessed 2019-05-10. doi:10.14284/170. [ Links ]
Received: September 05, 2018; Accepted: June 06, 2019











 text in
text in 



