INTRODUCTION
Within zooplankton, copepods belonging to crustaceans constitute the predominant group. Among them, pelagics are the most abundant in the oceans, due to three reasons: i) torpedo-shaped body, sensory antennas and the strong gear of the muscular motor for the detection and escape of predators, ii) the ability to detect prey remotely and capture them when they arrive in a current of exploration, or when they swim through the copepod's perceptual sphere and iii) to the efficient search for a partner in a diluted environment, allowing sexual reproduction with real mating in each generation, little common in plankton; This provides advantages in terms of eliminating bad mutations and promoting "good genes" through sexual selection (Kierboe, 2011). Due to this, copepods represent between 60 and 90 % of the biomass in coastal environments (Bradford-Grieve et al., 1999; Ruíz-Pineda et al., 2016; Neumann-Leitão et al., 2018).
Zooplankton presents detectable variations, a product of cyclical changes in environmental factors (Mclusky and Elliot, 2004; López-Peralta and Mojica-López, 2015; Rose et al., 2019); therefore, the patterns of variability in composition, abundance, and distribution of planktonic copepods are established based on their coupling with physical processes (i.e.: gradients of salinity, temperature, concentration of nutrients, and internal circulation patterns) at different scales (Marques et al., 2006; Escamilla et al., 2011; Ruíz-Pineda et al., 2016).
On the other hand, Magalháes et al. (2009, 2015) and Neumann-Leitão et al. (2018) mention that the seasonal and spatial variability observed in the occurrence and distribution of planktonic copepods in tropical coastal-estuarine systems is directly related to abiotic factors (i.e.: salinity, turbidity, and temperature) and biotic factors (i.e.: competition, predation, habits, availability, and quality of food) or a combination of both. Consequently, they cannot be considered as a homogeneous set of organisms, since their distribution will change not only in space and time, due to the influence of physical-chemical factors, but also due to biological processes (Seda and Devetter, 2000; Beisner, 2001; Vukanic et al., 2018).
In the coastal or estuarine zones, the biomass and diversity values (9 to 50 mg m-3 and 8-10 species, respectively) of the copepods, are relatively low when compared to the oceanic zones. These differences are attributed to the peculiar physiographic conditions of these areas, which favor a small group of copepod species (Vásquez-Yeomans et al., 2012; Rice et al., 2015; Ruíz-Pineda et al., 2016). In this sense, the salinity gradients resulting from the interactions between marine influence and freshwater runoff, often represent the main parameter to define the patterns of local distribution and species succession (Escamilla et al., 2011; Ruíz-Pineda et al., 2016). The eastern sector of the Gulf of Cariaco has a marked influence of freshwater, due to the Carinicuao and Cariaco rivers, which maintain communication with the gulf during some months of the year, originating an estuarine area (Caraballo, 1982). On the other hand, convection and outcrop processes in the Gulf of Cariaco tend to develop phenomena of renewal of warm surface waters by colder, nutrient-rich deep waters that stimulate the development of phytoplankton and zooplankton (Bonilla et al., 1985). Therefore, studying the variability patterns of copepods in estuarine zooplankton could reveal important information on the dynamics of the local community, with implications for other tropical estuarine-coastal systems. In this sense, the monitoring of the copepod community allows detecting and following up on levels of ecological disturbance and deterioration (Branco et al., 2007; Escamilla et al., 2011).
It is important to mention that due to all the aforementioned in the Gulf of Cariaco, copepods have been extensively studied, identifying a significant number of species. Of the 723 species indicated for the zone of Venezuela, 136 have been registered in that gulf (Legaré, 1961; Zoppi, 1961; Infante and Urosa, 1986; Márquez-Rojas et al., 2006, 2011, 2014a, 2014b; Razouls et al., 2005-2019). However, in the eastern sector or sac of the Gulf of Cariaco, knowledge about the copepod communities is relatively scarce, although several studies confirm a marked gradient of salinity in this area (Márquez et al., 2011; Martínez et al., 2011).
There is currently no published information on the structure of the copepod community in the eastern sector of the Gulf of Cariaco and how these are affected by seasonal variations in salinity, rainfall, and freshwater runoff. Therefore, the main objective of the present study is to evaluate the taxonomic composition of the copepod assembly in the eastern sector of the Gulf of Cariaco and to characterize patterns of seasonal abundance and dominance to annual variations in salinity.
MATERIALS AND METHODS
Study area
The eastern sector or sac of the Gulf of Cariaco (Figure 1), is approximately 9 km wide and 17 km long. The shallowest depths of the gulf are located in this sector, which is close to 40 meters. At its eastern end, the Carinicuao and Cariaco rivers flow out and in the south, the Oricoto and López ravines, making up the largest continental tributaries and probably the source of supply of much of the sedimentary materials deposited in the study area (Caraballo, 1982). The vegetation in this sector is characterized by the presence of mangroves and xerophytic zones.
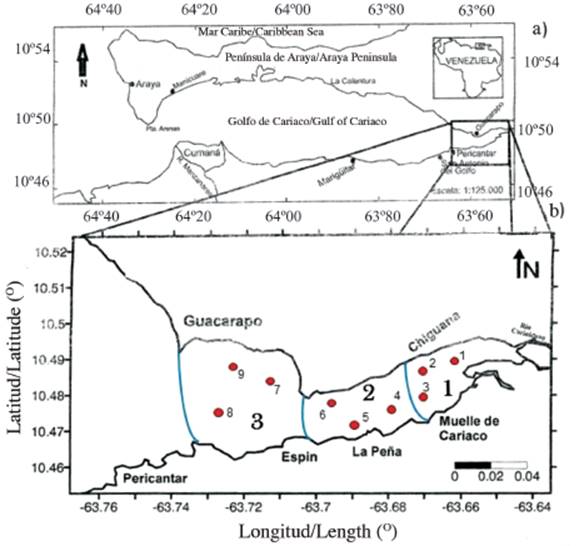
Figure 1 a) Geographical location of the Gulf of Cariaco and b) details of the eastern end or sac of the gulf, showing the sampling areas and stations.
The study area presents pronounced seasonality that can be observed in the physical parameters of the water column at the beginning of the year caused by the seasonal displacement of the intertropical convergence zone (ITCZ) that generates significant variability in the wind regime, river currents and discharge (Lorenzoni et al., 2013; López-Monroy and Tróccoli-Ghinaglia, 2015).
Field sampling
The design to take the samples was carried out utilizing a two-stage conglomerate sampling with a subsample (Cochran, 1977), which consisted of dividing the eastern sector or the Gulf sac into three zones; within each zone, three stations were chosen at random, for a total of 9 samples per outlet (Figure 1b). The samples were collected monthly between June 2009 and January 2010, guaranteeing representativeness in the rainy season (June-September) and drought (October-December and January), in the nine seasons in the eastern sector of the Gulf of Cariaco (Figure 1b).
The zooplankton samples were collected at the surface level, with a Bongo net (333 and 555 μm mesh opening and 60 cm in diameter), at a speed of 3.7 km/h for 15 min. For the present work, only the mesozooplankton samples (333 ^m) were used, this network was equipped with a flowmeter to estimate the volume of filtered water. All samples were immediately placed in 500 mL polyurethane bottles and stored in formalin buffered with sodium tetraborate, maintaining the final concentration at 5 %.
At each station, temperature, salinity, and surface dissolved oxygen were measured using a multi-parameter probe (YSI). Simultaneously, samples were collected in dark polyethylene bottles (2 L) for determinations of chlorophyll-α (Chlor.-α) using the spectrophotometric method described by Lorenzen modified by Strickland and Parsons (1972), the results were expressed in mg Clor.-α.m3.
Laboratory work
For zooplankton, each sample of the 333 mesh was homogenized and divided into two equal portions utilizing a Folsom separator; one portion was used for the study of community structure and the other for biomass analysis for other studies. For taxonomic determination, from the concentrated samples (400 mL), 3 sub-samples of 5 mL were obtained with a Stempel pipette, and a count and identification of adult and juvenile copepods was carried out under the lowest possible taxonomic level, using the specialized identification keys of Campos-Hernández and Suárez-Morales (1994), Suárez-Morales (1995), Bradford-Grieve et al. (1999), as well as the Razouls et al. (20052019). The number of organisms counted was divided by the volume of filtered water, so the results were standardized to ind.m-3. The rainfall data analyzed was provided by the Meteorological Service of the National Armed Forces of the Antonio José de Sucre Airport in the city of Cumaná.
Analysis of the information
The quantitative data obtained from the sample were used to calculate the absolute and relative abundance of the copepod species, while the ecological indices (diversity and equity) were calculated per month. Copepod diversity was calculated using the Shannon-Wiener index (H') and equity through the Pielou index (J) according to what Krebs (1999) pointed out.
The comparison of abiotic (temperature, salinity and dissolved oxygen), biotic (Chlor.-α), total abundance and diversity of copepods between stations and areas were compared with the Kruskal-Wallis non-parametric test, after verifying that the assumptions of the parametric analysis were not met using the Kolmogorov-Smirnov normality test and Levene's homoscedasticity (Sheskin, 2004). The comparisons of the periods studied were made with the Mann-Whitney test or the Wilcoxon test (Zar, 1999). Spearman's correlation coefficient was calculated to characterize the relationships between salinity, copepod abundance, and ecological indices. Statgraphics plus 5.1 statistical software was used for these tests.
To visualize the behavior of the diversity of the copepods between the different seasonal periods and the sampling months, K-dominance curves were performed (Lambshead et al., 1983). In its construction, the range of the species (on the abscissa axis) and the percentage of accumulated abundance (on the ordinate) are used.
Bray-Curtis dissimilarity matrices previously transformed to Log (x + 1) were constructed with the copepod data, while the environmental data (temperature, dissolved oxygen, salinity, and chlorophyll-α) were normalized and Euclidean distances were used for their construction. (Legendre and Legendre, 1998). The matrices generated from the copepods were used to analyze the similarity between the monthly abundances utilizing a classification analysis (Cluster) and the non-metric multidimensional scaling (nMDS) to visualize the order of the abundance of the copepods in two dimensions to the two epochs studied; The Primer 6 program was used for these analyzes (Clarke and Gorley, 2006).
To know the possible relationships between the most abundant copepod species and the environmental variables recorded during the two periods studied, a canonical correspondence analysis (ACC) was carried out. This procedure allowed to position the most abundant copepod species (> 10 %) with the variables temperature, salinity, dissolved oxygen, and chlorophyll-α, in a synthesized coordinate system (components 1 and 2) (Ter Braak and Verdonschot, 1995).
RESULTS
Abiotic variables
In the present study, temperature, dissolved oxygen, salinity, chlorophyll-α, as well as abundance and ecological indices did not show significant spatial differences (p > 0.05) between the nine stations sampled, nor in the three study areas (p > 0.05). Therefore, the values reported for each month of sampling were grouped and evaluated on seasonal scales (rainy period/dry period).
The surface temperature of the water did not show a marked variation between the periods studied (Mann-Whitney U-test = 556; p > 0.05), registering values between 24-28.6 °C in the rainy period and 23.4-27.6 °C for the dry period. Dissolved oxygen showed marked monthly fluctuations, registering significant differences between the two periods (Mann-Whitney U-test = 156; p < 0.05); the highest values were recorded in the rainy season (4.9-9.9 mLO2.L-1), while in the dry season they fluctuated between 1.4-7.8 mLO2.L-1.
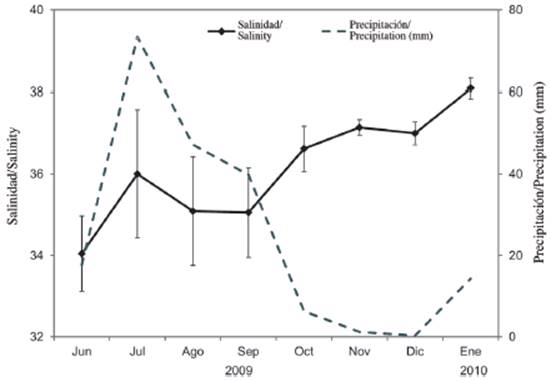
Salinity presented marked monthly variability, with differences of up to 6 units. Seasonal significant differences were detected (Mann-Whitney U-test = 1103.5; p < 0.05). The lowest value (32.5 ± 1.6; mean ± standard deviation) was recorded in June 2009 (rainy season), while the highest value (38.5 ± 0.2) was measured in January 2010 (dry season) (Figure 2). When salinity is related to precipitation, a contrary trend is observed, presenting the highest precipitation values (17.7 to 73.4 mm) in the rainy season (Figure 2).
Biotic variables
Chlorine concentration showed great fluctuation during the study, with values ranging from not detectable (ND) to 26.5 mg Chlor. α.m-3. The highest concentrations were measured at the beginning of the study (rainy season), registering magnitudes of 17.6 ± 6.4 mg Chlor. α.m-3 in July 2009. During the remaining months, the average Chlorine values were lower, ranging from 0.9 to 8.9 mg Chlor. α.m-3. Significant differences (Mann-Whitney U-test = 1008; p < 0.05) were detected between the periods studied.
Composition and abundance of copepods
A total of 45 species of copepods were identified (Table 1). The average monthly abundance of copepods varied between 513 ± 79 ind.m-3 in December 2010 (dry season) and 7156 ± 627 ind.m-3 in July 2009 (rainy season) (Figure 3a). Acartia tonsa (Dana) was the most abundant and representative of the entire study, contributing 83.2 ± 2.6 % in rain and 60.8 ± 4.7 % in the dry season of the total abundance of copepods (Table 1). On a monthly scale, the abundance of this species exceeded 90 % in July, November, and December 2009 (Figure 5c). This species was followed by Temora turbinata (Dana), with a relative abundance of 5.3 and 30.3 % for the periods of rain and drought, respectively. During the drought period (October 2009 and January 2010), the highest abundances of this species were obtained. Paracalanus quasimodo (Bowman) was the third species in order of abundance, excelling in the rainy season (2.15 %), mainly in June and September 2009.
Table 1 Taxonomic composition, absolute (mean ± SD), and relative abundance of the copepods collected during the rainy and dry season in the Gulf of Cariaco sack, Sucre state, Venezuela.
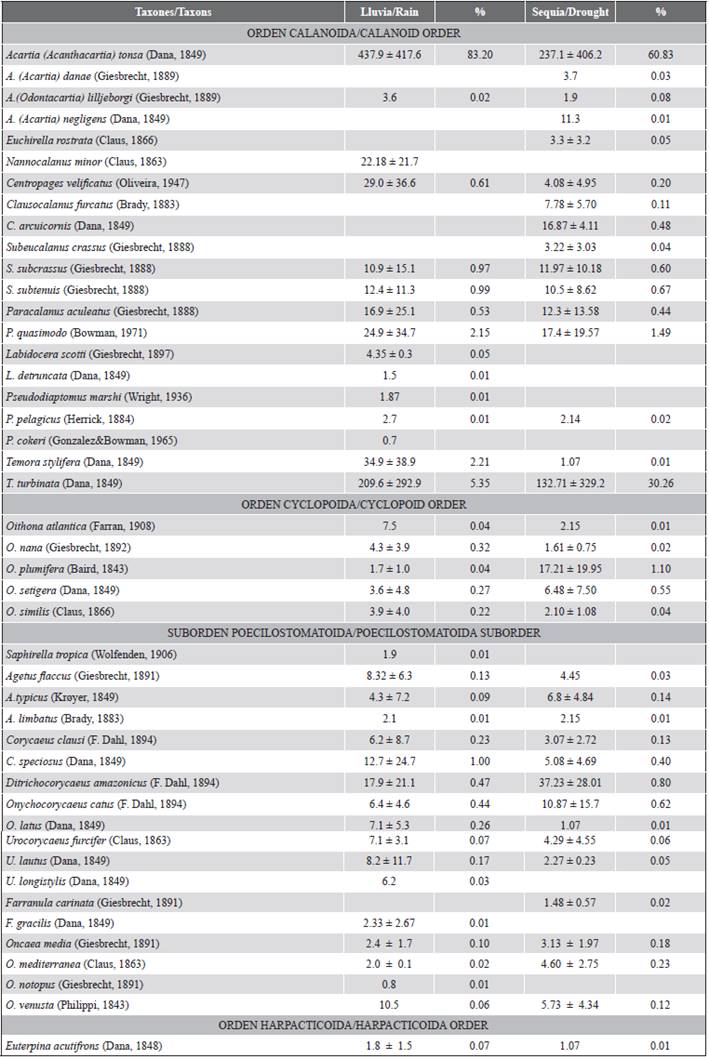
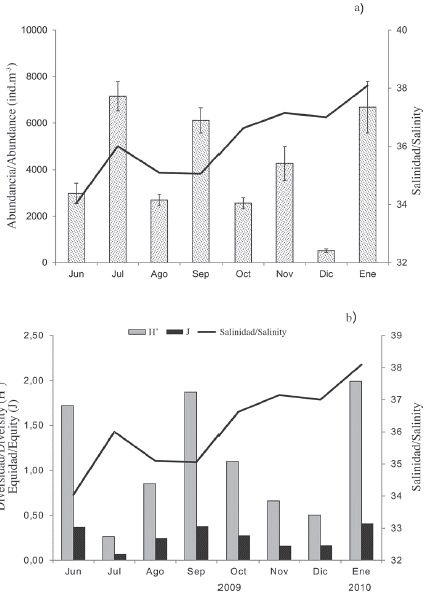
Figure 3 a) Monthly mean (± SD standard deviation) of the abundance of copepods and b) ecological indices of copepods collected in the Gulf of Cariaco sack, Sucre state, Venezuela. These data were always drawn with salinity (line).
Subeucalanus subcrassus and S. subtenuis followed in order of abundance, with relatively low representations (0.97 and 0.99 %, respectively) and standing out in the rainy season (Table 1, Figure 5c).
Among the main species, A. tonsa (K-W = 28.3, p < 0.001) and T. turbinata (K-W = 23.5, p < 0.001) showed significant seasonal differences in abundance. Seasonal variations were also observed in the total abundance of copepods (K-W = 31.4, p < 0.001). Spearman's coefficient showed significant relationships between salinity and abundance of T. turbinata (rs = 0.37, p < 0.05), between abundance of copepods and A. tonsa (rs = 0.72; p < 0.001) and T. turbinata (rs = 0.57; p < 0.01). On the other hand, significant negative correlations were found between diversity and equity with A. tonsa (rs = -0.57 and rs = -0.66; p < 0.001, respectively) and between abundance and equity (rs = -0.49; p < 0.01).
Community structure
The monthly fluctuations in the abundance, diversity, and equity of the copepods were plotted with respect to salinity (Figure 3b). No significant differences were detected in the diversity of the copepods to the periods studied (Mann-Whitney U-test = 641; p > 0.05). The values were similar during the two periods, registering average values of 1.2 ± 0.8 bits.ind-1 in the rainy season, with a minimum of 0.3 bits.ind-1 in July 2009 and a maximum of 1.9 bits.ind-1 in September 2009. In the dry season, an average value of 1.1 ± 0.9 bits.ind-1 was obtained, with a minimum of 0.5 bits.ind-1 in December 2009 and a maximum of 2.0 bits.ind-1 in January 2010. The slight decrease of the diversity values during the drought months coincided with the lower abundance of copepods (Figure 3b). Contrary results were found for equity, finding significant differences between the periods (Mann-Whitney U-test = 808.5; p < 0.05), but as in diversity, the highest values were recorded in rain (0.3 ± 0.1), while in drought the mean was 0.2 ± 0.1.
The K-dominance curve during the rainy season showed high dominance with less diversity during July and August, while June and September revealed less dominance with greater species diversity (Figure 4a). During the first three months of the drought period, they had similar behavior, high dominance with fewer species, while January 2010 registered the lowest dominance of the study with greater species diversity (Figure 4b). The K-dominance curve also exhibited a difference in the diversity and relative abundance of the species between the periods studied (Figure 4c), showing less dominance of the most abundant species in the dry season, this can be corroborated in Table 1.
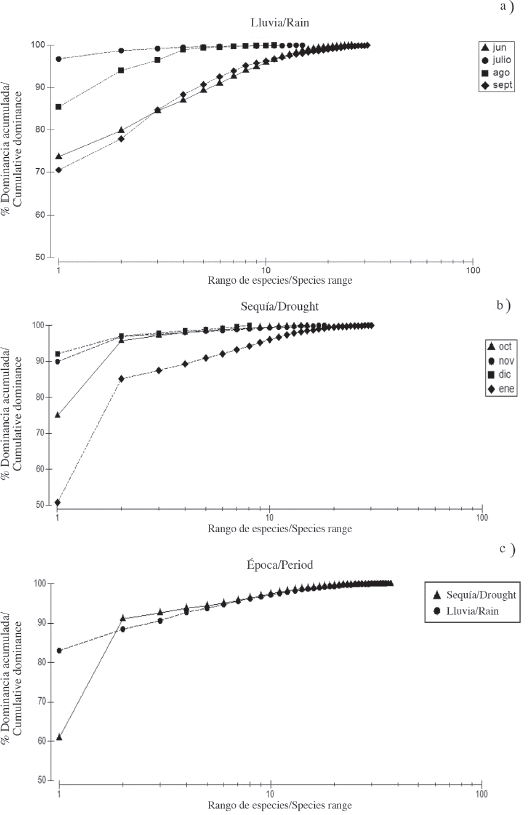
Figure 4 Cumulative dominance curve of the copepods in the rain a) and drought b) and during the two periods studied c) in the Gulf of Cariaco sack.
According to the Cluster and nMDS analyzes, we grouped the copepod community of the Gulf of Cariaco sack into two groups according to seasonal variability (Figure 5a, Figure 5b). In the Cluster, at a cutoff of 75 % similarity, these two groups are observed. The first between November and December 2009 (dry season). During these months a high percentage of abundance (> 80 %) of Acartia tonsa was observed, associated with relatively high salinity (Figure 5c). The second group can be divided into two subgroups, the first subgroup in July, August and October 2009 with high abundance percentages (75-95 %) of Acartia tonsa relating to high salinity and the second subgroup in June and September 2009 and January 2010 with a better distribution of the percentages of abundances among the other copepod species Temora turbinata and Paracalanus quasimodo (Figure 5c). In nMDS, group I mainly consisted of the rainy months, where T. turbinata and P. quasimodo registered their highest percentages of abundance; however, group II was associated with the dry season, when the percentage of the abundance of A. tonsa was almost absolute (Figure 5b).
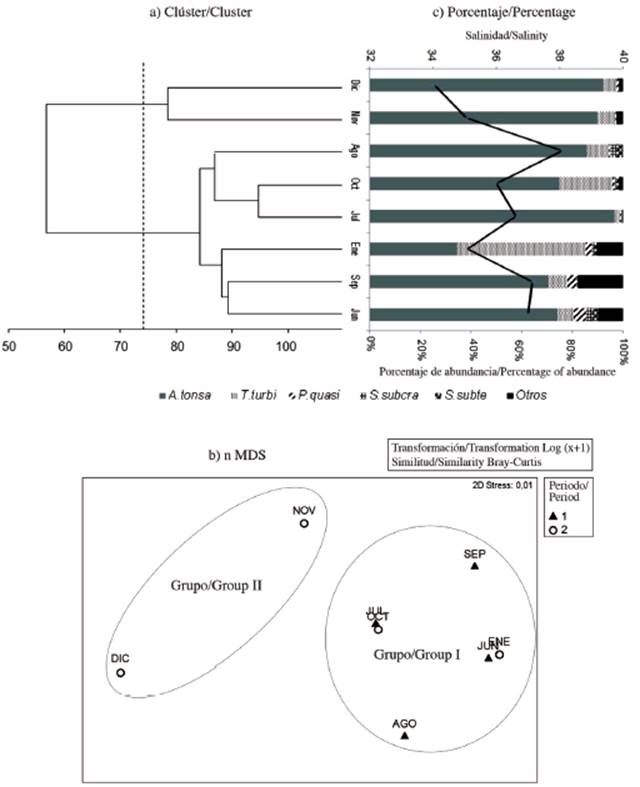
Figure 5 a) Cluster analysis and b) nMDS based on the abundance of the copepod taxa. c) Percentage of the abundance of copepod taxa with salinity (solid black line) sampled in the Gulf of Cariaco sack. A. tonsa: Acartia tonsa, T. turbi: Temora turbinata, P. quasi: Paracalanus quasimodo, S. subcra: Subeucalanus subcrassus, S.subte: Subeucalanus subtenuis. Rain period (black triangle) and drought (white circle).
The ACC considering the most abundant species, during the rainy season, showed a correlation between environmental variables and the set of species of 0.69, it was characterized by a direct relationship of A. tonsa with salinity and chlorophyll-α, while the component 2, which had an environment-species correlation of 0.45, was characterized by a direct relationship between T. turbinata and P. quasimodo with temperature and dissolved oxygen (Figure 6a).
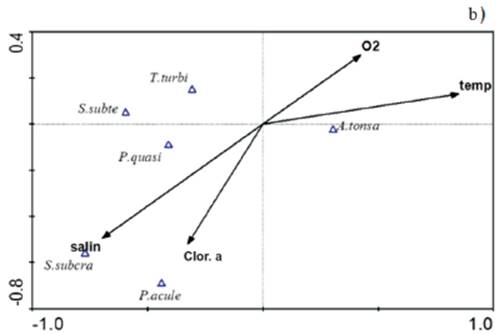
Figure 6 ACC orthogonal projection in the rainy season a) and drought b) among the most abundant copepod species and the environmental variables measured in the Gulf of Cariaco sack. salin: salinity, temp: temperature, O2: dissolved oxygen, Clor. a: chlorophyll-α, A. tonsa: Acartia tonsa, P. quasi: Paracalanus quasimodo, P. acule: Paracalanus aculeatus, S. subcra: Subeucalanus subcrassus, S. subte: Subeucalanus subtenuis, T. turbi: Temora turbinata.
On the other hand, in the dry season, it showed an environment-species correlation of 0.69, it was characterized by a direct relationship of A. tonsa with salinity and dissolved oxygen, while component 2, had an environment-species correlation of 0.43, was characterized by a direct relationship of S. subcrassus with salinity and chlorophyll-α with P. aculeatus. The ACC results corroborate what was found in Spearman's non-parametric correlations, which suggests that the abundance of the most abundant species of copepods showed significant seasonal variations, mainly in A. tonsa and T. turbinata and even in these species the relationships were demonstrated significant with salinity.
DISCUSSION
When considering tropical estuarine ecosystems in Venezuela (Zoppi, 1974; Gómez, 1983; Briceño et al.,, 2009; Márquez-Rojas et al., 2017; Villalba et al., 2017) and throughout the world (Sterza and Fernandes, 2006; Garboza da Costa et al., 2008; Magalháes et al., 2009; Da Costa et al., 2011) it is corroborated that seasonal changes in salinity are mainly influenced by rainfall. This statement was corroborated in the Gulf of Cariaco bag with the marked seasonal fluctuation that salinity presented. The high values in rainfall registered between June and September 2010 increased the flow rates of the rivers, especially Carinicuao and Cariaco, reflecting a decrease in salinity values in the rainy season. This temporal variation in salinity in the eastern end of the gulf coincided with that mentioned by Simpson and Griffiths (1967), Okuda et al. (1978) and more recently with Márquez et al. (2011), Martínez et al. (2011), Márquez-Rojas et al. (2017).
The seasonal distribution pattern observed in the total abundance of copepods, with the highest values recorded in the rainy season, was partly related to the behavior of Acartia tonsa, which was the most abundant species; this was also found in many other estuarine systems (Magalháes et al., 2009; Da Costa et al., 2011; Escamilla et al., 2011; Teiguel-C, 2015; Ruíz-Pineda et al., 2016). This planktonic calanoid copepod is a cosmopolitan species, dominant in many subtropical and temperate zones (Mauchline, 1998), it shows a high degree of tolerance to environmental changes, managing to normally withstand salinity intervals from 5 UPS (Cervetto et al., 1999) to 30 UPS (Calliari et al., 2006), exceptionally, samples have been found in systems with salinity from 0 UPS (Cronin et al., 1962) to 52 UPS (Rey et al., 1991).
The absence of a significant correlation between Acartia tonsa and salinity in the Gulf of Cariaco sack indicates that this species did not demonstrate a preference for estuarine waters. However, intraspecific tolerance to low and moderate salinities could explain the greater numerical amount of A. tonsa throughout the rainy season. For Temora turbinata, a species widely distributed in tropical and subtropical regions, a conspicuous member of superficial and near-surface mesozooplankton communities in estuaries and neritic and oceanic waters (Ara, 2002), the correlation between salinity and abundance was significant and positive. The high abundances recorded in the dry period suggest that the presence of this species in the studied area depends on the increase in salinity.
Ruíz-Pineda et al. (2016) mention that the genera Acartia, Temora, Paracalanus, and Labidocera are euryhaline and eurythermic fauna elements, with estuarine affinity. Therefore, A. tonsa and T. turbinata have been reported with great success in estuarine environments, in coastal lagoons with a strong marine influence and areas with a terrogenic influence (Peck et al., 2015), once again ratifying the presence of these species at the eastern end of the gulf. On the other hand, P. quasimodo was the third species in abundance in the present investigation, with higher increases in the rainy season; however, no correlation was found with salinity. The results regarding the abundance of this species coincided with that reported by Villalba et al. (2017) in the El Morro coastal lagoon, isla de Margarita, Venezuela, where it was counted as the second most abundant species during the relaxation period. Consequently, the copepods A. tonsa, T. turbinata, and P. quasimodo were present in both periods studied and in high abundances; therefore, it can be concluded that these species are permanent residents of this body of water.
The great variability of the physical-chemical conditions in estuaries requires that the species that inhabit them have a greater tolerance to environmental changes, resulting in less diverse ecological communities than in adjacent aquatic ecosystems, so not only the species physiological tolerance is important, but also feeding strategies (Elliott and Whitfield, 2011; Márquez-Rojas et al. , 2017). For example, typically estuarine copepods, A. tonsa, and T. turbinata are known to be euryhaline species (Paffenhõfer, 1991; Ara, 2002; Hwang et al., 2006), even the former has a wide tolerance range (0-50 UPS) (Paffenhõfer, 1991; Rey et al., 1991; Martínez-Barragán et al., 2009), while A. tonsa has a broader spectrum of food with a greater ability to select its prey among the material inorganic, even due to its omnivorous capacity, allows it to ingest large phytoplankton cells (which are common in environments influenced by inland waters) and control its potential competitors, preying on its nauplii and copepodites (Paffenhõfer, 1991), in contrast to T. turbinata that feeds mainly on diatoms with frustules (Eskinazi-Sant'anna, 2000). This A. tonsa feeding strategy is an advantage in the eastern end of the Gulf of Cariaco, due to the influence of inland waters, it is an area with high turbidity, high content of suspended matter, high concentrations of nutrients mainly of origin terrestrial (Márquez et al., 2011; Martínez et al., 2011). Furthermore, this premise was confirmed with the direct relationship of A. tonsa with salinity and chlorophyll-α in the ACC, during the rainy season. Similarly, Camatti et al. (2019) corroborated that the seasonal distribution of A. tonsa in the Venice lagoon, Mediterranean Sea, was positively and significantly associated with temperature, phytoplankton, chlorophyll-α and the salinity gradient, confirming that it is an opportunistic tolerant species that can take advantage of eutrophic ecosystems.
At the eastern end of the Gulf of Cariaco, diversity did not show significant seasonal differences; however, the copepod community showed slightly higher values in rain. This slight increase during the rainy months was probably related to the greater species richness recorded in this period, although during these months there was a greater contribution of A. tonsa in the total abundance of copepods. In this sense, the abundance of A. tonsa increased with the decrease in salinity, due to the increase in the discharge of the Carinicuao and Cariaco rivers that discharge precisely in the study area; however, no significant correlation was found between this species and salinity, where it can be deduced that salinity affects it directly. Numerous studies in estuaries have described the reduction in the diversity of copepods during the rainy season (Mwaluma et al., 2003; Osore et al., 2004; Magalháes et al., 2015); however, an opposite trend was verified in the Gulf of Cariaco sack. The low values of copepod diversity obtained in the eastern sector of the Gulf of Cariaco coincide with what was found by Magalháes et al. (2009) in the Curuçá estuary, Brazil (0.5-2.7 bits/ind-1) and by Ruíz-Pineda et al. (2016) in the Chetumal Bay estuarine system, Mexico (1.1-1.8 bits/ind-1).
Equity unlike diversity, if it presented seasonal differences; however, it followed the same pattern of diversity, with higher values in rainfall. This situation coincided with an increase in the frequency and abundance of the genus of estuarine habits Labidocera, Pseudodiaptomus, and Saphirella and could be the result of a phenomenon of "drift" of zooplankton from the adjacent Caribbean Sea. According to Bradford-Grieve et al. (1999) and Ordóñez-López and Ornelas-Roa (2003), these genera with their estuarine-coastal condition are frequent in the coastal zooplankton and their presence in the Gulf of Cariaco sac indicates fauna exchange between estuarine and marine environments. The slight increase in ecological indices of zooplankton in the rainy season supports this hypothesis.
Consequently, it was evident that during the rainy season the number of individuals and the occurrence of certain species increased significantly. This was also recorded in several tropical estuarine systems, registering high densities during the rainy season, such as the work of Sterza and Fernandes (2006) in Vitória Bay, southeast Brazil, that of Garboza da Costa et al. (2008) and Da Costa et al. (2011) in the Taperaçu and Caeté estuaries, respectively, northern Brazil. Studies on the copepod community carried out in estuarine areas of tropical and subtropical regions, mentioned above, agree that salinity is the determining factor in the spatial distribution of some species, while factors such as rainfall, temperature, and dissolved oxygen can determine the temporal distribution (Villate et al., 2017).
Various studies have long discussed the relationship between zooplankton and the estuarine environment, however, it is evident that the abundance of population changes (Day et al., 1989; Ordóñez-López and Ornelas-Roa, 2003). In this sense, the haline gradient, the temperature, and the freshwater discharge were not the only factors that determined the distribution patterns of the planktonic copepods, it is probable that biological factors such as the flow of early stages (naupliar or copepodites), the Competition and predation play an important role in the variation of the abundance of organisms for the copepod populations of the Gulf of Cariaco sack. These assumptions are also supported by Chaalali et al. (2013) and David et al. (2016), who mentioned that the coexistence and dominance of few species in the same system could be explained by spatial and temporal segregations caused by differences in reproductive strategies and diet.
The monthly and seasonal changes in the balance of the relative abundance of the main copepod species as visualized in the K-dominance curves directly influenced the behavior of the ecological indices. In particular, the changes in the contribution of A. tonsa in the periods studied were responsible for the changes in these indices.
The present study has not shown, in the eastern sector of the Gulf of Cariaco, a clear seasonal pattern in the abundance, diversity, and uniformity of the copepod community and the salinity values, which has also been visualized in the cluster and multidimensional scaling analysis (MDS). Therefore, the studied area can be considered spatially uniform and seasonally homogeneous with these parameters. However, it should be mentioned that the copepod community registered a greater abundance and species richness in the rainy season. Therefore, new research on the biomass and community structure of copepods in the Gulf of Cariaco sack should be evaluated to provide a better understanding of the trophic structure of this ecosystem.
Ultimately, copepods are known to be particularly sensitive to environmental changes, either as a result of natural environmental conditions or by anthropogenic action (Li et al., 2000; Camatti et al., 2019). The interaction between anthropogenic activity, climate change, and planktonic communities, focusing on systematic changes in community structure, abundance, and distribution in recent decades, is a key global issue, as well as the potential socio-economic impacts of these changes (Hays et al., 2005). In environments with constant changes, such as the eastern end of the Gulf of Cariaco, due to the influence of coastal upwelling, sewage discharge, and overfishing, monitoring the ecological dynamics of the species is of utmost importance. In particular, continuous modifications of copepod assemblies in response to anthropogenic and environmental stressors should be considered. Continuous monitoring of plankton will act as sentinel research to identify future changes in this highly impacted complex ecosystem and its related food web that provides sustenance for species such as sardines (Sardinella aurita), the main fishing resource in the gulf (Quintero et al., 2002).











 text in
text in