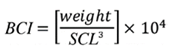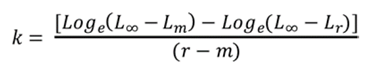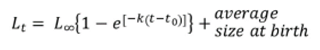INTRODUCTION
The hawksbill turtle, Eretmochelys imbricata, is classified globally as Critically Endangered (CR A2bd; Mortimer and Donnelly, 2008) since there has been a reduction of more than 80 % of its populations in the last three generations (IUCN, 2012). This reduction is mainly due to the use of their shell in jewelry and handicraft production, the consumption of their meat and eggs, and the loss of foraging and nesting habitats (National Research Council, 1990; Llamas et al., 2017). Until a decade ago it was believed that the species had been eliminated from the Eastern Pacific Ocean region (EPO; Campbell, 2014), but recently foraging habitats and nesting beaches have been found in this area (Altamirano et al., 2010; Gaos et al., 2012; Campbell, 2014; Chacón-Chaverri et al., 2015; Llamas et al., 2017).
The life cycle of hawksbill turtles after hatching and entering the sea begins in oceanic habitats. Upon reaching the juvenile stage, individuals migrate to neritic foraging habitats (Lutz and Musick, 1997) where they are mainly associated with coral reefs and other hard bottoms, as a consequence of their habitat and food requirements (Bjorndal and Bolten, 2010). There, they remain as residents for an extended period, and local conditions determine their survival (Blumenthal et al., 2009). Immature individuals have been documented to have a recruitment size in neritic habitats of between 20 to 35 cm Curved Carapace Length (CCL; Lutz and Musick, 1997; Blumenthal et al., 2009; Bjorndal and Bolten, 2010). Upon reaching sexual maturity, they move to other foraging and reproduction areas (Lutz and Musick, 1997) where they are associated with coral reefs and mangrove estuaries (Lutz and Musick, 1997; Chacón-Chaverri et al., 2015; Gaos et al., 2017). Sexual maturity in the EPO has been determined with the minimum size reported for nesting females in El Salvador and Nicaragua, of 66 cm CCL (Altamirano et al., 2010; Chacón-Chaverri et al., 2015). In the Caribbean (Puerto Rico), adult males with a Straight Carapace Length (SCL) greater than 68 cm were identified (Diez and van Dam, 2002).
Due to the permanence of juvenile hawksbill turtles in foraging areas for long periods (Blumenthal et al., 2009; Carrión-Cortez et al., 2013; Wood et al., 2013; Llamas et al., 2017) and these areas are vulnerable to pollution and climate changes (Muñoz and Zapata, 2013), it is important to diagnose the physical status of the animals, to assess the dynamics in the health of these populations. For this, the body condition index (BCI) is frequently used, which has significant predictive power in somatic growth models of the green sea turtle (Chelonia mydas) and has also been used in studies of habitat quality in hawksbills. (Bjorndal and Bolten, 2010). Some averages of the BCI values recorded for these turtles in the Caribbean, where it has been evidenced that individuals are in good physical condition, vary from 1.16 at Mona Cliff (Diez and van Dam, 2002) to 1.25 ± 0.17 in Little Cayman (Blumenthal et al., 2009), while this index has not been reported in the EPO region.
On the other hand, quantifying the body growth rates of individuals allows estimating the time it takes them to reach a height and/or the minimum age of sexual maturity, which is a fundamental attribute to understand their life histories and growth potential population, as well as for develop management strategies (van Dam, 2000; Bell and Pike, 2012). In studies carried out in the Great Barrier Reef (GBR) in Australia, a pattern in growth rates has been observed that suggests an adjustment to the von Bertalanffy model for hawksbill turtles (Chaloupka and Limpus, 1997; Bell and Pike, 2012). In the hawksbill turtle population in Hawaii, it was evidenced that the von Bertalanffy model was a better fit for the data: it estimated a characteristic growth coefficient (k) of 0.09 and an annual body growth rate for immature individuals of 4.59 cm/year (SD = 2.04) utilizing skeletochronology (Snover et al., 2012). This growth model usually has a better fit to the body growth data in turtles, in which juvenile growth rates are frequently higher than in adults and allows projecting how long it takes individuals to reach sexual maturity (Bjorndal and Zug, 1995).
Most growth studies for the hawksbill turtle do not use the von Bertalanffy growth model, but instead, report body growth rate (BGR). In these studies, a large spatial variation is observed in estimated BGRs for different categories of height and sex, in different regions of the Eastern, Western and Caribbean Pacific (Limpus, 1992; Boulon, 1994; Chaloupka y Limpus, 1997; Diez y van Dam, 2002; Blumenthal et al., 2009; Bell y Pike, 2012; Wood et al., 2013; Zárate, 2015; Bjorndal et al., 2016; Llamas et al., 2017). Additionally, in the Pacific region, Bjorndal and Bolten (2010) showed that hawksbill turtles have monotonic and relatively slow growth rates, which means that the growth rate increases in the same degree of variation, without having maximum peaks while Diez and van Dam (2002) showed a non-monotonic pattern in the Caribbean region, with lower growth rates in small individuals and with a higher growth peak in size categories between 30 and 40 cm in height SCL.
The EPO is considered one of the Regional Management Units with the greatest threat to the hawksbill turtle and there is still no knowledge of some fundamental demographic parameters to evaluate management plans for the species in this region (Wallace et al., 2011). Therefore, in this research, some of these parameters were evaluated, such as the proportion of different size categories, the BCI and the somatic growth rate, for the hawksbill turtle aggregation in the coral reefs Playa Blanca and La Azufrada of the Gorgona National Natural Park (GNNP) Colombia, through data obtained through the capture-mark-recapture method between 2004 and 2018. This information contributes to the improvement of the monitoring of sea turtles carried out in the GNNP and contributes new knowledge about the species in the EPO, generating in turn essential information for conservation and management plans in the region.
STUDY AREA
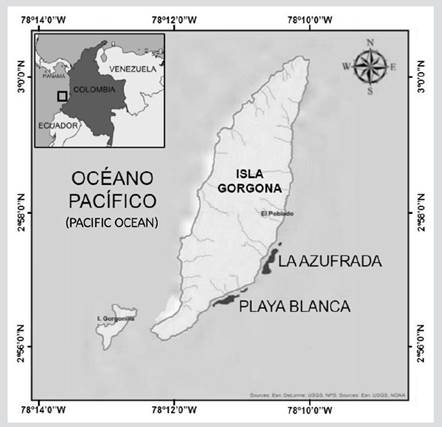
The sampling was carried out at the GNNP (02° 55′ 45″-03° 00′ 55″ N and 78° 09′ 00″-78° 14′ 30″ W), located in the department of Cauca in the Pacific Ocean in southwestern Colombia (Amorocho et al., 2015), on two well-developed edge reefs: Playa Blanca and La Azufrada, located on the eastern (leeward) side of the island (Figure 1). Both reefs generally have a similar structure and zoning (Muñoz and Zapata, 2013). Playa Blanca has an area of 9.9 ha, 930 m long and between 60 and 230 m wide, while La Azufrada has an area of 11.2 ha, 780 m long and between 80 and 180 m wide, both with depths that do not exceed 10 m at high tide (Muñoz and Zapata, 2013).
MATERIALS AND METHODS
Capture-mark-recapture
Data collection for this analysis began in 2004 with the support of CIMAD (Center for Research for Environmental Management and Development) and WWF-Colombia (World Wide Fund for Nature) and from 2008 to 2018 the GNNP (Amorocho et al., 2015). These episodes were not systematic, since a transect, a periodicity, or a specific time for the searches was not defined, nor is there a record of sampling events without individuals captured.ç
In both reefs, the personnel in charge of monitoring searched for individuals by lung (freediving). Each search episode lasted between 20 and 50 min and most of them took place between 20:00 and 21:00 h, during which time the turtles rested, which facilitates their capture. All individuals were measured, weighed, tagged, and released at El Poblado beach, located at a distance of approximately 3305 and 1407 m from the reefs of Playa Blanca and La Azufrada, respectively. The morphometric measurements taken were SCL and CCL, measured from the nuchal shield to the midpoint of the posterior notch between the supracaudal shields (Bolten, 2000). The straight measurements were taken with the help of a caliper (with a precision of 0.1 cm), the curved measurements with a flexible tape measure (with a precision of 0.1 cm), and the weight was taken with a continental brand Roman scale (with a precision of 0.5 kg). Each individual was marked with a metal tag (Monel or Inconel, n.° 681), on the second scale of the anterior fin, previously disinfected with povidone-iodine (Balazs, 2000).
Analysis of data
The turtles were grouped by size categories, arbitrarily determined as follows: category 1 (30 cm < CCL < 34.9 cm), category 2 (35 cm < CCL < 39.9 cm), category 3 (40 cm < CCL < 44.9 cm), category 4 (45 cm < CCL < 49.9 cm) and category 5 (CCL ≥ 50 cm). The characterization of the population structure for each reef was found with the proportion of turtles present in each size category. To determine if there was a difference in these proportions between reefs, an exact test of Fisher (1934) was performed. The BCI was calculated with the weight and the SCL, through the following equation (Bjorndal and Bolten, 2010):
Somatic growth was calculated utilizing the von Bertalanffy model for the aggregation of hawksbill turtles from both reefs, with the CCL measurements of the first and last catch because these are used more frequently in studies of sea turtles, which facilitates comparison with other studies. To calculate the characteristic body growth coefficient (k), which describes how fast an animal grows from its birth to its maximum length, the following equation was used (Munro, 1982):
Where:
L∞ is the maximum length that individuals in the population can reach (in this case, it was calculated for lengths between 60 and 66 cm),
Lm is the curved carapace length (CCL) at the first capture,
Lr is the CCL at the last recapture,
r and m are the dates of the last recapture and first capture, respectively, and
e is the base of the natural logarithm.
This coefficient was calculated for all the recaptured individuals. From these data, their average and the coefficient of variation (CV) was calculated.
Subsequently, utilizing the von Bertalanffy model, the length corresponding to each year of life of the individuals in this population (Lt) was estimated, from year 0, in which the individual measures approximately 4 cm CCL (Altamirano et al., 2010), until reaching the maximum length. In this study, a maximum length of 63 cm CCL was set, because at this size the lowest CV was obtained, and because it is a close value to 66 cm CCL, the minimum size of sexual maturity reported for females in the EPO (Altamirano et al., 2010). With these values, the time in years that it would take the individuals of the monitored population in the GNNP to reach the minimum maturity size was estimated.
Where:
t0 is the hatching time, and
Average size at birth is 4 cm
Also, the body growth rate (BGR) was calculated, this being the difference between the length of the last capture (Lr) and the first (Lm) over time (BGR = (Lr - Lm)/year; Lutz and Musick, 1997).
RESULTS
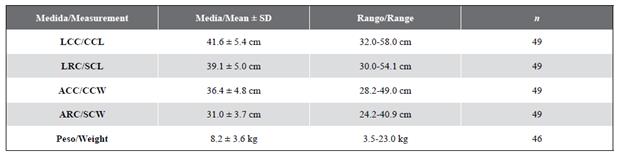
From 2004 to 2018, 66 successful nocturnal search episodes were carried out (at least one individual captured), 44 in the Playa Blanca reef, and 22 in La Azufrada. 49 individuals of hawksbill turtles were captured, of which 19 (38.8 %) were recaptured at least twice (nine individuals were captured twice, six individuals three times, two individuals four times, one individual five times, and one individual ten times). During the dives, the turtles were observed mainly resting (81.0 %), exploring (15.2 %), and to a lesser extent feeding (3.8 %). Furthermore, all recaptures (except one individual) always occurred on the same reef. The averages and standard deviations of the morphometric measurements in the first capture can be observed in Table 1.
Table 1 Average and range of morphometric measurements taken in the first capture of hawksbill turtles, Eretmochelys imbricata, in the GNNP.
In Table 2 you can see the measurements for each of the five size categories in each reef. Since the individuals do not exceed 66 cm of CCL they are considered immature; also, none of the captured individuals exhibited evident secondary sexual characteristics, such as a greater length of the tail in males (Bolten, 2000). The category with the highest proportion of individuals was category 2 (CCL between 35 and 39.9 cm) with 32.7 %, followed by category 3 (CCL between 40 and 44.5 cm) with 30.6 % (Figure 2). Category 1 (between 30 and 34.9 cm CCL) represented 12.2 % of the population and category 5 (CCL ≥ 50 cm) represented only 8.2 % of the population. Of the 49 individuals captured, 19 were in the La Azufrada reef and 30 in Playa Blanca. In La Azufrada a higher percentage of individuals is observed in category 2, while in Playa Blanca a higher percentage of individuals in category 3, such as can be seen in Figure 3. No significant difference was found in category proportions between reefs (Fisher’s exact test: P = 0.314). The average BCI (10-4 kg/cm3) of all the catches made on both reefs was 1.3 ± 0.11 (n = 46, range 1.0-1.5).
Table 2 Average morphometric measurements of hawksbill turtles, Eretmochelys imbricata, were captured between 2004 and 2018 for each size category and coral reef. CCL: curved carapace length. SCL: straight carapace length. SD: standard deviation. * Two outliers were found in the weight measurements, one in La Azufrada in category 3 and another in Playa Blanca in category 2.
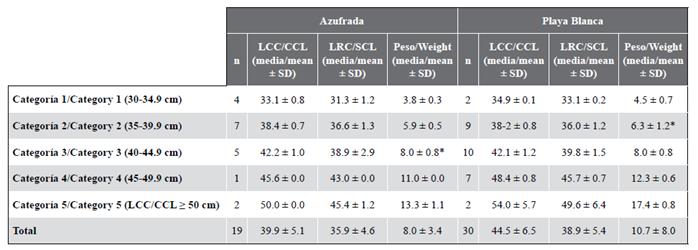
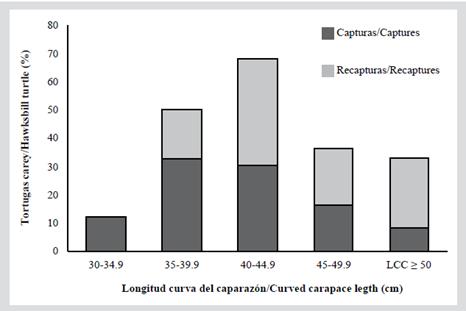
Figure 2 Percentage of individuals of hawksbill turtles, Eretmochelys imbricata, captured in each size category (CCL) on both reefs of the GNNP between 2004 and 2018.
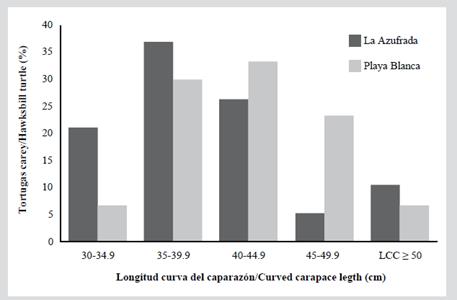
Figure 3 Percentage of individuals of hawksbill turtles, Eretmochelys imbricata, captured for the first time in each size category (CCL) captured on each coral reef of the GNNP between 2004 and 2018.
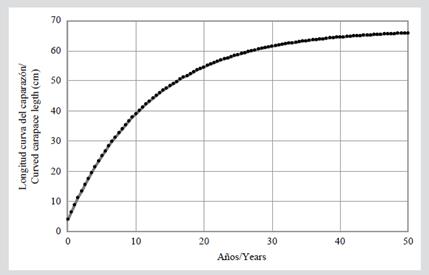
In the somatic growth analysis, the lowest CV was obtained with a maximum length of 63 cm CCL (CV= 0.96), and it yielded an average characteristic coefficient of body growth (k) of 0.081. The von Bertalanffy growth curve indicated that individuals in this population would reach this height between 33 and 34 years of age (Figure 4). In addition, an annual BGR of 1.5 ± 1.8 cm/year (range 0-7.3 cm/year) was estimated.
DISCUSSION
The hawksbill turtles found in this study are immature because they do not exceed 66 cm CCL, the minimum length described for reproductive females in the EPO (Witzell, 1983; Altamirano et al., 2010; Chacón-Chaverri et al., 2015) or that reported for males (SCL > 68 cm, Diez and van Dam, 2002). The documented curved carapace length range (32-60 cm) is similar to that found in other foraging areas on the Pacific, such as Coiba National Park, Panama (Table 3; Llamas et al., 2017) and Golfo Dulce, Costa Rica (34.8 and 81.1 cm of CCL; Chacón-Chaverri et al., 2015). Additionally, the distribution of height categories (32.7 % between 35 and 39.9 cm CCL) was similar to that found by Llamas et al., (2017) in Panama and the previous study carried out in the GNNP by Tobón-López and Amorocho (2014). No statistically significant difference was found in the category proportions between the two reefs, indicating that the size of hawksbill turtles is independent of the reef. The fact of having captured four small individuals (category 1 between 30 and 34.9 cm CCL) unmarked between 2014 and 2018, could be indicating the continuous recruitment of new individuals to this foraging area. All this suggests that the reefs of the GNNP provide an important development and foraging habitat for this species and that the absence of adult individuals suggests a migration to other breeding and foraging areas when they move from immature to adults during their development, as suggested by Tobón-López and Amorocho (2014).
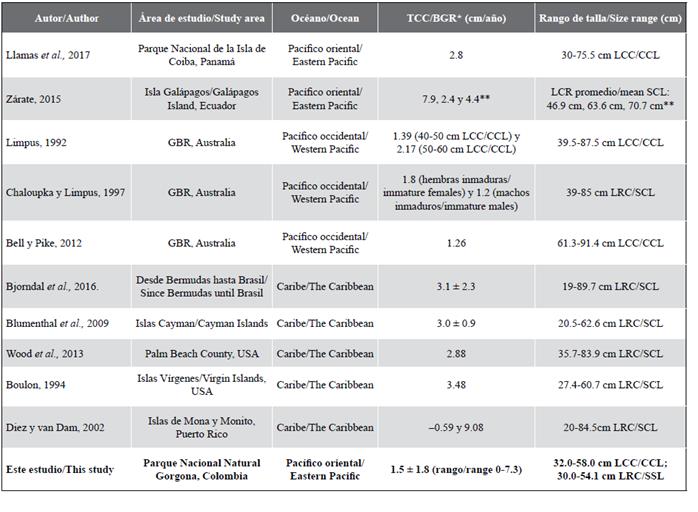
Table 3 Estimated annual body growth rates for different populations of Eretmochelys imbricata in different regions. *In some studies the growth rate is specified by size category and therefore two values appear. Some studies report standard deviation and others do not. **Three individuals were recaptured in this study.
Given that 98 % of the individuals tagged and released at El Poblado Beach were recaptured on the same reef, the fidelity of hawksbill turtles to each reef of the GNNP is hinted at, as mentioned by Tobón-López and Amorocho (2014) and the long-term residence of some individuals is evidenced (recapture of an individual after 10 years). The fidelity of hawksbill turtles to foraging areas has been documented in other studies (Carrión-Cortez et al., 2013; Wood et al., 2013; Llamas et al., 2017). Only one case was observed in which the individual was captured four times during 2011 in both reefs. This could be due to the connectivity between the two reefs since they are located relatively close. On the other hand, no previously tagged individuals were found in other foraging areas and turtles tagged in the GNNP were found in other foraging or nesting areas in neighboring countries where tagging and monitoring have been carried out (Altamirano et al., 2010; Liles et al., 2011; Carrión-Cortez et al., 2013; Heidemeyer et al., 2014; Chacón-Chaverri et al., 2015; Llamas et al., 2017). This warns of the importance of protecting the foraging area for this aggregation and suggests that monitoring should continue in the EPO to identify migration routes and complement regional conservation plans for the hawksbill population.
The BCI (1.3 ± 0.11) indicates that the hawksbill aggregation present in this protected area is in good physical and health condition since it is slightly higher than the indices estimated in the Caribbean region, such as in Little Cayman (1.25 ± 0.17) and Grand Cayman (1.24 ± 0.18; Blumenthal et al., 2009); in the Mona reef (1.18), the Mona cliff (1.16) and the Monito cliff (1.24) in Puerto Rico (Diez and van Dam, 2002), and in the Union Creek Reserve (1.17 ± 0.08), in Bahamas (Bjorndal and Bolten, 2010), where a good state of hawksbill turtles is reported. Even though the EPO region is strongly influenced by the latitudinal displacement of the Intertropical Convergence Zone (ITCZ) and by the El Niño phenomenon, which influences the climatic and oceanographic conditions of the GNNP (Zapata, 2017) that could have effects on food availability, the BCI indicates that the population remains healthy and does not have a food deficit. On the other hand, although one of the major physical disturbances in the reefs of the GNNP is the repeated aerial exposures during extreme low tides, which considerably decreased the live coral cover between 1998 and 2008, this coral cover has recovered (Zapata, 2017), which indicates that this phenomenon should not affect the development of hawksbill turtles in this foraging area. The BCI suggests that the environmental conditions that occur in the reefs of the GNNP are ideal to maintain the health of this aggregation of turtles in good condition, which reaffirms that this is an important foraging area for the conservation of the species in the EPO region.
The characteristic coefficient of body growth (k) estimated in this study (0.081) is comparable with the value found (0.09) by Snover et al. (2012) for hawksbill turtles in Hawaii using skeletochronology, where it suggests that turtles in this population will reach sexual maturity with an SCL of 78.6 cm between 17 and 22 years of age. In other studies carried out with sea turtles using the von Bertalanffy model, for Chelonia mydas has been reported values of k between 0.021 (US Atlantic Coast; Goshe et al., 2010) to 0.21 (San Diego Bay, California; Eguchi et al., 2012), for Caretta caretta values of k between 0.044 (Atlantic coast of Florida; Bjorndal et al., 2001) and 0.769 (coast of Italy; Casale et al., 2009), and for Lepidochelys kempii values of k between 0.115 (Florida Atlantic coast) and 0.25 (Gulf of Mexico; Avens et al., 2017). With the value of k that was estimated in this study using the von Bertalanffy curve, individuals in this aggregation could take around 33-34 years to reach a CCL of 63 cm (Figure 4), close to the minimum size of maturity for females reported for the EPO (Witzell, 1983; Altamirano et al., 2010; Chacón-Chaverri et al., 2015). This estimate is higher than that reported for hawksbill turtles in Hawaii by Snover et al. (2012), but comparable to Bjorndal and Zug (1995) and Bell and Pike (2012) in the northern GBR (Australia), which was estimated between 30 and 40 years. This indicates that the somatic growth rate of the individuals captured in the GNNP is within the ranges estimated for other populations of this species. On the other hand, this study found a BGR of 1.5 cm/year (range 0-7.3 cm/year), which is lower than the average rate of the other rates estimated in other regions of the Eastern, Western and Caribbean Pacific (Table 3). However, the range in BGR (0-7.3 cm/year) is within the observed in the Western Atlantic (-2.1-22.6 cm/year; Bjorndal et al., 2016).
The low somatic growth rates in the hawksbill turtle compared to other sea turtles such as C. caretta and L. olivácea, may be associated with a great variety of factors, both intrinsic to the individual (co-evolution with other attributes of its life history, phylogenetic restrictions, genetic variability, among others) as extrinsic (temperature, feeding), which affect growth in sea turtles (Sterns, 1992; Diez and van Dam, 2002; Bell and Pike, 2012). Because the BCI suggests that this aggregation is in a suitable foraging area for the proper development of hawksbill turtles, the GNNP was declared a protected marine area since 1984 and the Playa Blanca and La Azufrada reefs have remained closed to the public. Not scientific during the last years, since they are considered Conservation Object Values. The main cause of these slow somatic growth rates is their type of life history, since sea turtles in general, and E. imbricata in particular are characterized by a late sexual maturity age and a long life expectancy, under natural conditions (Sterns, 1992; Lutz and Musick, 1997). As a late age of sexual maturity is correlated with slow population growth rates (Doak et al., 1994) and with high annual survival rates of sub-adults and adults (Heppell et al., 1996; Heppell, 1998; Jonsson and Ebenman, 2001), a sustained reduction in the value of the survival rates of these two classes can lead to the local or global extinction of the species (Musick, 1999).
Because the EPO is considered one of the Regional Management Units with the greatest threat to hawksbill turtles, it needs immediate attention both in reducing threats and in increasing monitoring for more rigorous evaluations of their risks and threats (Wallace et al., 2011). To show whether the population is at risk and to strengthen management plans, it is necessary to know the demographic status of the sea turtle populations in foraging areas by documenting the dynamics with parameters such as abundance and survival (Bjorndal, 2000). Which are unknown for the hawksbill populations in the EPO. Because one of the authors of this study (LFP) estimates that in at least 20 % of the search episodes no individuals were found, the Mark program could not be used to estimate these parameters since these data are not found in databases. For this reason, it is recommended that the monitoring of this species be carried out systematically (time efforts and search areas consistent between years) and that unsuccessful sampling events (without captured individuals) be recorded in these databases. With this methodology, it will be possible to estimate the annual survival probability, the detectability, and the absolute abundance of the hawksbill turtle aggregation in the Playa Blanca and La Azufrada coral reefs of the Gorgona National Natural Park (GNNP).
CONCLUSIONS
This study contributes to the knowledge of the demographic characteristics of the hawksbill turtle aggregation that inhabits two coral reefs in the GNNP protected area in the EPO, presenting information necessary for the conservation and management of the species in the region. Evidently, the coral reefs of the GNNP are of great value as a foraging area for this species since they can maintain productive and healthy aggregations of hawksbill turtles based on the body condition index, which is close to the indices found in aggregations in the Caribbean. Also, the fidelity of these individuals to the coral reefs of the GNNP and their permanence for long periods during their development is observed. It is evident that these habitats are critical for the development of the juvenile hawksbill turtles of the Colombian Pacific. Since they spend a large part of their lives in neritic habitats, mainly on coral reefs, and efforts focused solely on nesting beaches may be insufficient (Lutz and Musick, 1997; Gaos et al., 2017; Llamas et al., 2017; Wildermann et al., 2018), the protection of anthropogenic mortality sources such as bycatch or boat strikes in the fishery around this region should be promoted for the recovery of the population.
On the other hand, a key component for the evaluation of the viability of the populations is the knowledge about their age of maturity to establish reasonable recovery periods in the management plans. This study presents data that shows the vulnerability of this species due to late maturity and slow growth in this aggregation of sea turtles in the EPO, which indicates that conservation efforts for this species must be long-term to ensure its recovery. Since this aggregation is made up of immature individuals and there are no growth data for size categories less than 30 cm or greater than 60 cm SCL, this extrapolation of growth to maturity should be interpreted with caution. For example, studies with freshwater turtles with known age reveal that the removal of small individuals from the analysis causes changes in the asymptote of the growth curve, which also modifies the estimation of the age of maturity (Bjorndal and Zug, 1995). The approach presented in this study on the age of maturity of hawksbill turtles in the GNNP is important as a baseline for future comparisons and also to strengthen management plans for the species in the EPO.











 text in
text in