INTRODUCTION
Red tides are natural phenomena that normally cause changes in the color of the water as a result of the increase in the size, density, or accumulation of pigmented planktonic organisms, which include microalgae, cyanobacteria, and microzooplankton ciliates. These events occur in different parts of the world and their apparent growth in both frequency and intensity, in recent decades, is increasingly evident on a global scale (Sar et al., 2002; Hallegraeff et al., 2004). Some studies indicate that several factors influence the formation of red tides, including salinity, water temperature, nutrient concentrations, and light availability (McGaraghan et al., 2012; California Ocean Science Trust, 2016).
These tides are usually harmless and infrequent when they occur in areas with a high rate of water exchange or where large-scale aquaculture is not used. However, in some cases, they can be considered a problem when they cause socio-economic impacts and affect trade and tourism. They can even generate a risk to public health, particularly if the blooms are produced by harmful species that represent a danger to organisms, including humans, as some of them can produce powerful toxins (Sar et al., 2002; Hallegraeff et al., 2004; Reguera et al., 2011, 2016).
In Colombia, red tide events have been evident for years at the local level in coastal areas, but most of this information is not public. However, some reports have been disclosed on the presence of potentially harmful microalgae and the negative impact produced by harmful algal blooms (HAP) (Mancera-Pineda et al., 2009, 2014; Rodríguez et al., 2010; Arbeláez et al., 2017; Ruiz and Mancera-Pineda, 2019). In recent years, an increase in the frequency of the appearance of red tides has been observed in the coastal zone of the Magdalena department (Colombia), particularly in the Santa Marta region (Invemar, 2010, 2015a, 2015b, 2017a, 2017b; Malagón and Perdomo, 2013). For this reason, the present study aims to analyze the possible influence of environmental and climatic variables on the appearance of red tide events that occurred between 2010 and 2017 and to try to identify temporal patterns of occurrence.
STUDY AREA
Santa Marta is located in the northeastern portion of the Colombian Caribbean (11°15'-22' N and 73°57'-74°12' W) (Figure 1; Díaz et al., 2000), in the area of influence of one of the country's most important ecosystem strategic points: The Sierra Nevada de Santa Marta, declared by UNESCO as a Biosphere Reserve since 1979. The region includes approximately ten bays that represent diverse ecosystems, most of which are located in the Tayrona National Natural Park (PNNT), one of the protected areas of the Colombian Caribbean.
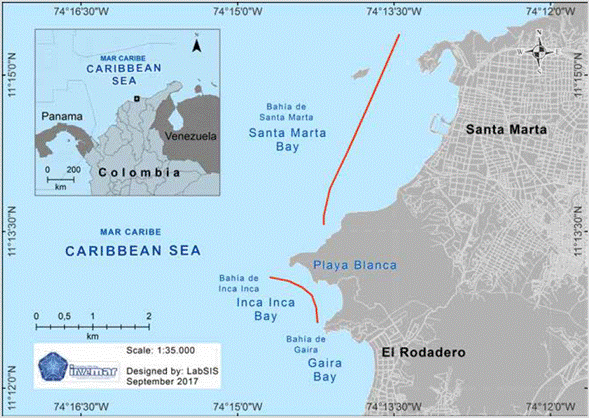
Figure 1 Map of Santa Marta (Colombian Caribbean) with the localities in which red tides have been detected (red line). Made by the Laboratory of Information Services (Labsis) of Invemar.
The urban area that makes up the bay of Santa Marta has a population of approximately 507 000 inhabitants (Santa Marta Chamber of Commerce, 2018). In this region, port and tourist activities predominate, which have experienced a rapid increase and have become the most important economic income of the city, along with local commerce and fishing, in order of importance (Vega-Sequeda et al., 2008).
The region's climate is governed by the climatic pattern of the Colombian Caribbean coast: a dry season from December to April and a rainy season from May to November. However, in detail, four climatic seasons have been described: major dry (from December to April), in which the presence of north trade winds generates upwelling events, as well as a decrease in seawater temperature (2025 °C) and increase in salinity (> 38) and wave intensity; less rainy (from May to June), characterized by a slight decrease in the trade winds and the appearance of light rainfall; minor dry season or "veranillo de San Juan" (from July to August), whose main characteristic is the intensification of the trade winds, which are again dominant but with less force, and more rainy (from September to November), which increases rainfall (more than 65 % of the annual volume), the trade winds disappear, the sea temperature tends to increase (2729 °C), the salinity of the water decreases (< 34) and the sediment load increases, which in turn increases the turbidity of the water due to the increase in continental contributions (Ramírez, 1983; Bula-Meyer, 1985; Díaz et al., 2000; Garzón-Ferreira and Díaz, 2003; Rodríguez-Ramírez and Garzón-Ferreira, 2003; Franco-Herrera, 2005). It should be noted that this pattern may vary, particularly due to the influence of climatic events such as the La Niña and El Niño phenomena (Franco-Herrera, 2005; Montealegre, 2014).
Regarding hydrodynamics, the Bay of Santa Marta is mainly influenced by two types of macrocurrents: The Caribbean current, formed by the action of the trade winds during the dry season, and the Panama-Colombia countercurrent (during the period of rains), coming from the southwest of the Caribbean basin. Both macrocurrents partially regulate local surface circulation patterns within bays located in the Magdalena region (Franco-Herrera, 2005). Likewise, the bay of Santa Marta receives continental contributions attributed not only to runoff but also to the mouth of the Manzanares River, which crosses the city and discharges its waters directly into the southern sector of the bay.
MATERIALS AND METHODS
The biological and physicochemical parameter information was collected during six red tide events that occurred in the bays of Santa Marta and Gaira between 2010 and 2017 (Invemar, 2010, 2015a, 2015b, 2017a, 2017b; Malagón and Perdomo, 2013). To define the characteristics of the water during these events, observations were made and the dispersion of the reddish patches was identified. Once the patches were located, three to four sampling points were established for each event, in which aspects such as water temperature, salinity, dissolved oxygen, and pH were measured, and samples of one liter of water were collected to perform the analysis of dissolved inorganic nutrients (nitrogen and phosphorus). These samples were deposited in previously washed plastic bottles and transported to the laboratory to perform the corresponding analyzes according to established methodologies (Strickland and Parsons, 1972; APHA et al., 2012).
To determine whether the change in the color of the water was caused by the flowering of microscopic organisms, at the same points in which the samples were collected for the physicochemical analysis, samples of 500 and 80 mL of surface water were taken (< 1 m). In each event, the 80 mL sample was stored unfixed and the 500 mL sample was fixed with neutral lugol in a 1/100 ratio (Reguera et al., 2016). The samples were then transported to Invemar's Marine Environmental Quality Laboratories Unit (Labcam) for analysis.
Samples without fixatives were immediately analyzed to observe cells in vivo. A quantitative analysis was carried out on the samples fixed with lugol. Aliquots of 1 to 3 mL of the sample were sedimented for three hours due to the high concentration of cells, following the Utermohl sedimentation method (Reguera et al., 2016); an inverted microscope was used to make the observations (Edler and Elbrächter, 2010).
The identification of organisms was carried out based on the Balech (1988) and Vidal (1995, 2010) guidelines. The precipitation data (monthly summation) and sea surface temperature were requested from the Institute of Hydrology, Meteorology and Environmental Studies (IDEAM, 2015; Simón Bolívar airport station) while the El Niño Oceanic Index (ONI) was consulted on the NOAA (2020) webpage.
The ONI index is an El Niño-Southern Oscillation (ENSO) measure used to detect warm and cold events such as El Niño and La Niña, respectively, in the tropical Pacific Ocean. This index is based on the average of three consecutive months of the superficial thermal anomalies of the sea, from measurements in the region of El Niño 3.4 (corresponding to 5 °N-5 °S, 120-70 °W). Neutral values correspond to those between ± 0.5 °C, values above 0.5 °C indicate El Niño events, and values below -0.5 °C indicate La Niña events for at least five consecutive periods (NOAA, 2020).
RESULTS
The dinoflagellate Cochlodinium sp. (= Margalefidinium sp.; Figure 2) was responsible for the three red tides events in October 2010, October 2011 (Malagón and Perdomo, 2013), and November 2015. The first two events were observed mainly in the bay of Santa Marta while the 2015 occurred in a neighboring bay (Bay of Gaira). These blooms were characterized by the presence of reddish spots in the water, which were kept away from the coastline and at a depth no greater than 2 m. The reddish patches showed a displacement from south to north, associated with the Panama-Colombia countercurrent, predominant between September and November. This period coincides with the annual rainy season, accompanied by light winds from the southeast.

Figure 2 Células de Cochlodinium sp. (= Margalefidinium sp.) observadas en las muestras recolectadas durante el evento de mareas rojas ocurrido en las bahías de Santa Marta y Gaira en noviembre de 2015. Barra de escala: 20 µm.
Cochlodinium sp. (= Margalefidinium sp.) was the most representative species among the microalgae observed during the samplings, with relative abundances (RA) higher than 95 %. The individuals were isolated or formed chains of four cells (Figure 2) and, less frequently, of eight cells. Maximum density (5 x 106 cells L-1) was observed in the bay of Santa Marta in October 2010. During the three events (October 2010, October 2011, and November 2015), the typical environmental conditions of the rainy period were characterized by increased rainfall (> 90 mm [total volume per month]) and the consequent decrease in the trade winds, as well as the increase in the contribution of inland waters from the Manzanares River and other contributions. The latter generated a decrease in salinity (< 35.5) and an increase in water temperature (> 29.7 °C) (Table 1).
Three more events were recorded during January 2015, January 2017, and March 2017 attributed to the ciliate Mesodinium cf. rubrum (Figure 3), which formed deep red spots. This study corresponds to the first record of red tides produced by said ciliate on the Colombian Caribbean coast. These organisms reached maximum densities of 5.5 x 104 and 9.2 x 106 cells L-1 and an RA of 90 %. The events were evident during the dry season, influenced by trade winds and total rainfall per month less than 0.1 mm. The higher cell density was associated with high phosphate concentrations (> 224 ng L-1), increased salinity (> 36.6), and water temperature lower than 26 °C (Table 1).
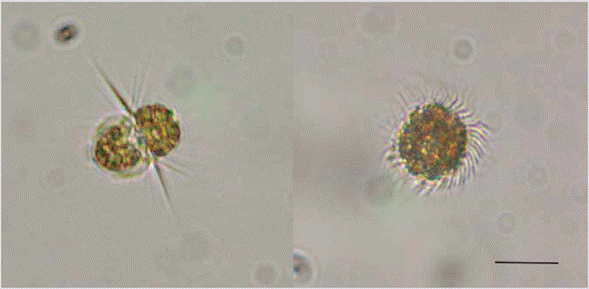
Figure 3 Cells of the ciliate Mesodinium cf. rubrum observed during the red tide occurred in the bay of Santa Marta in January 2017. Left: Side view of the cell. Right: Top view. Scale bar: 20 μM.
Table 1 Physicochemical variables recorded during red tide events (values related to maximum densities). Values with the < symbol refer to nutrient concentrations below the detection limit.
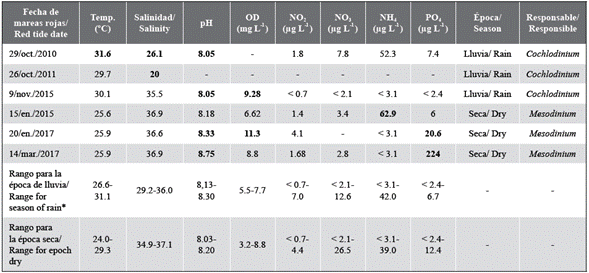
* Historical range of values recorded in the bay of Santa Marta from 2014 to 2017, in the rainy and dry season. Data from the monitoring of harmful microalgae in Colombia carried out by Invemar.
Note: The numbers in bold indicate the values observed outside the historical range recorded in the bay of Santa Marta ftom 2014 to 2017.
In most of the red tide events recorded in the bay of Santa Marta and other bays close to it, the physicochemical variables such as the concentration of dissolved oxygen in the water and some dissolved inorganic nutrients exceeded the range of concentrations typical of the area (Table 1), this being a key characteristic that allows formulating a hypothesis about the causes of the presence of an algal bloom. During the periods in which the red tides were recorded, climatic fluctuations were more evident on the Colombian Caribbean coast, particularly during 2010-2011 and 2014-2016. These multi-year periods were influenced by two climatic events: a La Niña event during 2010 that lasted until 2011 and in which rainfall increased dramatically (> 1000 mm) and an El Niño event, which began in 2014 with moderate intensity, strengthened in 2015, and lasted until mid-2016 (IDEAM, 2015; UNGRD, 2016).
It is worth mentioning that, despite the strong drought prevailing in 2015, during November of this year, the month in which one of the red tide occurred, there was an increase in rainfall in Santa Marta (IDEAM), which exceeded 139 mm. Analyzing the recorded red tide events and the ONI index as the main indicator to monitor El Niño and La Niña events, it becomes clear that the blooms of Cochlodinium sp. (= Margalefidinium sp.) occurred during years influenced by moderate-strong climatic events, with ONI values higher than ± 1.0 °C. On the other hand, the blooms caused by Mesodinium cf. rubrum occurred during neutral years, with values below ± 0.5 °C (Figure 4).
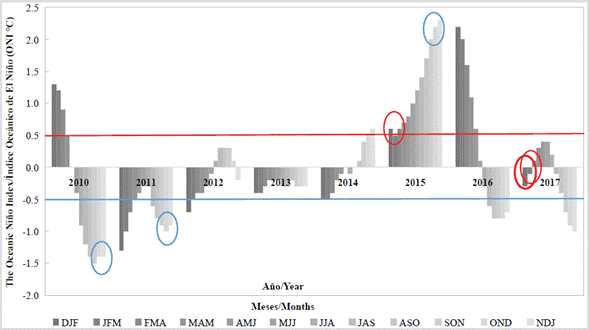
Figure 4 ONI index was calculated between 2010 and 2017. Climatic events above 0.5 °C are considered influenced by the El Niño phenomenon while events below -0.5 °C are considered influenced by the La Niña phenomenon. Values greater than ± 1.5 °C indicate strong climatic events. The blue circles correspond to the red tides of Cochlodinium sp. (= Margalefidinium sp.); the red circles, to those of Mesodinium cf. rubrum occurred in the bay of Santa Marta and neighboring bays.
DISCUSSION
A total of six red tide events were recorded between 2010 and 2017 in the Magdalena region: Three were produced by the dinoflagellate Cochlodinium sp. (= Margalefidinium sp.) and the other three by the ciliate Mesodinium cf. rubrum.Koray (1984) indicates that cell concentrations in red tides regularly vary from 106 cells L-1 to 108 cells L-1. Even densities higher than 5.0 x 104 cells L-1, similar values to those observed in the study area during the blooms of both species.
Red tides produced by Cochlodinium sp. (= Margalefidinium sp.) in October 2010, October 2011, and November 2015, during the rainy season in the Magdalena region, reflect a repetitive behavior in the proliferation of this dinoflagellate in the area, particularly during years in which moderate-strong climatic events (negative and positive) occurred. This could show the preference of these dinoflagellates for extreme conditions mainly related to high water temperatures (> 29.7 °C) and decreased salinity (< 35).
Morse et al. (2013) have observed repetitive blooms produced by the species Cochlodinium polykrikoides (= Margalefidinium polykrikoides) in the Chesapeake Bay, United States (Atlantic Ocean). The authors indicate that these red tides originate at the river mouths when the water temperature rises during periods of weak winds and low nutrient concentrations. Similar to what was observed in the Bay of Santa Marta, the blooms of C. polykrikoides in the Chesapeake Bay and La Paz Inlet, Gulf of California, coincided with periods of rain after a prolonged period of summer drought. In these events, the contribution of continental waters not only fertilized the waters but also generated vertical gradients of density and temperature that favored the stability of the water column and created suitable conditions for the survival and growth of C. polykrikoides (Alonso, 2004; Mulholland et al., 2009; López-Cortés et al., 2014). Likewise, other authors, including Gárate-Lizárraga et al. (2000, 2004) and Gárate-Lizárraga (2013) in the Gulf of California, Anton et al. (2008) off the coast of Malaysia, and Azanza et al. (2008) in the Philippines, also related the blooms of this dinoflagellate with a high concentration of nutrients caused by the influence of the rain and winds. For this reason, it is considered that the concentration of nutrients dissolved in the water, as well as the hydrodynamics of the area, has a significant influence on its high densities.
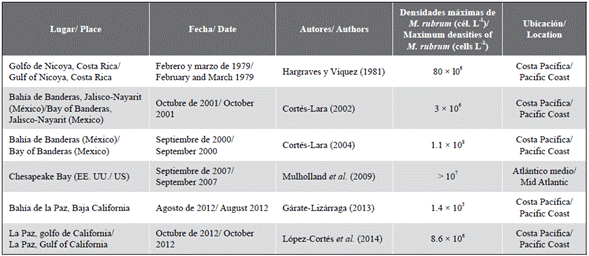
Most of the blooms or red tides produced by C. polykrikoides (= Margalefidinium polykrikoide) in North America have been recorded on the Pacific coast, including the bays of Mexico and Costa Rica and the Gulf of California (Table 2; Freer and Vargas-Montero, 2003; Gárate-Lizárraga and Muñetón-Gómez, 2008; Dorantes-Aranda et al., 2009; Calvo-Vargas and Arguedas-Rodríguez, 2012; López-Cortés et al., 2014). Some of these blooms have had drastic consequences for various species of fish. In Colombia, considering the information collected by Invemar through the monitoring system of harmful microalgae in the Magdalena region, the genus Cochlodinium (= Margalefidinium) is not usually common in the area: it is only observed when blooms occur.
Table 2 Cochlodinium (= Margalefidinium) densities recorded during different red tide events on the Pacific coast and the mid-Atlantic.
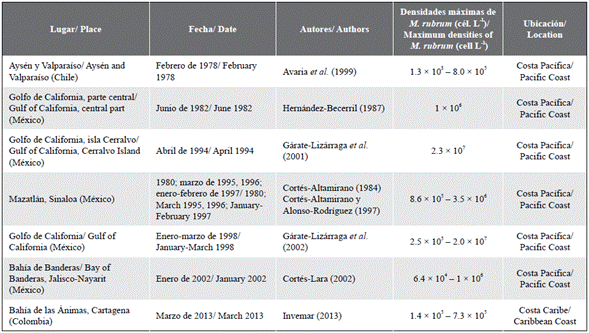
On the other hand, the red tides produced by Mesodinium cf. rubrum were repetitive during the first months of the year (dry season) when the presence of the trade winds generated the basic conditions for the occurrence of upwelling events in the coastal waters of the Colombian Caribbean region, a process that enriches the surface waters with nutrients like phosphorus and nitrogen, which are reincorporated into the water column from the bottom (Lindholm, 1985; Franco-Herrera, 2005; Jiménez and Gualancaña, 2006). The maximum densities reached by this ciliate during red tides in the Colombian Caribbean exceeded those recorded by other authors during various events that occurred in coastal cities of Chile and Mexico (Table 3). However, some authors recorded maximum densities higher than 2 x 107 (Gárate-Lizárraga et al., 2001, 2002) during the red tides in the Gulf of California (Table 3). This indicates that the densities ofM. cf. rubrum during the blooms recorded in Santa Marta are within the range of maximums recorded in North America. Also, in January 2013 a red tide of M. rubrum was observed in Bahía de las Ánimas, located in Cartagena de Indias (Colombian Caribbean), with maximum densities of 7.3 x 105 cells L-1 (Table 3).
Table 3 Densities of Mesodinium cf. rubrum recorded during different red tide events on the Caribbean and Pacific coast (modified from Gárate-Lizárraga et al., 2002).
Lindholm (1985) and Crawford (1989) mention that M. rubrum is a cosmopolitan species that produces occasional blooms in upwelling areas, estuaries, fjords, and temperate coastal waters. The proliferation of M. rubrum has also been recorded on different coasts of the world, including the United States (Johnson et al., 2013), England (Kifle and Purdie, 1993; Williams, 1996), and Brazil (De Oliveira, 2004), among others.
Red tides are recurrent in the bay of Santa Marta and nearby sectors and, so far, have not represented an apparent risk to public health or other organisms. However, some species of the genus Cochlodinium (= Margalefidinium) have caused a high number of deaths of marine animals in other coastal countries due to their hemolytic activity and the production of oxidizing compounds (ROS = reactive oxygen species). Although its toxicity is not clear and, to date, there is controversy around its mechanism of action (Kim et al., 2002; Gobler et al., 2008), some studies have confirmed its adverse effects that include hemolysis, osmoregulatory alteration, and asphyxia of organisms due to mucus production (Lee, 1996; Kim et al., 1999, 2000, 2002). For this reason, the species Cochlodinium polyclicoides (= Margalefidinium polykrikoides) is included in the Unesco IOC taxonomic reference list as a toxin-producing species.
On the other hand, the blooms of Mesodinium cf. rubrum are generally harmless (Johnson and Stoecker, 2005). In extreme cases, they have been associated with the death of fish and some marine invertebrates due to hypoxic and anoxic conditions caused by the decrease in dissolved oxygen concentrations in the water (Hayes et al. , 1989; Cortés-Lara, 2002) and the plugging of their gills (Horstman, 1981). The blooms of this ciliate could also represent an indirect risk considering that these organisms are the essential food of the mixotrophic dinoflagellates of the genus Dinophysis, which produces diarrheic shellfish poisoning(DSTs). Thus, the blooms of M. cf. rubrum could trigger the growth in Dinophysis densities and increase the risk of diarrheic poisoning through the consumption of shellfish exposed to these populations.
Furthermore, the appearance of red tides in places such as bays can generate environmental problems, since, being a concavity in the coastline, the flow of water in a bay tends to be less than in an open system and, therefore, water retention is longer and situations may occur that endanger the system, such as the death of marine organisms by anoxia (Alonso et al., 2016). Likewise, red tides can generate socio-economic problems, particularly in sectors with a high tourist influx, such as the bays in the Magdalena region. Changes in the color of the water and the strong shellfish smell that occurs may not be pleasant for bathers when the event occurs.
CONCLUSIONS
High biomass blooms were detected (> 106 cells L-1, red tides) of Mesodinium cf. rubrum in the dry season (first months of the year) and of Cochlodinium sp. (= Margalefidinium sp) in the rainy season (October and November). These red tides showed a cyclical pattern of occurrence in the bay of Santa Marta and nearby bays, influenced by the environmental conditions and the climatic variability.
Through the ONI index, it was possible to show that the red tides produced by Mesodinium cf. rubrum occurred in years with the typical climatic conditions of the area, while the tides of Cochlodinium sp. occurred in years with moderate-strong climatic events (La Niña and El Niño), which indicates the preference of these dinoflagellates for extreme environmental conditions.
Although the red tides detected in the bay of Santa Marta and other nearby bays have not been classified as harmful to human and other organisms health, these events must continue to be monitored and studied to avoid future inconveniences. This information, together with the permanent monitoring of potentially harmful microalgae in the Magdalena region, could be considered as a relevant input for the construction of an early warning system for harmful algae blooms (HABs) in the Colombian Caribbean region, which would help the country's environmental authorities to take action when these events occur.











 text in
text in 


