INTRODUCTION
On the north coast of the Chocó department (Colombian Pacific), there are rock formations known as riscales and morros, which are submerged rocky reefs and small islets or cays, respectively (Guzmán et al., 2004). These formations are composed of mafic and ultramafic basaltic igneous rocks having different levels of topographic complexity, shaped by centuries of exposure to marine erosion factors that have created rock faces and large rock clusters with cracks and caves of varying size. The cliffs and hills provide hard substratum, for the settlement of benthic biotas such as soft corals, algae, and sponges, as well as shelter for a wide variety of mobile invertebrates and fish (Velandia and Díaz, 2016).
Among the characteristic biota of these rock formations, sponges (Porifera) are among the most conspicuous and important sessile benthic organisms. They are filters feeders that incorporate particulate and dissolved organic matter from the water column to the bottom, which then, through a process of cellular regeneration, return in the form of inorganic nutrients and particulate detritus to the environment (Goeij et al., 2008, 2013). In the Caribbean and the seas of the Western Indian Ocean and Indonesia, sponges are the most conspicuous and diverse organisms in coastal waters, after corals and algae, as they can be found in different habitats such as mangrove roots, coral reefs, and soft bottoms, among others (Zea, 1987, 1998; Van Soest, 1994; Hooper et al., 2002). In comparison with other taxonomic groups of invertebrates, the systematic study of sponges is underdeveloped due to the scarcity of specialized taxonomists, the difficulty of identifying the species, the ambiguity of the characters, and the dispersion of the literature (Hooper et al., 2002). However, the popularization of autonomous diving, the development of underwater photography, and the implementation of histological and molecular techniques have helped in alleviating the problem (Worheide et al., 2005; Cárdenas et al., 2012).
Although this has made it possible to increase the group's biodiversity inventory in recent decades, it is believed that the number of valid species described corresponds, at most, to half of the existing species (Van Soest et al., 2012). It is therefore important to continue making efforts to consolidate the inventory of global Poriferan biodiversity, especially in less explored regions such as the Eastern Pacific and, in particular, on the Pacific coast of Colombia.
The first descriptions of sponges on the American Pacific coast were made from the material obtained from research vessels such as the Challenger expedition, where Ridley and Dendy (1887) described 23 species of monoaxonic sponges for southern Chile and Patagonia. Subsequently, Vosmaer and Vernhout (1902)) studied six species of the genus Placospongia collected in Central and South America by the Siboga expedition, and von Lendenfeld (1910) described 46 species of the Geodidae family collected by the ship Albatross. In the 20th century, more exhaustive local studies were developed. De Laubenfels (1932) described 101 freshwater and marine species from California and De Laubenfels (1935) added eight new shallow-water species from Baja California, Mexico. Green and Gómez (1986) recorded 14 species of sponges in the Bay of Mazatlán (Sinaloa, Mexico), this being the first work for the rocky coast of the tropical Pacific. Desqueyroux-Faúndez and Van Soest (1997) described 45 species of sponges from shallow waters in the Galapagos Islands, Van Soest and Hajdu (2000) described six species of sponges from the Pacific coast of Panama, and Díaz et al. (2005) described a new species of shallow-water sponge in the Gulf of Chiriquí in Panama.
Recently, work has focused on the Mexican Pacific coast. For example, Carballo et al. (2004) carried out a complete study of coral burrowing sponges, and Carballo and Cruz-Barraza (2008) recorded a new species for Isla Isabel National Park in the Sea of Cortez. Later, Vega (2012) recorded 88 species of sponges in the Mexican Pacific, of which 18 were new records and possibly 33 are species new to science. The most recent work is that of Carballo et al. (2019), which includes 87 species of sponges associated with coral reefs throughout the Mexican Pacific.
On the other hand, some studies have addressed the question of the similarity in the sponge species on both sides of the Isthmus of Panama, a topic of great interest in the evolutionary context of the marine fauna of the Caribbean and the Eastern Tropical Pacific (ETP). For example, De Laubenfels (1936b) made a comparison between shallow-water sponges on the Pacific side of the Panama Canal with those on the Caribbean side and found differentiable morphological characteristics, a work subsequently corroborated by Wulff (1996).
For the Colombian Pacific, only three unpublished works are known that, in sum, have recorded 33 species of sponges (Narváez, 1999; Escobar, 2000; García-Suárez et al., 2012). These studies have focused on the southern portion of the Colombian Pacific and, mainly, on sponges associated with coral ecosystems. Considering these antecedents, this study seeks to contribute to the knowledge of the biodiversity of sponges of the Colombian Pacific and the ETP in general, with emphasis on the sponges associated with the rocky reefs of the northern portion of the Chocó.
STUDY AREA
The study was carried out in Colombian North Pacific, between Cape Corrientes (5°29' N, 77°32' W) and Cape Marzo (6° 49' N, 77°41' W). In this area, the seabed is characterized by a continental shelf of variable extension, narrow and steep in front of the headlands and wide and lying in the gulfs. This underwater topography is the result of the interaction between an abundant land runoff and marine erosive forces that have acted for centuries on ancient rocky coasts of igneous origin, associated with the formation of the Baudo mountain range (Posada et al., 2009).
In this rather broken seabed there are dozens of rocky reefs, known locally as "morros" and "riscales", which arise from variable depths and at different distances from the coast. In these rock formations, composed of mafic and ultramafic igneous basalt rocks, different levels of topographic complexity are observed; but, for the most part, they consist of rocky walls and groups of large rocks with caves and holes of varying size. These submerged structures offer an ideal hard substratum, for the settlement of benthic fauna and flora (e.g. soft corals, sponges, erect and encrusting algae, tunicates and bryozoans, among others), as well as shelter for hundreds of fragile invertebrates and fish, which makes these important artisanal fishing grounds (Velandia and Díaz, 2016). The sampling effort was concentrated on the shallow rocky reefs in the sectors of Cape Corrientes, Gulf of Tribugá, Solano Bay, Cupica Bay, Punta Cruces and Cape Marzo (Figure 1).

Figure 1 Study area in the northern sector of the department of Chocó (Colombian Pacific). The sampling locations are numbered: 1) Piedra de Rodrigo (6° 47' 2.07" N. 77° 41' 36.6" W). 2) Piedra de Eroito (6° 48' 52.84" N. 77° 41' 32.99" W). 3) La Foca (6° 40' 42.7044" N. 77° 41' 42.71" W). 4) La Viuda (6° 37' 58.872" N. 77° 29' 58.92" W). 5) La Mina (6° 41' 11.76" N. 77° 32' 57.48" W). 6) La Parguera-Piñas (6° 41' 30.084" N. 77° 32' 29.76" W). 7) Morros de Jurubidá (5° 48' 47.16" N. 77° 17' 49.56" W). 8) Morromico Norte (5° 48' 47.16" N. 77° 17' 49.56" W). 9) Punta Arusí (5° 36' 39.996" N. 77° 29' 6.36" W). 10) Punta Orión (5° 37' 50.664" N. 77° 23' 18.59" W). 11) Piedra de Jairo (5° 36' 36.04" N. 77° 30' 2.52" W). 12) Roñosa 5° 35' 6.22" N. 77° 30' 50.76" W). 13) Parguera-Corrientes (5° 36' 35.60" N. 77° 30' 14.76" W). 14) Piedra de Colo (5° 30' 43.87" N. 77° 32' 1.32" W).
MATERIALS AND METHODS
During an expedition carried out in 2015, 14 sampling stations between 11-19 m depth were evaluated, where all the sponges present were photographed and complete specimens or fragments were collected that were fixed in 96 % ethanol. Permanent mounts of clean spicules and skeletons were made in the laboratory, as described in Zea (1987), which were observed and photographed using an optical microscope (Zeiss, Axio Lab. A1). Up to 25 spicules of each class and the most relevant skeletal structures were measured. Spicule measurements are presented as minimum-average-maximum length x width in nm. The identifications were carried out by consulting the keys of the Systema Porifera (Hooper et al., 2002) and the descriptions of materials from the Pacific, especially the Eastern Pacific, with the help of the species lists from the World Porifera Database (http://www.marinespecies.org/porifera; Van Soest et al., 2018). The material studied was deposited in the sponge collection (acronym INV POR) of the Museum of Marine Natural History of Colombia (MHNMC) of Invemar while the information was entered in the databases of the Information System on Marine Biodiversity of Colombia (SIBM).
RESULTS
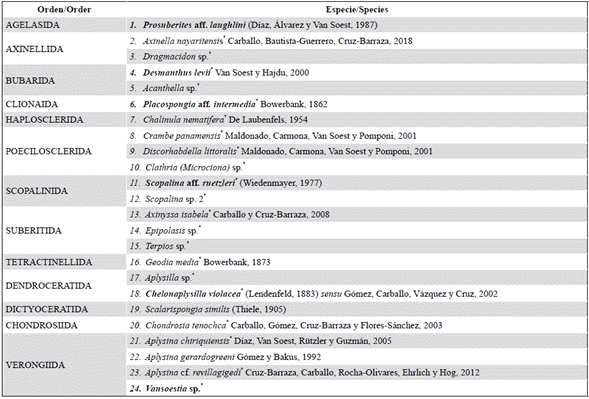
24 species of 13 orders, 17 families, and 21 genera were identified, of which 21 are new records for the Colombian Pacific (*). Twelve (12) species/morphotypes were identified at the genus level or with a tentative assignment at the species level. In Table 1 the list of species is presented and the description of each one is given below.
Table 1 List of sponge species in the Colombian North Pacific, grouped by order. Species that have a morphologically similar counterpart in the Caribbean are indicated in bold.
Class Demospongiae Sollas, 1885
Subclass Heteroscleromorpha Cárdenas, Pérez and Boury-Esnault, 2012
Order Agelasida Hartman, 1980
Family Hymerhabdiidae Morrow, Picton, Erpenbeck, Boury-Esnault, Maggs and Allcock, 2012
Species 1. Prosuberites aff. laughlini (Díaz, Álvarez and Van Soest, 1987)
Figure 2; Plate 1, figs. 1, 4.
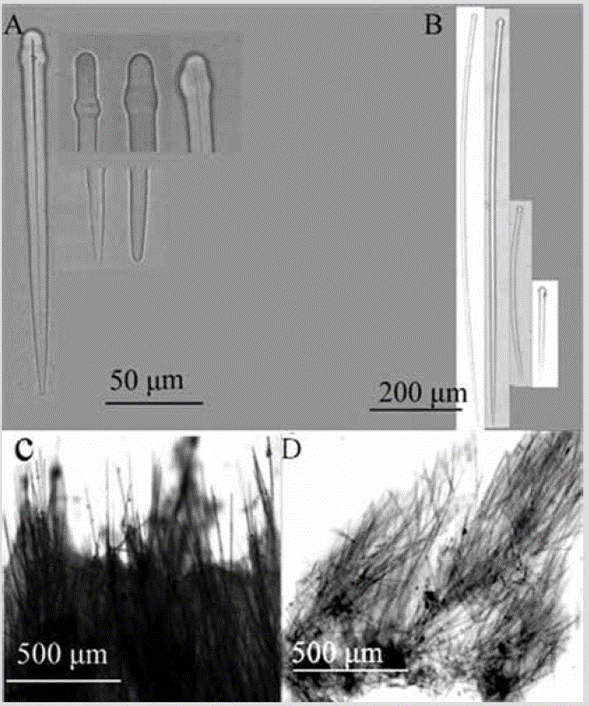
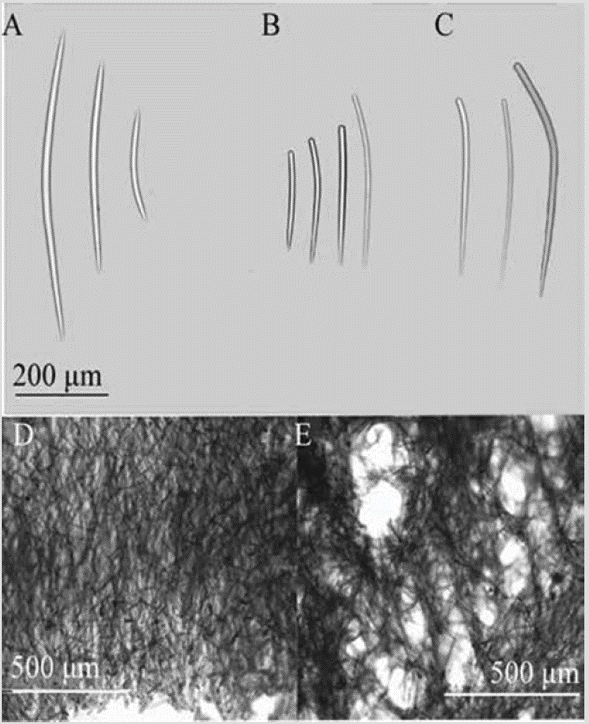
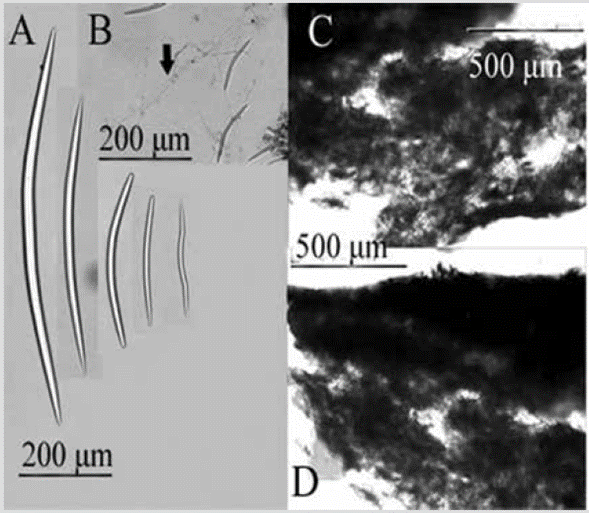
Figure 2 Prosuberites aff. laughlini. A) Tylostyles with different endings in head and tip. B) Tylostyles. C) and D) Perpendicular section showing ectosome and choanosome.
?Eurypon laughliniDíaz, Álvarez and Van Soest, 1987: 33, Plate I-A, fig. 2.
Eurypon aff. laughlini;Escobar, 2000: 62, figs. 22-23.
Prosuberites laughlini;Nichols, 2005: 91, Appendix A (Specimens: UCMPWC952 and UCMPWC875).
Prosuberites aff. laughlini;García-Suárez et al. 2012: 21.
Studied material: INV POR1361, Punta Cruces, La Parguera-Piñas, 13 m, col. L. Chasqui, 08-16-2015. INV POR1375, Gulf of Tribugá, Morromico, 11.9 m, col. L. Chasqui, 08-20-2015. INV POR1389, Punta Cruces, La Viuda, 13-15 m, col. L. Chasqui, 08-14-2015. INV POR1395, Punta Cruces, La Mina, 15 m, col. L. Chasqui, 08-16-2015. INV POR1407, Cape Marzo, Piedra de Rodrigo, 18.5 m, col. L. Chasqui, 08-15-2015.
Shape: thick encrustation, widely spread on the rocky substratum; corrugated surface, with erect spicules traversing the skin; scattered oscules with a transparent raised collar. Color: bright orange; light brown, sometimes pale yellow in alcohol. Consistency: soft, fragile; hispid to the touch. Ectosome: thin pinacoderm, pierced by large spicules. Choanosome: erect tracts or individual spicules emerging from the base of fixation, 38-115 µm thick, separated 46-230 µm (Figure 2C, 2D). Spicules: tylostyles, 184-568-1221 µm by 5.0-11.0-18.3 µm (Figure 2A, 2B).
Habitat: exposed vertical rock substrata and in cave walls.
Distribution in the Pacific: Pacific of Panama (Nichols, 2005); Malpelo Island (García-Suárez et al., 2012); Palma Island in Malaga Bay (Escobar, 2000); Colombian North Pacific.
Comments: when reviewing the descriptions by Díaz et al. (1987) for specimens from the Caribbean and Escobar (2000) for specimens from the Pacific, it can be hypothesized that these are populations of the same species that were separated by the uplift of the Isthmus of Panama about three million years ago. Alternatively, it could be a recent dispersal through the Panama Canal. Although there are molecular studies with the species (Nichols, 2005), these do not allow comparisons of the populations on both sides of the isthmus (see also Holmes and Blanch, 2007; Schuster et al., 2015). More detailed studies should be carried out to determine if there are morphological differences between the Pacific and Caribbean populations and if different, give the Pacific species a new name. García-Suárez et al. (2012) record Prosuberites aff. laughlini for Malpelo Island based on an underwater photograph, for which that locality is included here in the distribution of the species; however, that record must be confirmed with biological material.
Order Axinellida Lévi, 1953
Family Axinellidae Carter, 1875
Species 2. Axinella nayaritensis Carballo, Bautista-Guerrero and Cruz-Barraza, 2018
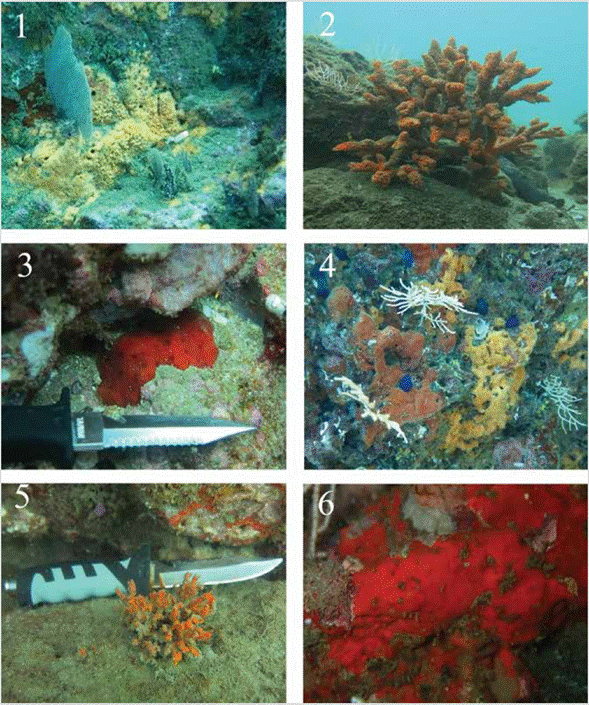
PLATE 1 fig. 1.Prosuberites aff. laughlini INV POR1361 with octocoral, La Parguera-Piñas, 13 m; fig. 2.Axinella nayaritensis INV POR1372, Gulf of Tribugá, 7.8 m; fig. 3.Dragmacidon sp. INV POR1362, La Parguera-Piñas, 6 m; fig. 4.Desmanthus levii (reddish-orange, left), INV POR1376, Gulf of Tribugá, 12 m, with Prosuberites aff. laughlini (pale orange, right); fig. 5.Acanthella sp., INV POR1373, Gulf of Tribugá, 7.1 m; fig. 6.Crambe panamensis., INV POR1365, La Parguera, 6 m.
Axinella nayaritensisCarballo, Bautista-Guerrero and Cruz-Barraza, 2018: 114, figs. 2-3.
Material studied: INV POR1372, Gulf of Tribugá, Punta Orión, 7.8 m, col. L. Chasqui, 08-20-2015. INV POR1404, Cape Corrientes, La Roñosa, 14.4 m, col. L. Chasqui, 0821-2015.
Shape: erect, arborescent, 15-30 cm tall; branches like fingers, tapering apically; very uneven and corrugated surface. Color: bright orange; pale yellow in alcohol. Consistency: firm, not very compressible. Ectosome: pinacoderm supported by tufts of spicules from the end of the ascending tracts. Choanosome: diffuse tracts of spicules, 32-139 µm thick, separated 46-223 µm, irregularly interconnected by interspersed spicules. The tracts rise from the central part of the branch and diverge towards the surface, arriving perpendicular to it (Figure 3D, 3E). Spicules: oxeas, 241-362-632 µm by 6.9-11.5-17.5 µm (Figure 3A); styles I: thin and straight, the tip gradually tapers, 223-307-388 µm by 7.8-12.4-17.8 µm (Figure 3B); styles II: thick and slightly bent like a rhabdostyle, 407-518642 µm by 4.6-9.0-12.3 µm (Figure 3C).
Habitat: in non-exposed areas of the rocky reef.
Distribution: Mexican tropical Pacific (Carballo et al. , 2018), Colombian North Pacific.
Comments: the spicules and the structure of the skeleton are characteristic of bushy or branchy species of the genus AxinellaSchmidt, 1862. Carballo et al. (2018) described it as a new species for the Mexican tropical Pacific. In vivo photographs and laboratory-processed material of the specimens collected in this study were sent to Professor J. L. Carballo, who confirmed the similarity to A. nayaritensis.
Species 3. Dragmacidon sp.
Material studied: INV POR1362, Punta Cruces, La Parguera-Piñas, 6 m, col. L. Chasqui, 08-16-2015.
Shape: thick encrustation (several mm), 10 x 5 cm; smooth surface, scattered oscules. Color: scarlet red; pale yellow in alcohol. Consistency: firm, not very compressible. Ectosome: organic pinacoderm supported by tufts of spicules from the ascending tracts. Choanosome: a prismatic network of ascending and divergent plµm,ose spicular tracts, 181-549 µm thick, separated 74-155 µm, interconnected by transverse tracts, 79-133 µm thick (Figure 4C, 4D); mesh size 119-255 µm (Figure 4B). Spicules: curved and thick styles, folded in a rhabdostyle fashion, with the head slightly pronounced, 152-302-374 µm by 8.7-18.8-26.1 µm (Figure 4A).
Habitat: shallow rocky reef.
Distribution: Colombian North Pacific.
Comments: the specimen was placed in this genus due to its growth form, the color, and the organization of the choanosome (Álvarez and Hooper, 2002), despite not presenting oxeas. Austin et al. (2013) described a new species from British Columbia and adjacent waters in Canada and named it Dragmacidon kishinensis, but its characteristics do not match material from Colombia. For this reason, a more detailed study (including molecular markers) must be done to confirm it as a new species.
Order Bubarida Morrow and Cárdenas, 2015 Family Desmanthidae Topsent, 1893
Species 4. Desmanthus levii Van Soest and Hajdu, 2000
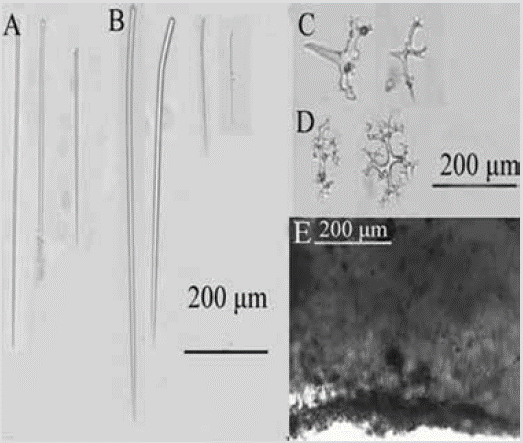
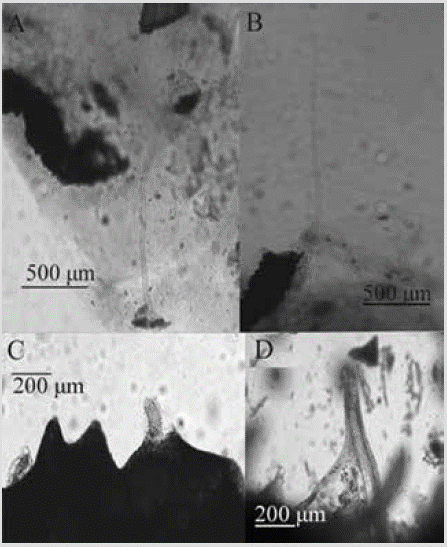
Figure 5 Desmanthus levii. A) and B) Styles. C) Desmas II (tetracrepids). D) Desmas I. E) Choanosome.
Desmanthus leviiVan Soest and Hajdu, 2000: 22, figs. 1F-I.
Studied material: INV POR1376, Gulf of Tribugá, Morromico, 12 m, col. L. Chasqui, 08-20-2015.
Shape: thin encrustation broadly covering the substratum; smooth, finely grained surface.
Color: reddish-orange, dull purple in alcohol. Consistency: undefined from being thin. Ectosome: pinacoderm pierced by long, erect styles, arranged between desmas II. Choanosome: a basal layer of desmas I, followed by one or more layers of desmas II, with the cladome predominantly facing upward and the rhabdom downward; scattered erect styles (Figure 5E). Spicules: smooth styles, 177-434-846 µm by 4.5-8.5-16.5 µm, straight or curved in a rhabdostyle fashion (Figure 5A, 5B); Desmas I: multiple thin branches, 56-100-141 µm by 5.2-10.8-18.8 µm (Figure 5D); Desmas II (tetracrepids): conical rhabdomes, robust, carrot-shaped, 43-72-112 µm by 10.5-20.6-36.7 µm, cladome 60-93-166 µm by 21-15-10 µm (Figure 5C).
Habitat: rocky reef walls in shallow water.
Distribution: Pacific coast of Panama (Van Soest and Hajdu, 2000); Colombian North Pacific.
Comments: the identification was made based on the previous work of Van Soest and Hajdu (2000) of the Pacific coast of Panama. It is possible that Desmanthus incrustans (Topsent, 1889) from the Caribbean is a sister species to D. levii due to its similarity in morphology and spicular structure; however, comparative work with molecular markers is required to establish this affinity.
Family Dictyonellidae Van Soest, Díaz and Pomponi, 1990
Species 5. Acanthella sp.
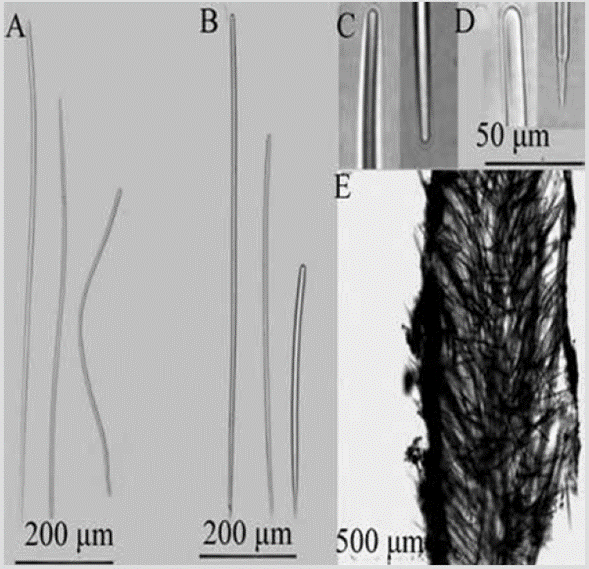
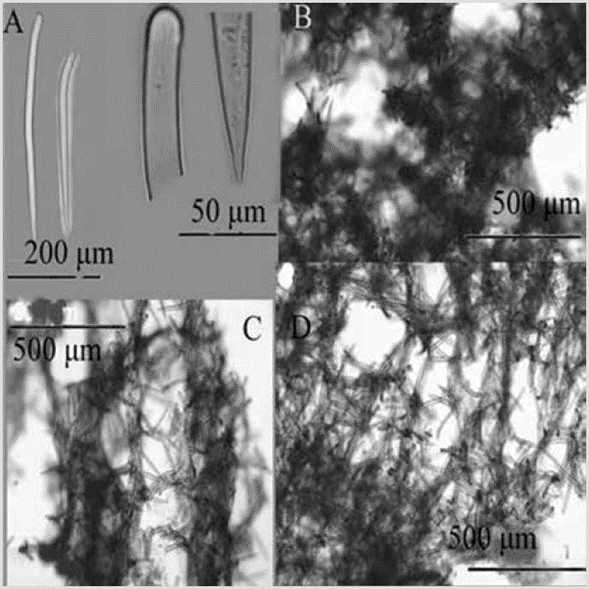
Figure 6 Acanthella sp. A) Sinuous strongyles. B) Styles. C) Winding strongyle ends. D) Extremes of styles. E) Cross-section of the skeleton.
Material studied: INV POR1381, Gulf of Tribugá, Morros de Jurubidá, 12 m, col. L. Chasqui, 08-20-2015. INV POR1383, Morros de Jurubidá, 13.3 m, col. L. Chasqui, 0820-2015. INV POR1386, Morros de Jurubidá, 10 m, col. L. Chasqui, 08-20-2015. INV POR1373, Gulf of Tribugá, Punta Orión, 7.1 m, col. L. Chasqui, 08-20-2015.
Shape: encrusting mass with lobes to low bushes up to about 5 cm in height; branches 1-5 mm in diameter, little divided, coming out almost from the base; velvety or corrugated surface, microhispid, often with trapped sediment, especially towards the base. Color: bright orange; pale yellow in alcohol. Consistency: soft, compressible. Ectosome: pinacoderm supported by erect tracts and tangential spicules. Choanosome: plumose spicule tracts, 42-138 um thick (Figure 6E), dendritic, diverging from the center of the branches towards the surface, reaching it diagonally, interconnected very irregularly by simple spicules in all directions. Spicules: smooth and straight styles, the smallest slightly curved, 348-590-813 µm by 4.49.0-15.1 µm (Figure 6B, 6C, 6D); sinuous strongyles, 36651-910 µm by 3.6-6.0-9.6 µm (Figure 6A).
Habitat: shallow rocky reefs in murky waters.
Distribution: Colombian North Pacific.
Comments: the generic location of this sponge is tentative, based on the presence of dendritic tracts and spicules with curved styles and sinuous strongyles (Van Soest et al. , 2002), although there are other genera with the same type of spiculation (e.g. AulettaSchmidt, 1870, PhakelliaBowerbank, 1862, PhycopsisCarter, 1883), but of the order Axinellida (Van Soest et al., 2018). Acanthella daneriiCosta, Barvastrello, Pansini, and Bertolino, 2020 was described from the Chilean fjords in the southeastern Pacific. It differs from Acanthella sp. in having sinuous, curved, or bent oxeas, in addition to sinuous styles and strongyles (Costa et al., 2020). The structure of the skeleton in plumose tracts and the spicules it presents are very similar characteristics to Acanthella cubensis (Alcolado, 1984) from the Caribbean, but it differs from it in the shape of growth. It should be subjected to a more exhaustive review to consider it as a new species since there are several species of the genus described for the Western Pacific and the Indian Ocean (Van Soest et al., 2018; Costa et al., 2020).
Order Clionaida Morrow and Cárdenas, 2015
Family Placospongiidae Gray, 1867
Species 6. Placospongia aff. intermedia Sollas, 1888
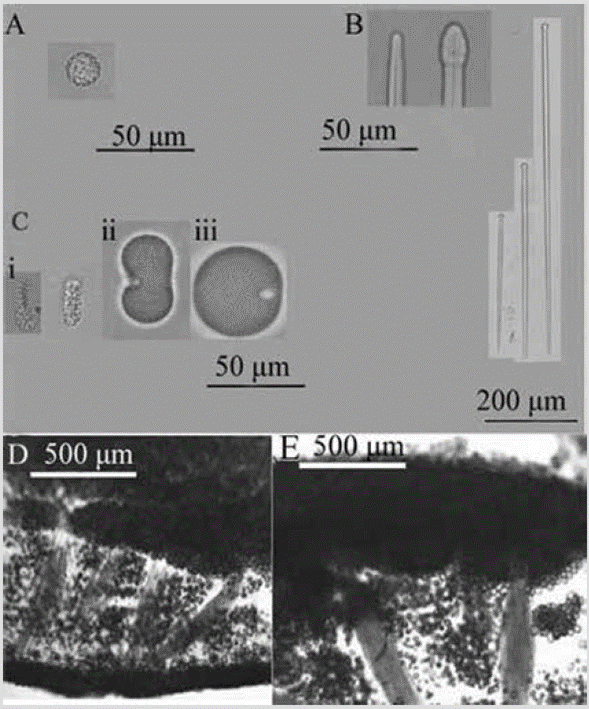
Figure 7 Placospongia aff. intermediate. A) Spheraster. B) Ends of tylostyles and tylostyles. C) Selenasters (i, ii, iii stages). D) and E) Choanosome.
?Placospongia intermediaSollas, 1888: 273 (valid species from the Caribbean).
Placospongia intermedia;De Laubenfels, 1936b: 454.
Material studied: INV POR1364, Punta Cruces, La Parguera-Piñas, 6 m, col. L. Chasqui, 08-16-2015.
Shape: encrusting, surface divided into polygonal plates by irregular elevations or grooves. Color: rose preserved in alcohol. Consistency: tough, tenacious. Ectosome: plates formed by densely packed selenasters, up to 2 mm thick. Choanosome: basal layer of selenasters, 202-341 µm thick, from which multispicular tylostyle tracts arise, 137-290 µm thick, separated 163-711 µm, like columns, supporting the ectosomal plates. Numerous selenasters are scattered among the columns (Figure 7D, 7E). Spicules: megascleres: tylostyles, 180-657-874 µm by 5.2-10.6-17.6 µm (Figure 7B). Microscleres: spherasters, 9.2-10.9-13.6 µm in diameter (developmental stages 16.1-18.3-19.5 µm) (Figure 7A); selenasters in various stages of development, small, 20.3-26.5-32.8 µm by 7.3-10-13.1 µm (Figure 7C-i); medium, 43-36-55 µm by 16.9-25.0-33.4 µm (Figure 7C-ii); and developed, 55-66-76 µm by 42-52-62 µm (Figure 7C-iii).
Habitat: on shallow rocky substrata.
Distribution: Pacific of Panama (De Laubenfels, 1936b); Colombian North Pacific.
Comments: Sollas (1888) described Placospongia intermedia from Punta Arenas, which was later confused with a locality of that name in the Pacific of Costa Rica. However, in the section "Species of the faunistic provinces", p. 383, Sollas (1888) located P. intermedia in the Caribbean province. In the World Porifera Database (http://www.marinespecies.org/porifera/porifera.php?p= distribution&id=651010) the type locality of P. intermedia is located in Punta Arenas, Margarita Island. De Laubenfels (1936b) described the material of P. intermedia from both the Pacific and Caribbean sides of the isthmus of Panama, considering them the same species. Van Soest (2009) argued that the De Laubenfels material from the Caribbean was different from that from the Pacific, but Rützler et al. (2014: 35) reviewed the original slides without finding differences. The Pacific material may be a different species that requires a new name, although Nichols and Barnes (2005) did not find, from molecular analysis of the ITS region that encodes ribosomal RNA, important differences between various specimens of the Caribbean and Pacific of Panama (Clade C8, unidentified). This could indicate that the two populations have not diverged enough since they were separated by the isthmus, but it will be necessary to examine this material morphologically to reach a more precise conclusion. In the Colombian Pacific, Escobar (2000) described P. carinata (Bowerbank, 1858), which is undoubtedly another species, since it lacks spheresters and its color is dark brown. The record of an underwater photograph of Placospongia carinata from García-Suárez et al. (2012) from Bahía Málaga cannot be confirmed as there is no material to examine.
Order Haplosclerida Topsent, 1928
Family Chalinidae Gray, 1867
Species 7. Chalinula nematifera (De Laubenfels, 1954)
Nara nematiferaDe Laubenfels, 1954: 76, fig. 46.
Chalinula nematifera;De Weerdt, 2002: 853; Cruz-Barraza and Carballo, 2008: 748, figs. 5.7. C; Vega, 2012: 105, figs. 7.2.57. A, B, C (genus transfer).
Material studied: INV POR1380, Gulf of Tribugá, Morromico, 12 m, col. L. Chasqui, 08-20-2015.
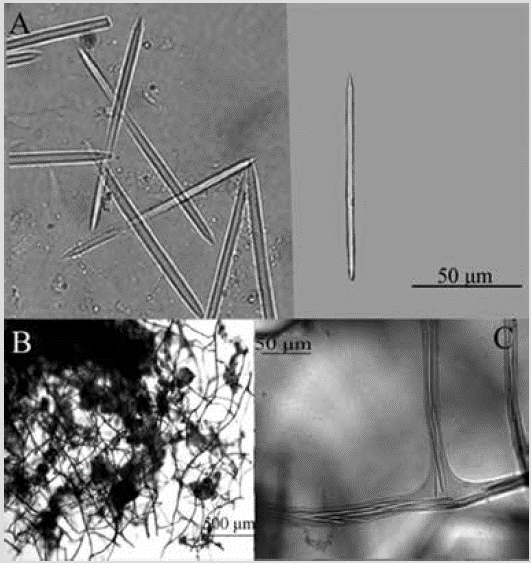
Shape: encrusting thin cushion, approx. 1 cm in diameter. Finely hispid surface. Color: light purple in alcohol. Consistency: soft and compressible. Ectosome: lacking an ectosomal tangential network; formed by unispicular ascending fiber ends. Choanosome: square to a polygonal network of spongin fibers cored with one or two spicules, 6-22 µm thick, with meshes 65-326 µm in diameter (Figure 8B, 8C). Spicules: oxeas, 63-80-94 µm by 2.6-3.7-5.1 µm (Figure 8A).
Habitat: shallow rocky reefs.
Distribution: Tropical central and western Pacific (De Laubenfels, 1954); Sea of Cortez (Mexico) (Cruz-Barraza and Carballo, 2008); Colombian North Pacific.
Comments: it was first described by De Laubenfels (1954: 76) as Nara nematifera with material from the central-western Pacific. Later, De Weerdt (2002) transferred it to the genus Chalinula, which is defined as having a finely encrusting growth form, in pillow-shape, and presenting short oxeas, ranging from vestiges to cigars. The identification of this species was carried out by comparing the characteristics described by Cruz-Barraza and Carballo (2008) of the Mexican Pacific. According to Ávila and Carballo (2009), C. nematifera is an invasive species native to the central and western Pacific that has been recorded since 2003 in the Mexican Pacific, where it is considered a potential threat to coral reefs because it grows on live Pocillopora spp. colonies.
Order Poecilosclerida Topsent, 1928
Family Crambeidae Lévi, 1963
Species 8. Crambe panamensis Maldonado, Cardona, Van Soest and Pomponi, 2001
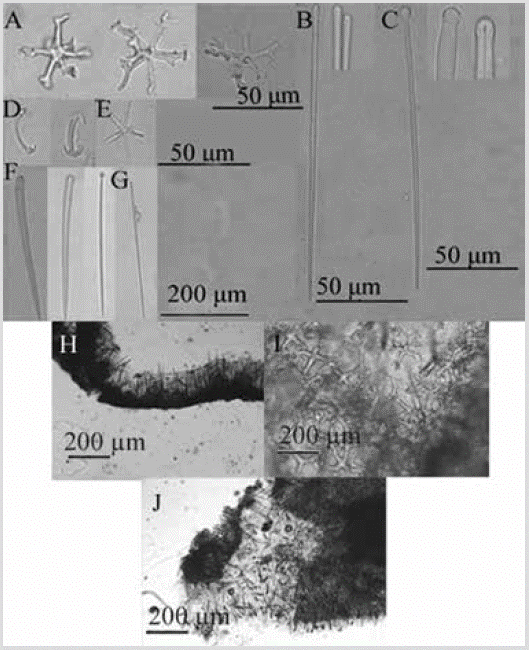
Figure 9 Crambe panamensis. A) Desmas astroclones. B) Thin subtylostyles. C) Tylostyles. D) Isochelae. E) Oxyaster. F) Tylostyles. G) Thin subtylostyle. H) and I) Section perpendicular to the surface (top left) and view of the basal astroclone crust (top right). J) Partially macerated section showing part of the ectosome (vertical spicules and tufts) and the choanosome.
Crambe panamensisMaldonado, Carmona, Van Soest and Pomponi, 2001: 1264, fig. 1.
Material studied: INV POR1365, Punta Cruces, La
Parguera-Piñas, 6 m, col. L. Chasqui, 08-16-2015.
Shape: 15-20 cm diameter thin encrustation. Color: Red-orange; white in alcohol.
Consistency: hard, not very compressible. Ectosome: tylostyles and subtylostyles, erect, sometimes tufted, support the pinacoderm. Choanosome: basal stratum, of astroclones, from which subtylostyles and tylostyles ascend, turning into hispid tufts upon reaching the surface (Figure 9H). Spicules: megascleras: slightly curved tylostyles with well-defined head, sometimes irregular, 217-308-427 µm by 8.8-17.8-29.7 µm, tyle diameter 10.1-16.5-27.6 µm (Figure 9C, 9F); slender, straight subtylostyles with slightly oval heads, 77-185-232 µm by 2.4-4.0-5.8 µm (Figure 9B, 9G); Desmas astroclones, with a well-developed center, from which four to six actins radiate, 50-84-117 µm (total diameter) (Figure 9A). Microsclera: oxyasters, 4-5 radii, 9.9-21.1 -29.8 µm in diameter (Figure 9E); isochelae with three teeth, 19.7-23.9-29.9 µm (Figure 9D).
Habitat: on vertical walls of the rocky reef.
Distribution: Pacific of Panama (Maldonado et al., 2001); Colombian North Pacific.
Comments: the record of Crambe panamensis was confirmed by the presence of astroclone desmas together with a category of choanosomeles tylostyles, small ectosoal subtylostyles, and a category of isochelae (Maldonado et al., 2001). The type locality is close to the Colombian North Pacific.
Species 9. Discorhabdella littoralis Maldonado, Carmona, Van Soest and Pomponi, 2001

Figure 10 Discorhabdella littoralis. A) Main tylostyle. B) Secondary subtylotyle. C) Acanthoestyle. D) Sigma. E) Choanosome.
Discorhabdella littoralisMaldonado, Carmona, Van Soest and Pomponi, 2001: 1270, fig. 3.
Material studied: INV POR1401, Cape Marzo, La Foca, prof. 17 m, col. L. Chasqui, 08-15-2015.
Shape: thin encrustation. Color: white in alcohol. Consistency: too thin to notice; hispid texture to the touch. Ectosome: spicules piercing the pinacoderm. Sigmas are found primarily below the surface. Choanosome: acanthostyles clustered densely at the base on a layer of spongin, from which the main tylostyles project upward, then traverse the surface of the sponge. The secondary subtylostyles are arranged in the shape of branches or tufts around the tylostyles (Figure 10E). Spicules: megascleras: slightly curved main tylostyles with oval head, 128178-247 µm by 2.9-5.9-9.2 µm (Figure 10A); secondary subtylostyles, 118-144-171 µm by 3.2-5.3-7.2 µm (Figure 10B); acanthostyles, 20-30-42 µm by 4.1-6.1-8.7 µm and with a large head, 9.2-12.3-18.1 µm in diameter (Figure 10C). Microscleres: sigmas, 11.2-22.5-32.1 µm (Figure 10D).
Habitat: shallow rocky reefs.
Distribution: Pacific of Panama (Maldonado et al., 2001); Colombian North Pacific.
Comments: it was compared with the Discorhabdella species described by Maldonado et al. (2001) for the Pacific coast of Panama. It was defined as D. littoralis by the presence of tylostyles, substylostyles, and sigma.
Family Microcionidae Carter, 1875
Species 10. Clathria (Microciona) sp.
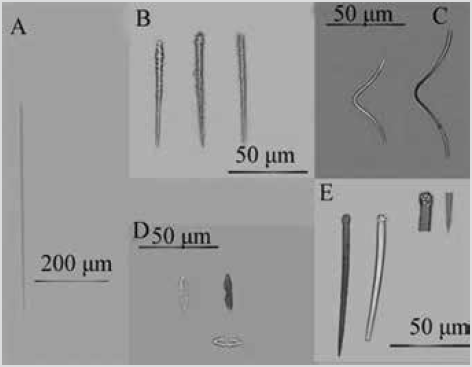
Figure 11 Clathria (Microciona) sp. A) Subtilostyles. B) Auxiliary acanthostyles. C) Toxas. D) Isochelaw. E) Main styles.

PLATE 2 fig. 1.Clathria (Microciona) sp., INV POR1400, Cape Marzo, 17 m; fig. 2.Scopalina sp. 2., INV POR1396, Punta Cruces, 15 m (orange cladding on snail); fig. 3.Axinyssa isabela, INV POR1397, Cape Marzo, 19 m; fig. 4.Terpios sp., INV POR1403, Cape Marzo, 17 m (yellow encrustation on octocoral Carijoa riisei);fig. 5.Aplysilla sp., INV POR1366, Cape Corrientes, 18 m (with octocoral Carijoa riisei);fig. 6.Chelonaplysilla violacea sensu Gómez et al., 2002, INV POR1368, Cape Corrientes, 19 m.
Material studied: INV POR1400, Cape Marzo, La Foca, 17 m, col. L. Chasqui, 08-15-2015.
Shape: thin encrustation, widely covering the rocky substratum,. Color: scarlet red, white in alcohol. Consistency: fragile. Spicules: megascleres: main styles slightly curved, slightly robust at the base, head with spiny projections and ending in blunt or pointed tips, 176-247354 µm by 4.5-11.2-15.9 µm (Figure 11E); thin and straight subtylostyles, 140-196-248 µm by 3.2-6.2-8.2 µm (Figure 11A); straight auxiliary acanthostyles, with spines along the entire spicule, and gradually tapered towards the tip, 6893-120 µm by 3.8-5.8-8.0 µm (Figure 11B). Microscleres: isochelae with a thin axis, 16.5-19.1-30.0 µm (Figure 11D); V-shaped smooth toxas, 49-114-148 by 1.4-2.6-4.1 µm (Figure 11C).
Habitat: on shallow rocky reef walls.
Distribution: Colombian North Pacific.
Comments: reference was made to Aguirre et al. (2011), who made a comparative table of the species of the genus ClathriaSchmidt, 1862 from the ETP. The spicular composition is typical of the subgenus MicrocionaBowerbank, 1862, with main styles, mostly having spines only on the head, smooth auxiliary subtylostyles, smooth toxas in one or two length categories and often more than 100 |im in length, and isochelae in a single category, usually less than 15 |im long. More detailed analysis with additional material should be done to confirm if it is a new species.
Order Scopalinida Morrow and Cárdenas, 2015
Family Scopalinidae Morrow, Picton, Erpenbeck, Boury-Esnault, Maggs and Allcock, 2012
Species 11. Scopalina aff. ruetzleri (Wiedenmayer, 1977)
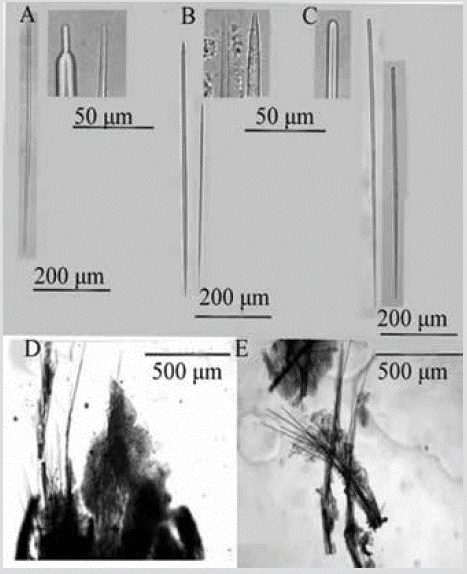
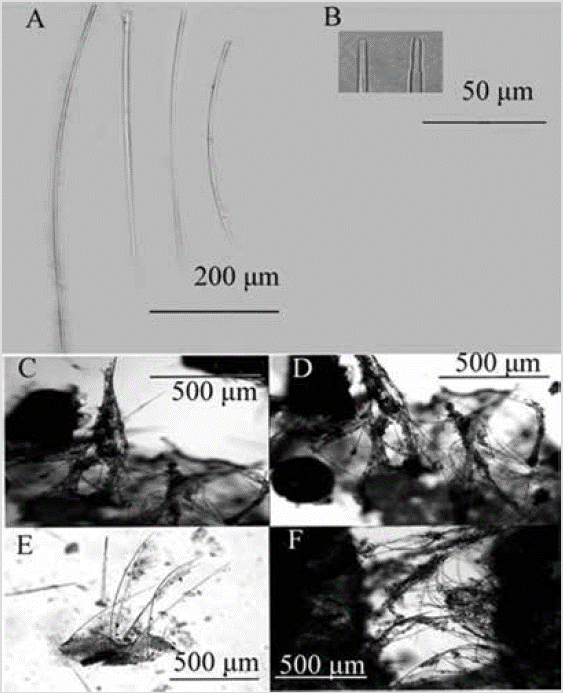
Figure 12 Scopalina aff. ruetzleri. A) and B) Anisoxeas. C) Styles. D) and E) Tracts of the choanosome that cross the ectosome.
?Ulosa ruetzleriWiedenmayer, 1977: 145; Plate 30, figs. 6-7.
Scopalina ruetzleri;Vega, 2012: 103, figs. 7.2.55. A, B.
Material studied: INV POR1378, Gulf of Tribugá, Morromico, 12 m, col. L. Chasqui, 08-20-2015.
Shape: thin encrustation; conulose surface, with scattered membranous oscules. Color: beige in alcohol. Consistency: weak, softly compressible. Ectosome: organic pinacoderm supported by the ends of the ascending tracts of the Choanosome, which make up the conules. Choanosome: skeleton of ascending multispicular fibers (4 to 7 spicules) 18-55 µm thick, branching towards the surface. Little spongin covers the tracts (Figure 12D). Spicules: long, slightly curved styles of simple tips, 299-552-761 µm by 4.1-6.9-11.6 µm (Figure 12C); anisoxeas (oxeas with a thicker and shorter end), 85-333-618 µm by 3.0-6.5-11.6 µm (Figure 12B, 12C).
Habitat: shallow rocky reefs.
Distribution: Mexican Pacific (Vega, 2012); Colombian North Pacific.
Comments: the skeletal and spicule characteristics are similar to Scopalina ruetzleri (Wiedenmayer, 1977) from the Caribbean (see Zea, 1987); however, a more detailed analysis should be made to determine if it is the same species or sister species that have not diverged phenotypically enough since reproductive isolation occurred due to the uprising of the isthmus of Panama, or if it is trans-isthmus colonization, to define the use of a new name for Pacific specimens.
Species 12. Scopalina sp. 2
Material studied: INV POR1396, Punta Cruces, La Mina, on gastropod shell, 15 m, col. L. Chasqui, 08-16-2015. INV POR1408, Cape Marzo, Piedra de Rodrigo, on wood, 18.5 m, col. L. Chasqui, 08-15-2015.
Shape: thin encrustation extending approx. 60 mm irregularly on the substratum, and on a gastropod shell, conulose. Color: orange; pale yellow in alcohol. Consistency: soft, hispid to the touch. Ectosome: organic pinacoderm; conules supported by erect spicular tracts. Choanosome: skeleton of ascending multispiculate tracts with 2 to 7 spicules in cross-section, 34-91 µm thick, branching on the surface; high presence of foreign material (Figure 13C). Spicules: long, thin styles, slightly curved, 463-845-1265 µm by 4.36.5-8.9 µm (Figure 13A, 13B).
Habitat: on gastropod shells and on wood on rocky reefs.
Distribution: Colombian North Pacific.
Comments: the generic location of this specimen was made using the Systema Porifera (Hooper et al., 2002). Except for Scopalina aff. ruetzleri described here and in Vega (2012), there are no other species of the genus Scopalina recorded for the ETP. Although they have the same color, Scopalina sp. 2 is less porous and transparent, in addition to only having more elongated styles.
Order Suberitida Chombard and Boury-Esnault, 1999
Family Halichondriidae Gray, 1867
Species 13. Axinyssa isabela Carballo and Cruz-Barraza, 2008
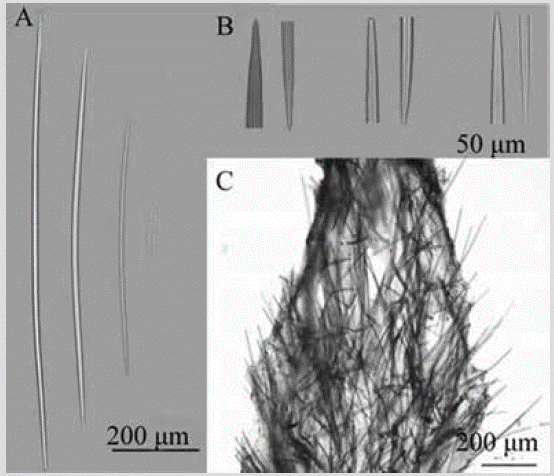
Figure 14 Axinyssa isabela. A) Oxeas. B) Ends of oxeas. C) Section perpendicular to the surface showing ectosome and choanosome.
Axinyssa isabelaCarballo and Cruz-Barraza, 2008: 60, figs. 2-5.
Material studied: INV POR1385, Gulf of Tribugá, Morros de Jurubidá, 13.3 m, col. L. Chasqui, 08-20-2015. INV POR1397, Cape Marzo, Piedra de Eroito, 19 m, col. L. Chasqui, 08-15-2015.
Shape: massive amorphous, with rounded low lobes, spreading irregularly on the substratum,. Smooth surface, round or oval shaped oscules of approx. 1-4 mm diameter. Color: dark yellow, brown to ocher in alcohol. Consistency: toughly compressible and fleshy, in life; when preserved, it crumbles. Ectosome: partially detachable pinacoderm, without specialized skeleton; tracts and spicules of the peripheral choanosome cross it, but are also organized in cross or paratangential fashion. In the subectosomal region, there is a dense collagen layer with individual spicules or tracts. Choanosome: irregular and plumose tracts, 48-94 µm, rise and diverge towards the surface; many spicules in confusion (Figure 14C). Spicules: large, thin oxeas, slightly curved (Figure 14A), with highly variable tips: sharp, mucronated, stepped, and blunt (Figure 14A, 14B), 526-647-765 µm by 5.6-10.6-16.1 µm.
Habitat: on vertical rocky reef walls, caves, and ledges.
Distribution: Mexican Pacific (Carballo and Cruz-Barraza, 2008); Colombian North Pacific.
Comments: Professor J.L. Carballo examined the photos of the specimen taken in the field in vivo and of the skeleton and spicules in the laborataory and confirmed that it is this species by the shape of growth and morphological characteristics.
Species 14. Epipolasis sp.
Material studied: INV POR1387, Gulf of Tribugá, Morros de Jurubidá, 10 m, col. L. Chasqui, 08-20-2015.
Shape: thin encrustation with a smooth surface, slightly corrugated, approx 6mm thick. Color: purple in alcohol, possibly from being preserved alongside an Aplysina. Consistency: compressible to crumbly. Ectosome: cortex of spicules, smaller and closer together than in the choanosome. Choanosome: confused skeleton of oxeas of various sizes (Figure 15C, 15D). Spicules: megascleres: oxeas slightly curved in the center and frequently bent in three portions, robust, with pointed or blunt tips, 149-195290 µm by 6.4-10.4-16.9 µm (Figure 15A). Microscleres: straight and thin raphides, 94-253-386 µm by 1.0-1.7-2.9 µm (Figure 15B).
Habitat: on shallow rocky reefs.
Distribution: Colombian North Pacific.
Comments: the generic location was based on the work of Díaz et al. (1993), who reviewed the genera of Halichondriidae from the central Atlantic. The diagnosis of the genus Epipolasis in De Laubenfels (1936a) agrees with that of the specimen described here. This is the first record of the genus in the ETP.
Family Suberitidae Schmidt, 1870
Species 15. Terpios sp.

Figure 16 Terpios sp. A) Tylostyles. B) and C) Sections perpendicular to the surface showing ectosome and choanosome.
Material studied: INV POR1403, Cape Marzo, La Foca, octocoral envelope, 17 m, col. L. Chasqui, 08-15-2015.
Shape: smooth thin coating. Color: bright yellow; pale yellow in alcohol. Consistency: soft. Ectosome: pinacoderm crossed by tufts or rows of choanosomal spicules, perpendicular to the surface. Choanosome: confused skeleton with tracts or groups of spicules without definite direction (Figure 16B, 16C). Spicules: short tylostyles, slightly curved, with irregular wrinkled, bulging, narrow, flattened, or lobed head, 137-219-339 µm by 2.13.1-4.1 µm (Figure 16A).
Habitat: on octocoral Carijoa riisei on rocky reef.
Distribution: Colombian North Pacific.
Comments: no sponge with lobed or irregular tylostyle heads has been described for the Eastern Pacific, so the identification was made based on Rützler and Smith (1993), who described new species of the genus Terpios in the Caribbean. More material is required to define its taxonomic status.
Order Tetractinellida Marshall, 1876 Suborder Astrophorina Sollas, 1887 Family Geodiidae Gray, 1867 Subfamily Geodiinae Gray, 1867
Species 16. Geodia media Bowerbank, 1873
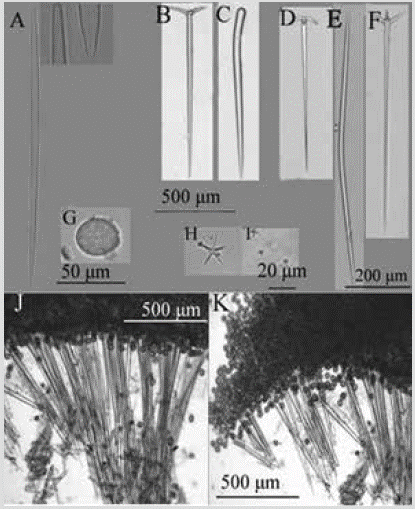
Figure 17 Geodia media. A) Strongyloxea. B) Ortotriaene. C) Style. D) Anatriena. E) Oxea. F) Plagiotriaena. G) Sterraster. H) Oxyaster. I) Strongylasters. J) and K) Perpendicular section showing the ectosomal cortex and the choanosome.
Geodia mediaBowerbank, 1873: 13, Plate II; Lendefeld, 1910, Plate 16, figs. 1-21, Plate 17, figs. 1-22; Desqueroux-Faúndes and Van Soest, 1997: 408, figs. 69-76; Vega, 2012: 56, figs. 7.2.8. A, B, C.
Material studied: INV POR1363, Punta Cruces, La Parguera-Piñas, 6 m, col. L. Chasqui, 08-16-2015.
Shape: massive amorphous, small in size, no more than 1-2 cm in diameter and 0.9 cm thick. Micro-rough, even surface. Color: white in alcohol. Consistency: hard cortex, fragile choanosome. Ectosome: cortex of tightly packed sterrasters, 1576-1940 µm thick, also containing oxyasters and strongylasters. Choanosome: orthotriaene and anatriaene tracts, 123-422 µm wide, 60-671 µm apart, supporting the cortex (Figure 17J). Spicules: megascleres: slightly curved oxeas, 288-938-1445 µm by 10.2-21.3-32.7 µm (Figure 17E); strongyloxeas with uneven tips, with sharp and blunt ends, slightly curved, 132-182-313 µm by 4.1-6.4-17.4 µm (Figure 17A); robust styles, rare, 993-10491106 µm by 36.5-38.9-41.2 µm (Figure 17C); orthotriaenes, rhabdome 512-1012-1497 µm by 35-44-62 µm, cladome 140-270-405 µm, clades 73-154-200 µm by 15.6-28.940.6 µm (Figure 17B); plagiotraenes, rhabdome 382-9561301 µm by 16.5-23.9-38.0 µm, cladome 64-176-314 µm, clades 43-65-85 µm by 10.5-16.8-21.5 µm (Figure 17F); anatriaenes, rhabdome 382-727-879 µm by 16.5-28.6-40.6 µm, cladome 64-156-246 µm, clades 22.6-26.2-30.0 µm by 10.5-13.4-17.5 µm (Figure 17D). Microscleres: sterrasters, 37-68-91 µm by 40-55-70 µm (Figure 17G); strongylasters, 3.2-4.9-7.2 µm (Figure 17I); oxyasters, 18.6-27.2-37.8 µm (Figure 17H).
Habitat: on shallow rocky reef walls.
Distribution: Pacific Coast of Mexico (Bowerbank, 1873; Vega, 2012); Panama (Lendenfeld, 1910); Galapagos Islands (Desqueyroux-Faúndez and Van Soest, 1997); Colombian North Pacific.
Comments: the identification was based on Lendenfeld (1910) and Desqueyroux-Faúndez and Van Soest (1997). The sample is more similar to that described by Lendenfeld (1910) for presenting styles and ortho-plagiotriaenes; however, the measurements differ somewhat from those of Colombia. The differentiation between species of this genus is quite difficult since many have been described to date.
Subclass Keratose Grant, 1861
Order Dendroceratida Minchin, 1900
Family Darwinellidae Merejkowsky, 1879
Species 17. Aplysilla sp.
?Aplysilla sulphurea;Thiele 1905: 488, figs. 112, 114.
Non Aplysilla sulphureaSchulze, 1878 (Valid Mediterranean and Eastern Atlantic species).
Non Aplysilla "sulphurea";Escobar 2000: 101, fig. 39.
Non Aplysilla sulphurea;Vega, 2012: 113, figs. 7.2.65. A, B.
Material studied: INV POR1366, Cape Corrientes, Piedra de Jairo, 18 m, col. L. Chasqui, 08-18-2015. INV POR1367, Cape Corrientes, Piedra de Jairo, 18 m, col. L. Chasqui, 0818-2015. INV POR1369, Cape Corrientes, Piedra de Colo, 14 m, col. L. Chasqui, 08-19-2015.
Shape: encrustation of a few mm thick; conulouse surface. Color: pale yellow, beige in alcohol. Consistency: soft; elastic but somewhat rigid fibers. Ectosome: spongin fibers support the pinacoderm, but can pierce it and protrude beyond it. The conules in the preserved material have a height of 118-342 µm (Figure 18C, 18D). Choanosome: single, erect spongin fibers 35-89 µm in diameter emerging from a basal plate; some branch out and rise to the surface (Figure 18A, 18B).
Habitat: over rocky reef along with octocoral Carijoa riisei.
Distribution: Colombian North Pacific.
Comments: Aplysilla polyraphisDe Laubenfels, 1930 recorded in California is distinguished by being dark purple in life and preserved, which differs from the Chocó samples. The spongin fibers of Aplysilla lendenfeldiThiele, 1905 from Chile are thicker, so it would not correspond to that species either. The characteristics of A. sulphurea, described for the Strait of Magellan (Thiele, 1905), are similar, but a more detailed analysis must be made to ensure that it is the same species, especially due to the great distance from the Colombian Pacific. Since A. sulphurea is originally found in the Eastern Atlantic and the Mediterranean, it would be incorrect to call the Eastern Pacific material that. Escobar (2000) and Vega (2012) describe an Aplysilla sulphurea (sulphurea in the case of Vega, 2012 and "sulfurea" in the case of Escobar, 2000), which is yellow in life and oxidizes to purple when fixed in alcohol, so it follows that it would not be the same species either.
Species 18. Chelonaplysilla violacea (Lendenfeld, 1883) sensuGómez, Carballo, Vázquez and Cruz, 2002
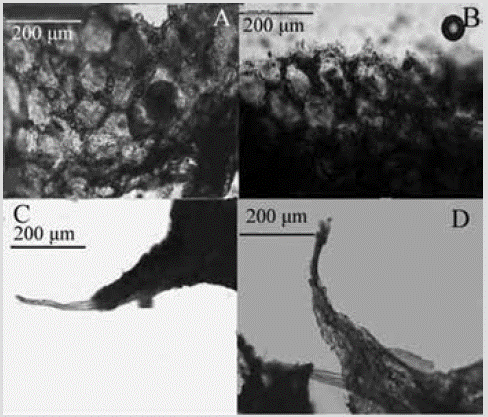
Figure 19 Chelonaplysilla violacea sensu Gómez et al., 2002. A) and B) Sediment network reinforcing the ectosome. C) and D) Spongin fibers in the conules.
?Aplysilla violaceaLendenfeld, 1883: 237, Plate X, figs. 5, 7.
Chelonaplysilla violacea;Gómez, Carballo, Vázquez and Cruz, 2002: 234, fig. 7 (with additional synonymy); Vega, 2012: 114, figs. 7.2.66. A, B.
Material studied: INV POR1368, Cape Corrientes, Piedra de Colo, 19 m, col. L. Chasqui, 08-19-2015. INV POR1379, Gulf of Tribugá, Morromico, rock fragments, 11.9 m, col. L. Chasqui, 08-20-2015.
Shape: thin cushions a few centimeters in diameter, conular, with scattered oscules, slightly raised with an organic collar. Color: greyish purple, with darker dark collar; dark purple in alcohol. Consistency: soft and brittle. Ectosome: conulose surface reinforced by a 33-72 µm thick polygonal debris network and 50-164 µm mesh size (Figure 19A, 19B). Choanosome: solitary spongin fibers 37-80 µm thick (Figure 19C, 19D), arising from a basal plate and rising to the surface; the fibers are pithed and the pith occupy 3767 % of the diameter.
Habitat: on a shallow rocky reef.
Distribution: Pacific coast of Mexico (Gómez et al., 2002); Colombian North Pacific.
Comments: the material conforms to that described by Gómez et al. (2002) from the Pacific coast of Mexico. It was originally described from the Western Pacific, so it could be a complex of cryptic species. Hence the decision to ascribe it only to the meaning of these authors. It is very similar to Chelonaplysilla americanaVan Soest, 2017 from the Caribbean, described for Colombia by Zea (1987) as C. erectaRow, 1911, suggesting that they would be sister species.
Order Dictyoceratida Minchin, 1900 Family Thorectidae Bergquist, 1978 Subfamily Thorectinae Bergquist, 1978
Species 19. Scalarispongia similis (Thiele, 1905)
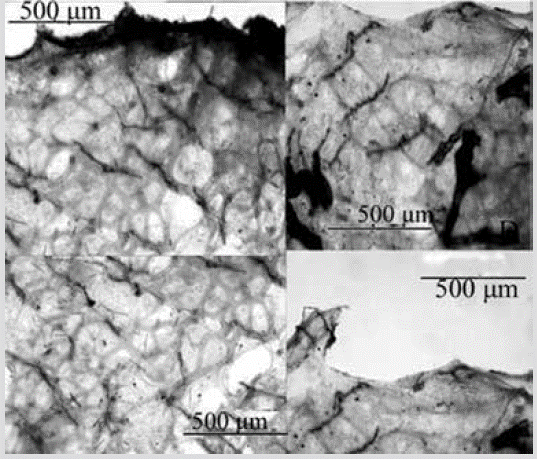
Figure 20 Scalarispongia similis. Sections perpendicular to the surface showing the ectosome and the spongin networks of the choanosome.
Cacospongia similisThiele, 1905: 481, fig. 108; Desqueyroux-Faúndez and Van Soest, 1997: 455, figs. 200205.
Scalarispongia similis;Cook and Bergquist, 2000: 398 (genus transfer).
Material studied: INV POR1392, Punta Cruces, La Viuda, 13-15 m, col. L. Chasqui, 08-14-2015.
Shape: thin encrustation, conulose surface. Color: dull pink in alcohol. Consistency: firm, little compressible. Ectosome: thick and fibrous pinacoderm; conules 114-281 µm high, separated 314-1173 µm, shaped by primary fibers protruding from the choanosome. Choanosome: Regular prismatic spongin network with ascending primary fibers, 20-80 µm thick, filled with foreign spicules and sediment, separated 143-373 µm, interconnected by transverse secondary fibers, 25-7 µm thick, or a secondary network with meshes 55-521 µm in diameter (Figure 20).
Habitat: on a shallow rocky reef.
Distribution: Galapagos Islands (Desqueyroux-Faúndez and Van Soest, 1997); Juan Fernández and Desventuradas Islands of Chile (Thiele, 1905); there is a record from the Argentina coast (Burton, 1940) that requires confirmation; Colombian North Pacific.
Comments: the rectangular network organization and the presence of spicules or other foreign particles within the fibers are diagnostic of this species; the diameter of the fibers is very similar to the original, although the secondary fibers are thicker in the Colombian sample. Thiele (1905) described it for the first time in Chile, which may indicate that this species is distributed throughout the Eastern Pacific. If Burton's record (1940) is confirmed, it would also extend to the south of the American Atlantic.
Subclass Verongimorpha Erpenbeck, Sutcliffe, De Cook, Dietzel, Maldonado, Van Soest, Hooper and Wörheide, 2012
Order Chondrosiida Boury-Esnault and Lopes, 1985
Family Chondrosiidae Schulze, 1877
Species 20. Chondrosia tenochca Carballo, Gómez, Cruz-Barraza and Flores-Sánchez, 2003
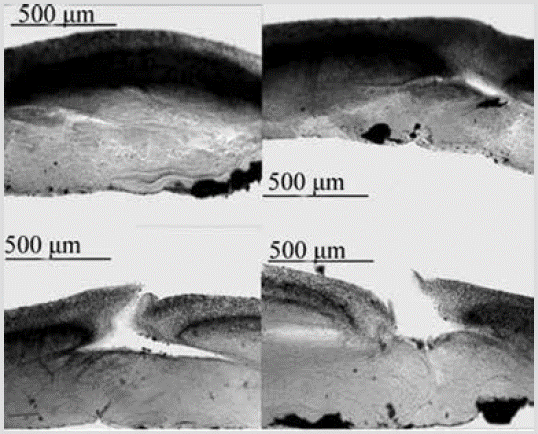
Figure 21 Chondrosia tenochca. Perpendicular sections showing the ectosome and choanosome, with canals and oscules.
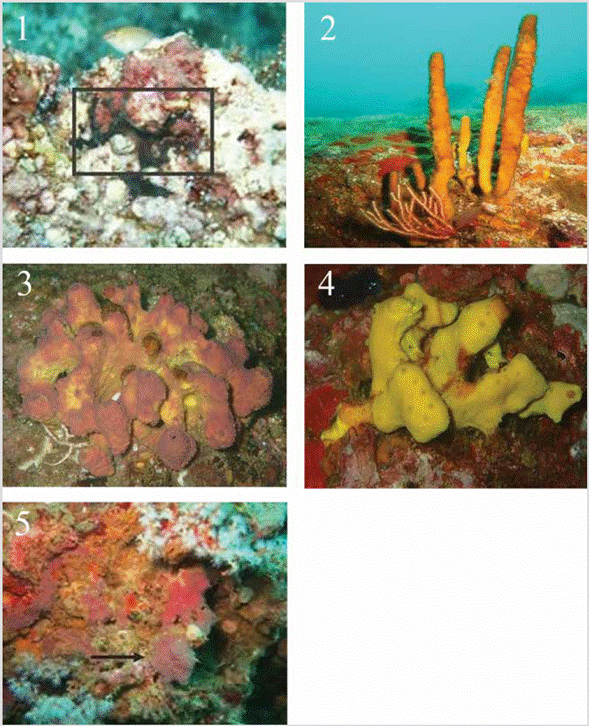
PLATE 3 fig. 1.Chondrosia tenochca, INV POR1371, Cape Corrientes, 11 m. (in box); fig. 2.Aplysina chiriquiensis, INV POR1402, Cape Marzo, 18.3 m; fig. 3.Aplysina gerardogreeni, INV POR1388, Punta Cruces, 13-15 m; fig. 4.Aplysina cf. revillagigedi, INV POR1390, Punta Cruces, 13-15 m; fig. 5.Vansoestia sp., INV POR1370, Cape Corrientes, 14 m (indicated with arrow).
Chondrosia tenochcaCarballo, Gómez, Cruz-Barraza and Flores-Sánchez, 2003: 517; Vega, 2012: 87, figs. 7.2.39.A, B, C.
Material studied: INV POR1371, Cape Corrientes, Piedra de Colo, 11 m, col. L. Chasqui, 08-19-2015.
Shape: encrustation 1-2 mm thick, stretched over the substratum, and partially fragmented; smooth, flat, shiny surface. Color: dark purple to black, beige on the surface, and grayish-purple on the inside in alcohol. Consistency: tough, leathery. Ectosome: without a skeleton. In sections, an organic crust of about 300 µm thick is observed, composed of two layers, the clearest superficial one and the inner denser one, an apparent product of granular cells. Choanosome: very dense, fibrous, but less granular than the ectosome, without skeleton of spongin fibers or spicules; some canals that flow into the oscules are observed (Figure 21).
Habitat: on a shallow rocky reef.
Distribution: Pacific coast of Mexico (Carballo et al., 2003; Vega, 2012); Colombian North Pacific.
Comments: initially it was believed that it was a colonial ascidian, but it was discarded when a dense cortex of cells that decreases towards the basal part was evidenced, with some fleshy channels in the choanosome. So far, this is the second record of this species for the ETP.
Order Verongiida Bergquist, 1978
Family Aplysinidae Carter, 1875
Species 21. Aplysina chiriquiensis Díaz, Van Soest, Rützler and Guzmán, 2005
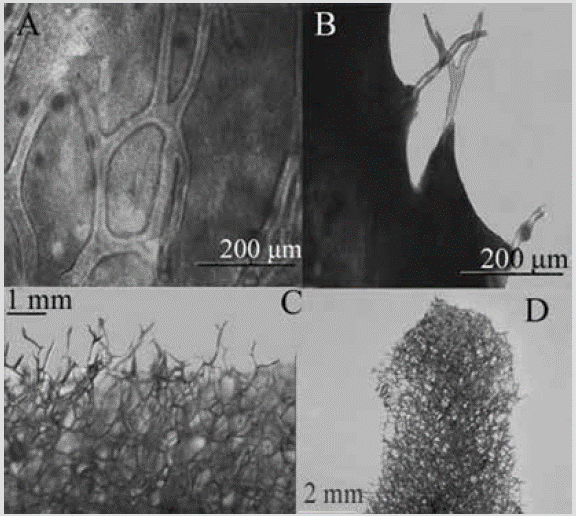
Figure 22 Aplysina chiriquiensis. A) and B) Histological section of the skeleton. C) and D) Three-dimensional skeletal reticulµm, (tissue removed in a bleach bath).
Aplysina chiriquiensisDíaz, Van Soest, Rützler and Guzmán, 2005: 3, figs. 1-3.
Material studied: INV POR1402, Cape Marzo, La Foca, 18.3 m, col. L. Chasqui, 08-15-2015. INV POR1405, Cape Marzo, Piedra de Rodrigo, 18.5 m, col. L. Chasqui, 0815-2015.
Shape: erect, thickened, or laterally compressed cylindrical branches with annular swellings, finger-shaped or rounded projections, and knotty ends emerging from the main stem. Height between 10 and 45 cm; branches 3-12 mm thick and 21-71 mm long. Circular oscules, distributed in one or more rows along the branches, with a collar-like membrane. Color: ocher yellow exterior, intense yellow interior; dark purple in alcohol. Consistency: slightly compressible and elastic. Ectosome: microconulous surface; conules regularly distributed, shaped by the growing ends of the fibers of the choanosome, which support the pinacoderm. Choanosome: skeleton as a polygonal or oval network of amber fibers (Figure 22C, 22D), concentrically laminated, 49-117 µm thick, forming meshes of 135-535 µm in diameter. The fibers have a dark granular pith that occupies 31-57 % of the diameter. Towards the surface, the fibers tend to be dendritic, with a length of 189-584 µm (Figure 22A, 22B), thinner than those located towards the main stem.
Habitat: abundant on shallow rocky reefs.
Distribution: Costa Rica, Panama, Galapagos Islands (Díaz et al., 2005); Colombian North Pacific.
Comments: it is recorded for the first time for the Colombian North Pacific and the Colombian Pacific in general. It is the only species of the genus with the shape of vertical branches in the ETP.
Species 22. Aplysina gerardogreeni Gómez and Bakus, 1992
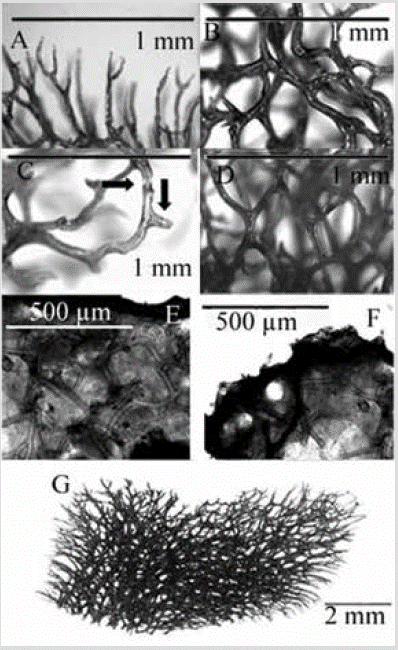
Figure 23 Aplysina gerardogreeni. A) Dendritic fibers bifurcated on the surface. B) Pith of the spongin fiber. C) Detail of fibers with nodular cortex and pith and short protrusions (indicated with arrows). D) Three-dimensional polygonal network. E) and F) Histological section. G) Three-dimensional skeletal reticulation (in G and A-D the tissue was removed in a bleach bath).
Aplysina gerardogreeniGómez and Bakus, 1992: 176, Plate 1-2; Cruz-Barraza, Carballo, Rocha-Olivares, Ehrlich and Hog, 2012: 4, figs. 1E, F; 2I, J; Vega, 2012: 116, figs. 7.2.68. A, B.
Suberea gerardogreeni;Escobar, 2000: 108, figs. 41-42.
Material studied: INV POR1374, Gulf of Tribugá, Morromico, 11.7 m, col. L. Chasqui, 08-20-2015. INV POR1377, Morromico, 11.9 m, col. L. Chasqui, 08-202015. INV POR1388, Punta Cruces, La Viuda, 13-15 m, col. L. Chasqui, 08-14-2015. INV POR1393 and 1394, Punta Cruces, La Mina, 15 m, col. L. Chasqui, 08-16-2015. INV POR1398 and 1399, Cape Marzo, Piedra de Eroito, 17.5 m, col. L. Chasqui, 08-15-2015.
Shape: massive and lobular, up to 10-15 cm in diameter and about 5-10 cm in height; conuloses surface; oscules at the top of the lobes, usually with a membrane as a collar. Color: externally dark yellow (ocher) with purple hues in vivid, yellow interior; dark purple in alcohol. Consistency: firm and slightly compressible. Ectosome: easily detachable pinacoderm, supported by the end of the fibers of the choanosome, which conform the conules. Choanosome: three-dimensional polygonal network of spongin fibers with a mesh size of 148-1037 µm in diameter (Figure 23D). Fibers 53-156 µm in diameter, with amber crust with small protrusions and pith 33-84 % of the diameter (Figure 23C). Near the surface, the fibers become dendritic and generally bifurcate and form the conular surface, ending in rounded tips, 571-643 µm long.
Habitat: abundant in the rocky reefs of the Colombian North Pacific.
Distribution: southern Baja California to Oaxaca (Mexico) (Gómez and Bakus, 1992; Cruz-Barraza et al., 2012; Vega, 2012); Málaga Bay in the Colombian Pacific (Escobar, 2000); Colombian North Pacific.
Comments: the studies by Gómez and Bakus (1992) and Cruz-Barraza et al. (2012) from the Mexican Pacific were reviewed. The presence of a regular three-dimensional polygonal lattice and the diameter measurements are similar in both descriptions; however, comparing the mesh size, it is smaller than that recorded by Gómez and Bakus (1992), 1.11.9 mm vs 0.2-1 mm in our material. The species described by Gómez et al. (2018) from the Gulf of California was also compared. The growth form and the external color of our material is similar to A. airapii, but the skeleton, instead of a uniplanar mesh, has a polygonal network from which dendritic fibers arise, which also differs from A. sinuscaliforniensis, which has a fully cross-linked skeleton, without dendritic fibers.
Species 23. Aplysina cf. revillagigedi Cruz-Barraza, Carballo, Rocha-Olivares, Ehrlich and Hog, 2012
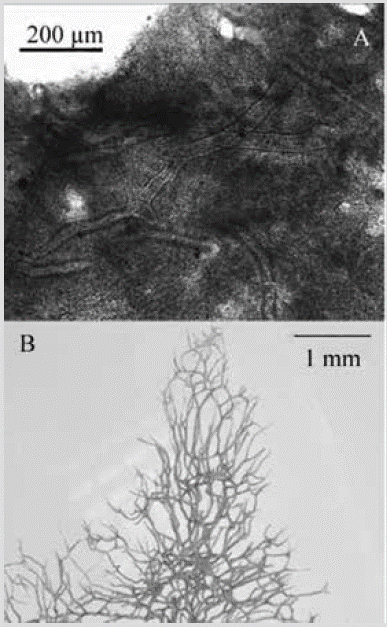
Figure 24 Aplysina cf. revillagigedi. A) Histological section. B) Three-dimensional skeletal reticulation (tissue removed in a bleach bath).
Aplysina revillagigediCruz-Barraza, Carballo, Rocha-Olivares, Ehrlich and Hog, 2012: 3, figs. 1B, C; 2F-H.
Material studied: INV POR1382, Gulf of Tribugá, Morros de Jurubidá, 14.5 m, col. L. Chasqui, 08-20-2015. INV POR1390 and 1391, Punta Cruces, La Viuda, 13-15 m, col. L. Chasqui, 08-14-2015. INV POR1406, Cape Marzo, 18.5 m, col. L. Chasqui, 08-15-2015.
Shape: massive amorphous, lobulated, about 5 cm in diameter and up to about 3 cm high. Smooth surface, conules evident in preserved specimens, soft to the touch. Scattered or aligned oscules at top of lobes, 1.6-6.7 mm diameter. Color: bright yellow; dark purple in alcohol.
Consistency: flexible, slightly compressible. Ectosome: end of the choanosomal network supports the pinacoderm. Choanosome: three-dimensional cross-linked, polygonal to irregular, and very open skeleton, with meshes of 153-1037 µm in diameter. Amber or dark brown fibers, 119-60 µm in diameter, with pith 29-74 % of the diameter (Figure 24A). Towards the surface, they terminate as dendritic fibers 3261749 µm long, with rounded tips (Figure 24B).
Habitat: shallow rocky reefs.
Distribution: Mexican Pacific (Cruz-Barraza et al., 2012); Colombian North Pacific.
Comments: identification is tentative, as it is difficult to separate the massive Aplysina species from the ETP. The shape of growth and the organization of the spongin network is confused with that of A. gerardogreeni, since some samples are tubular lobes with an apical osculum, but, in these, the organization of the skeleton near the surface shows fibers that branch out and end in rounded tips like those of A. revillagigedi.
Family Ianthellidae Hyatt, 1875
Species 24. Vansoestia sp.
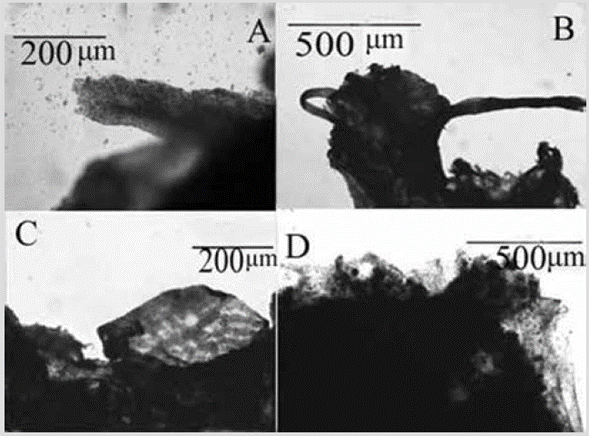
Figure 25 Vansoestia sp. A) and C) Grainy strands. B) Ectosomic network of granular strands. D) Foreign particles included in a fiber.
Material studied: INV POR1370, Cape Corrientes, Piedra de Colo, 14 m, col. L. Chasqui, 08-19-2015. INV POR1384, Gulf of Tribugá, Morros de Jurubidá, 10 m, col. L. Chasqui, 08-20-2015.
Shape: thin encrustation spreading irregularly and fractionally over the substratum, for several centimeters. Color: light purple; dark purple in alcohol. Consistency: soft. Ectosome: pinacoderm with a sort of net of strands of higher cell density, with a mesh size of 17-57 µm in diameter (Figure 25C). Choanosome: grainy fiber-like strands of cells, 96-182 µm in diameter (Figure 25A, 25B). Short vertical fibers with a large accumulation of debris are visible in sections and macerates (Figure 25D), but they are difficult to discern due to the pigmentation of the cells.
Habitat: on a rocky reef and other organisms.
Distribution: Colombian North Pacific.
Comments: this species was included in the genus Vansoestia due to its similarity with Vansoestia caribensisDíaz, Thacker, Redmond, Pérez, and Collins, 2015 both in external morphology and in the presence of granular strands.
There do not appear to be Aplysilla-type fibers (clean), but there could be Pleraplysilla-type fibers (filled with debris); therefore, Zea et al. (2014) called Pleraplysilla sp. V. caribensis samples, due to the apparent presence of short, undivided vertical fibers, filled with detritus. Confirming this requires different histological methods such as ground and polished sections.
DISCUSSION
The known diversity of sponges in the ETP region, with no more than a hundred species (Van Soest et al., 2012), is much lower than that of the Caribbean, with at least 519 species (Miloslavich et al., 2010). The first and most obvious reason is an uneven sampling effort between both regions, which was noted by Van Soest et al. (2012). While in the Caribbean the group is considered to be well known (> 70% of the described species, at least four identification guides; Miloslavich et al., 2010), the sponge fauna of the ETP is among the least known globally, with only four recent studies that include lists of species (Escobar, 2000; Berman, 2004; Lizarazo, 2018; Carballo et al., 2019).
In Colombia, this bias is even more marked, because while for the Caribbean there are more than 25 published works on sponge biodiversity (Zea, 1987; Zea et al., 2014), as well as an identification guide (Zea, 1987), for the Colombian Pacific, only three unpublished works are known (Narváez, 1999; Escobar, 2000; Lizarazo, 2018). Consequently, while for the Colombian Caribbean more than 150 sponge species have been recorded (Silva and Zea, 2017), for the Pacific only 46 species are known. However, this difference in sponge biodiversity between the two coasts goes beyond mere sampling bias, as suggested by the results of Carballo et al. (2019). They compared the diversity of sponges in coral reefs of the ETP, the Caribbean, and the Western Pacific, and showed large differences in abundance and richness of species, as well as in growth forms (massive and exposed in the Caribbean and Western Pacific versus small, encrusting and cryptic in the ETP). Observations made during fieldwork in this study compared with the authors' observations at "similar" habitats (i.e. reefs and rocky islets) off the Caribbean coast suggest that the observations of Carballo et al. (2019) on the marked difference between the coral reef sponge communities of the Mexican Pacific and the Caribbean also apply to the rocky reef ecosystems in Colombia.
Several explanations have been proposed for these differences in sponge communities between the two regions, involving biological (e.g. predation, competition) and physical (e.g. sedimentation, turbulence, abrasion) factors on a local scale, as well as phenomena on a regional and global scale. (e.g. upwelling, ENSO) (Wulff, 1997; Carballo et al., 2019). Regarding the difference in the predominant growth forms on both coasts (i.e. encrusting and massive small hidden between the reef in the ETP versus large colonies growing upright into the water column in the Caribbean), it is believed that a strong predation pressure in ETP, especially of spongivorous fish, on species that mostly lack chemical defenses, restricts colonization and further development in exposed environments (Bakus, 1964; Wulff, 1997). Carballo et al. (2019) attribute this strong difference in sponge communities between the Caribbean and the ETP to the effect of an accentuated regime of local and large-scale natural disturbances that act in the long term on a biota that was perhaps never particularly exuberant [i.e. speaking specifically of sponges associated with coral reefs, see López-Pérez (2017) for a general context on the origin of the coral biota of the ETP] and that was virtually isolated around three million years ago with the uprising of the Isthmus of Panama. Then, natural disturbances of great impact such as changes in surface temperature and nutrient regime caused by ENSO (Wang et al., 2017), as well as recurring events of extreme low tides, among others, could generate local and regional extinction processes in sponge communities, which added to an absence of rescue effect due to the uplift of the isthmus and the virtual impossibility of natural colonization of new sponge species through the Pacific barrier (i.e. more than 7000 km of open and deep water) could be the main forces that have sentenced an impoverished Porifera fauna in the ETP.
On the other hand, the marked difference in marine biodiversity between the ETP and the Caribbean is well known, especially with regard to the biota of coral reefs (Veron, 1995). In general terms, a scarce presence and development of coral formations in the ETP greatly affect any comparison of biodiversity with the Caribbean, the second region in coral development in the world, in any taxon that is strongly associated with these ecosystems, as in the case of sponges. Even, not only the low presence of coral reefs but of reefs in general (i.e. including rocky reefs) in the Colombian Pacific (e.g. Posada et al., 2009) affects the diversity indicators of sessile biota that requires hard substratum to settle.
Of the sponges identified, six species are morphologically similar (geminated species) with species from the Colombian Caribbean, which could indicate that: 1. They are sister species with a common ancestor before the closure of the isthmus; 2. These are populations of the same species, perhaps in a slow process of evolutionary divergence via vicariant speciation; 3. Some of the two populations are a biological invasion mediated by maritime transport (i.e. as biofouling) via the Panama Canal. The answer necessarily goes through the molecular genetic analysis of several specimens of these "geminated species", which is outside the scope of this work; however, this issue shows that the evolutionary history of populations of marine species on both sides of the Isthmus of Panama continues to be of total relevance and matter of interest.
Regarding the geographical distribution of the sponges found in the Colombian Pacific, they were grouped into five biogeographic sectors (*Species recorded by Escobar (2000), **Species recorded by Narváez (1999): Eastern Tropical Pacific and Californian coastal province: Spheciospongiasp. (possibly S. raromisclerosa)*; Eastern Pacific and Chile-Peru coastal province: Scalarispongia similis; Tropical Eastern Pacific (from Mexico to Ecuador): Axinella nayaritensis, Desmanthus levii, Crambe panamensis, Discorhabdella littoralis, Axinyssa isabela, Geodia media, Chondrosia tenochca, Aplysina chiriquiensis, A. gerardogreeni, Spirastrella sabogae*, Niphates perforata*, T. (Tedania) "nigrescens" (possibly T. (Tedania) tropicalis)*, A. azteca* (as Suberea), Aplysina cf. revillagigedi; Tropical Eastern and Western Pacific: Chalinula nematifera, Chelonaplysilla violacea, Mycale cecilia*, Pseudosuberites "sulcatus"*; Tentatively only in Colombia while its taxonomic status is defined (possibly new species, but requires detailed studies): Prosuberites aff. laughlini, Dragmacidon sp., Acanthella sp., Placospongia aff. intermedia, Clathria (Microciona) sp., Scopalina aff. ruetzleri, Scopalina sp. 2., Epipolasis sp., Terpios sp., Aplysilla sp., Vansoestia sp., Xestospongia sp. **, Anphimedon sp. **, Aaptos sp.**, Geodia sp.**, Desmanthus aff. incrustans*, Geodia sp. *, Amorphinopsis sp. *, Topsentia aff. ophiraphidites*, Mycale sp. *, Haliclona sp. 1, Haliclona sp. 2, Haliclona sp. 3, Haliclona sp. 4*, Placospongia carinata (P. intermedia in the Eastern Pacific), Aplysilla "sulphurea"*.
Finally, with the description made in this work of 24 species found in the rocky reefs of the Colombian North Pacific (21 are new records for Colombia), the number of sponge species for the Colombian Pacific rises to 46. This richness of species is considered low compared to the known biodiversity of the Colombian Caribbean (> 150 species). Some of the sponges recorded here have skeletal and external morphological characteristics very similar to Caribbean Species, which indicates that they could be geminated species; however, the analysis carried out based on exclusively morphological characters does not allow to conclude whether or not they are different species and whether they require a new name; therefore, the question remains open pending more detailed analyzes, which include the review of a greater number of samples of these species from both coasts, and of course, the analysis of molecular characteristics.











 text in
text in