INTRODUCTION
Acropora palmata and A. cervicornis are two species of branched coral whose populations have declined significantly throughout the Caribbean since the late 1980s, leading them to be included in the list of threatened species (Aronson et al., 2008a, 2008b). In Colombia, A. palmata is recorded as Endangered, and A. cervicornis as Critically Endangered (Ardila et al., 2002). The deterioration has been attributed to factors such as white band diseases (EBB) (Gladfelter, 1982; Aronson and Precht, 2001; Porter et al. 2001), Acropora serriatiosis (WPX) (Patterson et al., 2002), events of bleaching (Hoegh-Guldberg, 1999; Rodríguez-Ramírez et al. 2008; Navas-Camacho et al. 2010), as well as overfishing, coastal development and climate change (Buddemeier et al., 2004).
In the Colombian Caribbean, for the 1990s and early 2000s, the reduction of the populations of A. palmata and A. cervicornis was also recorded (Díaz et al., 1995, 2000; Garzón-Ferreira, 1997; López-Victoria and Díaz, 2000; Cendales et al., 2002), with mortalities in the Tayrona National Natural Park (TNNP) of up to 60 % for A. palmata and 80 % for A. cervicornis (Garzón-Ferreira and Cano, 1991). Garzón-Ferreira et al. (2004) evaluated these two species in 2001 in the TNNP regarding the location, extension, composition, and coverage of the coral community and other sessile organisms. For A. palmata, 29 formations were studied that had average coverage of 10 %, an area of 14 000 m2 of living tissue, while of 12 formations of A. cervicornis only four exhibited live colonies with an average coverage of 5 % for a living area of only 1200 m2. In general, within the formations of these corals, the algae presented coverage of 80%, followed by the stony corals. The results indicated that the populations of these two species still did not show signs of recovery.
In the Caribbean, in the last decade, assessments have been carried out to establish changes in the populations of the acroporids, mainly to have evidence of whether they have shown signs of recovery or, on the contrary, their deterioration has continued to increase. These works include 1) the construction of maps of potential habitats of the two species for Florida, Puerto Rico, and the Virgin Islands (Wirt et al., 2013, 2015), and 2) the evaluation of the state of the A. palmata populations along the Mesoamerican reefs (Mexico, Belize, Guatemala, and Honduras), of the 107 reefs studied, only 15 showed recovery and only in one an increase in coverage was observed. (Rodríguez-Martínez et al., 2014). In the reef system of Veracruz in the Gulf of Mexico, Larson et al. (2014) suggested recovery of A. palmata by finding healthy, abundant colonies with a wide distribution and greater density compared to other regions of the Caribbean. In Jamaica, after the bleaching event of 2005, A. palmata populations showed a recovery of 2 % to 22 % in 2008 (Crabbe, 2009). In the Virgin Islands between 2003-2010, Rogers and Muller (2012) found that acroporid serriatosis disease continues to be one of the main causes of deterioration in A. palmata. In Los Roques, Venezuela, Croquer et al. (2016) documented how 50 % of the original distribution of A. palmata has been lost due to massive bleaching, while in the reefs of Cuba Caballero-Aragón et al. (2020) concluded that A. palmata had a fair to poor status in about 90 % of the reefs.
Regarding population studies, as the acroporids are branched and fast-growing species, they are susceptible to breaking and fragmentation (Hughes and Connell, 1999), although this is a form of asexual reproduction and dispersal that allows rapid colonization of the substrate (Hughes et al., 1992; Hall and Hughes, 1996). The difficulty lies in determining demographic rates, which are based on growth, mortality, and fertility, factors depending on the size of the colonies (Hughes and Jackson, 1985; Soong, 1993), and it is not easy to distinguish if the small colonies came from partial mortality, recruit or have become fragmented. Therefore, variables such as bias and kurtosis are used as indicators of the preponderance in the distribution of small or large sizes, as well as the logarithmic transformation that is used to visualize the small size classes more finely and combine fewer size classes (Meesters et al., 2001). According to Bak and Meesters (1998), demographics can change with gradients and environmental conditions, which is reflected in the contrasting size distributions between different populations; For this reason, knowing the disturbance regime can help infer demographic processes. Hence, studies of the size structure of populations allow examining spatial, temporal, or taxonomic differences in life history, evaluating responses to disturbances such as bleaching, or evaluating age structure (Adjeroud et al., 2007; Crabbe, 2009; Anderson and Pratchett, 2014).
In Colombia, only one assessment of the status of the populations of the two Acropora species has been carried out: this was done 15 years after the loss of coverage began (Garzón-Ferreira et al., 2004). Taking into account the above, the present study, almost 20 years after the said assessment, presents information on the current state, coverage, condition, areas of the formations and also includes aspects of demography such as the size structure. These data are a baseline that can contribute to future studies that made it possible to estimate possible spatial and temporal changes of the populations of acroporids in the TNNP.
STUDY AREA
The study was conducted at the TNNP located on the north coast of Colombia between 11° 17′-11° 22′ N and 73° 53′-74° 12′ W. This includes several bays with coral reefs, pastures marine, mangrove forests, and sandy beaches (Garzón-Ferreira and Díaz, 2003). Detailed information on the climatic and oceanographic conditions of the area can be found in various publications (Andrade et al. 2003; Bayraktarov et al., 2014a, 2014b). The Acropora formations in the bays of Chengue, Gayranca, Nenguange, and Cinto were evaluated, and the formation of A. palmata located in El Torín on Aguja Island was included, which, although not previously evaluated, is part of the TNNP (Figure 1).
MATERIALS AND METHODS
Estimation of the areas of each formation
A reference map was made with the coral formations of A. palmata (FAP) and A. cervicornis (FAC) registered for the bays of Cinto, Nenguange, Gayraca, and Chengue in the TNNP by Garzón-Ferreira et al. (2004). The samplings were carried out between October 2016 and May 2018 and to evaluate the area of the populations of the two species, the same bays were visited and the geographical location of each formation was verified. The formations were delineated with buoys that were then georeferenced using a 3 m precision Garmin eTrex 20 GPS. In the small patches, direct measurements of the area were taken with tape measures. Additionally, missions were set up with a Phantom 4 drone to record the shallowest formations.
With the GPS points of the boundaries of each patch, the polygons that represented the estimated area of each formation were calculated; For this, the ArcGIS 10.4 geographic information system (GIS) was used. In the formations where visibility conditions are allowed, aerial images were used for the construction of orthophotomosaics using the Pix4D professional mapping program, with which the areas were calculated with greater precision.
Population structure, coverage, and condition
In each formation where living colonies were found, an assessment of their coverage, sizes, and condition was carried out. Depending on the size of each formation, between one and five 10 m linear transects (between 2 and 10 m deep) were arranged, and videos were made along the transect. To calculate the coverage of the substrate, the video record was analyzed using the Coral Point Count program. Initially, 20 images were captured at 5 s intervals, and each one was randomly assigned 50 points. Based on them, the coverage of the substrate components was estimated (Kohler and Gill, 2006). To assess whether there were differences in coverage of the components of A. palmata, macroalgae, and other coral species (except Acropora) between this study and that of Garzón-Ferreira et al. (2004) the Wilcoxon test of ranges was used for related samples, as the data did not meet the assumption of normality for the cover of A. palmata and macroalgae, while for the cover of other corals the paired t-Student test with the data transformed to arcsine, after fulfilling the normality assumption (Shapiro-Wilks). For A. cervicornis it was not possible to make comparisons as there was only one FAC of those previously recorded.
During each training, the largest diameter (L) and the diameter perpendicular to the largest diameter (l) of the highest number of single colonies was measured. The area of each colony was calculated by approximating an ellipse (A = (L / 2 × l / 2) × π; Yap et al., 1992; Linares et al., 2011). The difference in the size of the colonies (cm2) between bays was compared by the Kruskal-Wallis failure to meet the assumption of normality (Shapiro-Wilk P < 0.05) and the post-hoc Dunn test was performed. The size structure for each bay was examined through a frequency distribution table, with which the average size was used to calculate standard deviation and coefficient of variation (CV). Bias (g1) was used to describe the proportion of colonies that are smaller or larger than average and kurtosis (g2) to describe whether the frequency distributions are flatter peaks, more pronounced, or normal distribution (Anderson and Pratchett, 2014). Then the data were transformed to the logarithm (log10) for normal distribution and the resolution of small sizes increased (Meesters and Bak 1998; Vermeij and Bak 2003). To check whether the distributions came from a normal distribution, the Kolmogorov-Smirnov test was used with correction Lilliefors.
To evaluate the condition in terms of health in each formation, a video-photographic record was made on each colony. For A. palmata, healthy colonies, presence of diseases (WPX or EBB), damsel territories, evidenced as lesions on living tissue, and the presence of signs of predation by fireworms and snails were recorded. For A. cervicornis, healthy colonies, EBB, competition with macroalgae, damsel territories, signs of fireworm and snail predation, and bleaching were recorded. With the total number of colonies sampled, the incidence (%) of each type of condition in each of the formations was calculated.
RESULTS
Distribution and areas
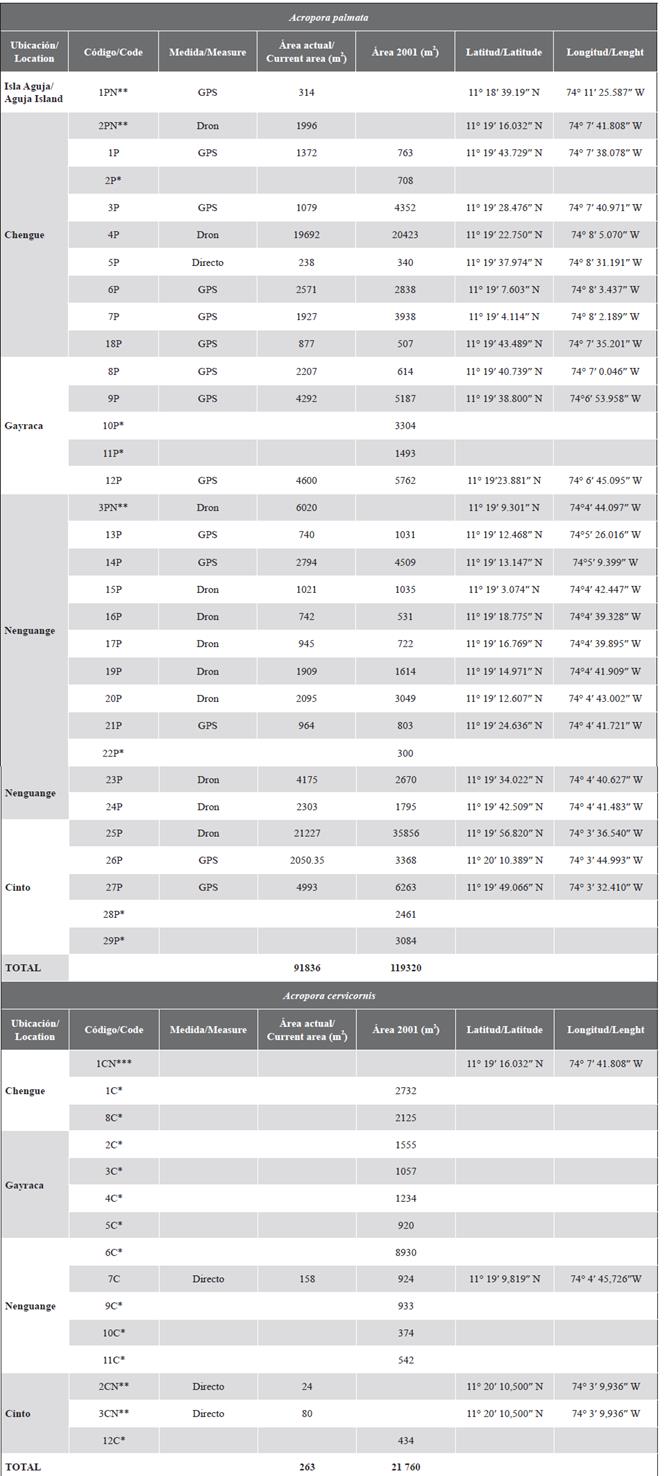
A total of 30 formations were evaluated, covering an area of 93 157 m2. Of the 29 FAPs described by Garzón-Ferreira et al. (2004), 23 were found with an area of 84 825 m2 and of the 4 FACs only one was found in Nenguange with 158 m2. Two additional A. palmata formations are included in the NTP; one in Nenguange with 6020 m2 and one in Chengue with 1996 m2, in addition to the A. palmata formation in Aguja Island in the El Torín sector with an area of 315 m2. A. cervicornis complete colonies were distinguished and two small formations are included in Cinto Bay with 24.0 and 81.0 m2 and in Chengue the presence of the species was georeferenced inside the bay, but they were small isolated colonies that do not constitute a formation. The area, location, and identification code of each training appear in Supplementary Material 1.
Taking into account only the comparison of the area for previously detected and still existing formations, the data, in general, show a reduction of the PAF in the bays of the TNNP of 34 494 m2 (28.9 %). However, an increase in area was obtained for nine formations equivalent to 5480 m2, while the reduction occurred in 14 formations (28 031 m2). Formations 4P in Chengue and 25P in Cinto continue to be the largest in the entire TNNP area. However, there was a significant reduction of 40.7 % in Cinto, while only 3.6 % in Chengue. For the FACs, the reduction in the general area was 99.3 %, and only the Nenguange 7C formation presented live colonies and went from 924 m2 to 158 m2 (83 % reduction).
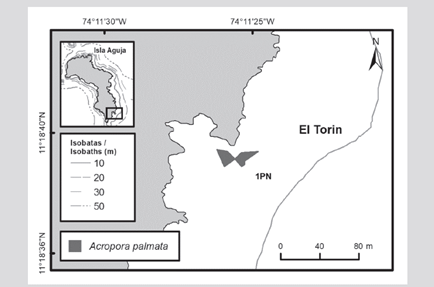
In the formation of the El Torín sector, southeast of Aguja Island, it is possible to find scattered colonies of A. palmata between 5 and 8 m deep. These continue until they reach the shallow part, very exposed to the impact of the waves at a depth of 1 m (Figure 2). In Chengue Bay, the 2P formation was not found, but an additional formation was recorded in the protected sector (3PN) at 2 m depth, which shares sectors with important patches of Millepora spp., Madracis decactis, and massive colonies such as Orbicella faveolata and Pseudodiploria strigosa (Figure 3). Formation 10P on the exposed side of the bay and 11P on the protected side were not found at Gayraca (Figure 4). In Nenguange there is still the highest number of formations (n = 10) of those registered by Garzón-Ferreira et al. (2004). In this bay it was not possible to locate the 22P formation, although the protected side formation is included, which it was called 3PN, considering that it could be part of the previously described 20P formation, but that according to the method used could be left out of the evaluation in 2001 (Figure 5). At Cinto, no two formations were found on the protected side (28P and 29P) and, although the 25P formation has been reduced, it still covers an important area (Figure 6).
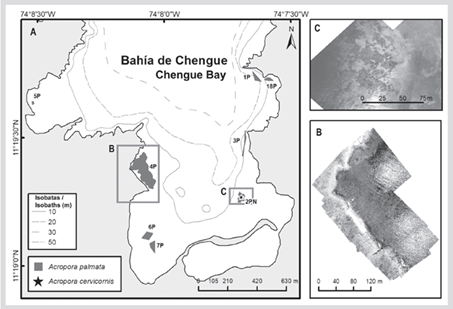
Figure 3 A) Location of Acropora palmata formations and isolated colonies of A. cervicornis in Chengue Bay, 2016-2018. B) Mosaic of aerial photographs to delimit 2PN (new patch of A palmata). C) Mosaic of aerial photographs to delimit 4P.
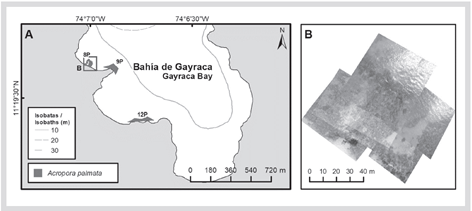
Figure 4 A) Location of Acropora palmata formations in Gayraca Bay, 2016-2018. B) Mosaic of aerial photographs to delimit 8P.
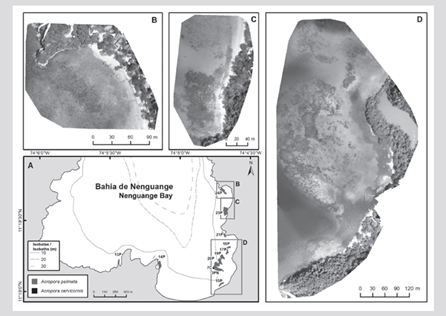
Figure 5 A) Location of the Acropora palmata and A. cervicornis formations in Nenguange Bay, 2016-2018. B) Mosaic of aerial frames to anchor 24P. C) Mosaic of aerial frames to anchor 23P. D) Mosaic of frames to anchor polygons 16P, 17P, 20P, 3PN and 15P.
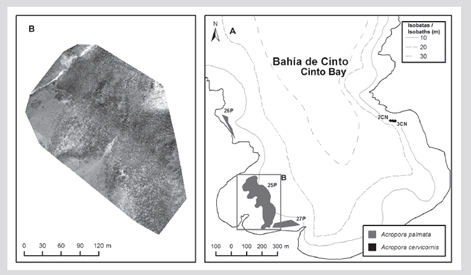
Figure 6 A) Location of the Acropora palmata and A. cervicornis formations in Cinto Bay, 2016-2018. B) Mosaic of aerial frames to outline 25P.
As for the FACs, only the 7C formation was found in Nenguange Bay (Figure 5). The registry made in 2009 of training in good health is included, in 2010 with a whitening registry and in 2011 its deterioration was already observed (Figure 7).
Acropora palmata cover
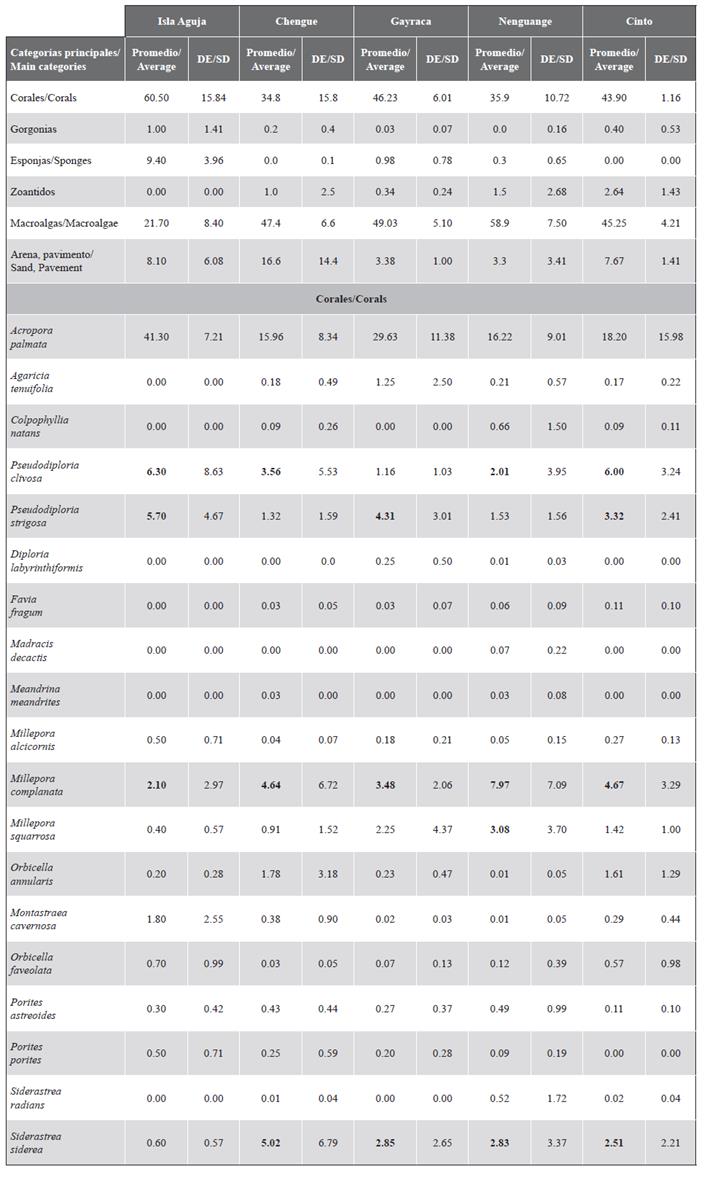
The average coverage (± standard deviation) of A. palmata in the TNNP was 19.9 ± 9.2 %; macroalgae 52.0 ± 11.1 % and corals in general 19.1 ± 11.6 %. The lowest coverage was found in the Chengue 6P formation with only 1.8 % and the highest in Gayraca 8P with 42.3 % (Figure 8). Of the 23 FAP in which it was possible to compare the A. palmata coverage with respect to the 2001 valuation, only in five was no increase in coverage observed, highlighting the greatest loss in 6P (64.0 %), 4P (50 %), and 5P (50.0 %) in Chengue Bay. The analysis of the 23 FAPs compared showed a significant increase in average coverage (Wilcoxon test T23 = 2.49; P = 0.012) of 11 % in Garzón-Ferreira et al. (2004) to 19.9 % in this study. Macroalgae showed a significant decrease in all PAFs except for 8P in Gayraca. The difference in total was 27 % compared to 2001 (Wilcoxon T23 test = 3.92; P < 0.001). Regarding the coverage of the other coral species, the increase was 12.8 % and was seen equally in all the FAP except for 6P in Chengue and 16P in Nenguange (t-Student test for paired samples t23 = 5.491; P < 0.001) (Figure 8). The main species with covers of more than 2 % were Millepora complanata, M. squarrosa, Siderastrea siderea, Pseudodiploria strigosa and P. clivosa (Supplementary Material 2).
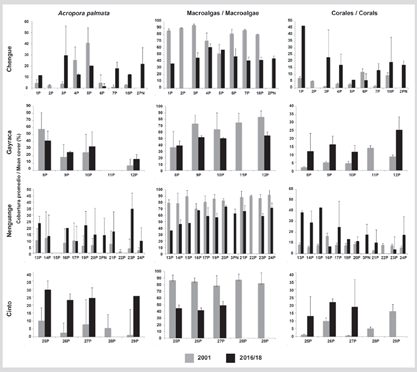
Figure 8 Average coverage (%) + DE of the main components of the substrate (A. palmata, macroalgae, and other corals) in TNNP between 2001 (Garzón-Ferreria et al., 2004) and 2016/18.
Regarding the formations registered as additional to the interior of the TNNP bays, the 2PN formation in Chengue presented a percentage of A. palmata, corals, and macrolagas of 21.0, 17.5, and 44.7 %, respectively, while in Nenguange the 3PN formation presented values of 14.3, 17.8 and 63.1 %, respectively (Figure 8). For the El Torín sector in Aguja Island, the 1PN formation presented coverage of A. palmata of 41.3 %, macroalgae of 31.1 %, and other corals of 19.2 %, the latter composed mainly of the Pseudodiploria clivosa and P. strigosa species.
Population structure of Acropora palmate
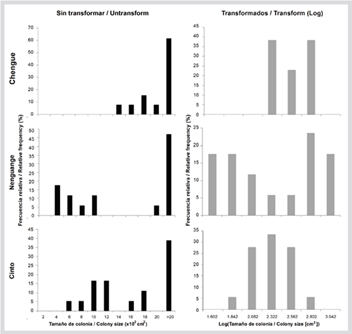
A total of 606 colonies were measured. Colonies > 10,000 cm2 represented 32.0 % of the colonies in the entire TNNP. In the bays this percentage ranged between 25.0 and 57.3 %, being Aguja Island where the highest proportion of large colonies was found. Small colonies (< 100 cm2) were found in all bays, except for Cinto bay (Figure 9). There were significant differences in the size of the colonies between the different bays (Kruskal-Wallis H3 = 30.2 P < 0.001) and Dunn’s test by pairs showed Aguja and Cinto islands as the sites where the largest colonies were found, and Nenguange, Gayraca, and Chengue as similar, with colonies in all length intervals (untransformed data; Figure 9).
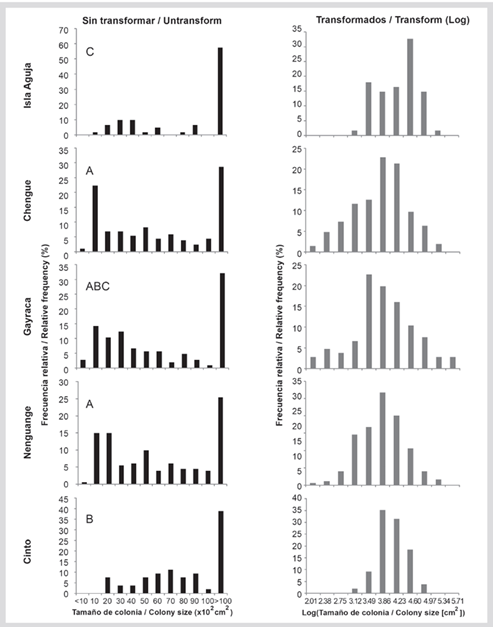
Figure 9 Frequency distribution (%) of Acropora palmata for the untransformed data and logarithm transformed in base 10 in the bays of TNNP. Untransformed data above indicate the result of Dunn’s post hoc analysis of colony sizes.
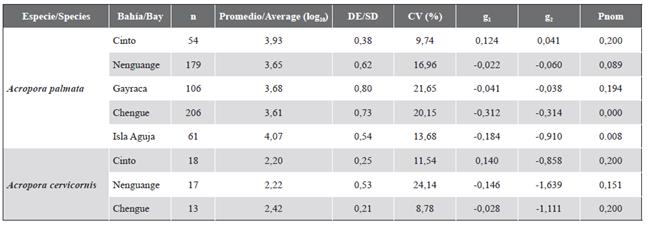
The logarithmically transformed length data were distributed in 11 class intervals, between 1.74 and 5.71, and were normally distributed in the bays of Cinto, Nenguange, and Gayraca. In Chengue and Aguja Island, the sizes did not show a normal distribution (Table 1). Although large colonies were found in all bays, the highest percentage was found on Aguja and Gayraca islands (16.3 and 13.2 %, respectively). Small colonies (i.e. in the first three-class intervals) were present in Nenguange 1.7 %, Chengue 6.3 %, and Gayraca 7.5 % and absent in Cinto and Aguja Island (Figure 9). The negative bias was dominant in all bays with values between -0.022 and -0.312, indicating the predominance of large colonies. For the bay of Cinto, the bias was positive (0.124) although close to the mean value, and with the lowest coefficient of variation (9.75 %), which is reflected in colonies of intermediate size. The kurtosis was mainly negative except for the Bay of Cinto (0.041). In the rest of the bays, the coefficient of variation (CV) ranged between 16.7 and 21.7 % (Table 1).
Table 1 Statistical summary of length frequency distribution data for Acropora species in the TNNP. Conventions: n = number of colonies. log10 = average value of the size of the colonies after transformation to logarithm. SD = standard deviation. CV = coefficient of variation. g1 = bias. g2 = kurtosis. Pnom (Kolmogorov-Smirnov test with Lilliefors adjustment) = probability that the data present a normal distribution.
Acropora palmata condition
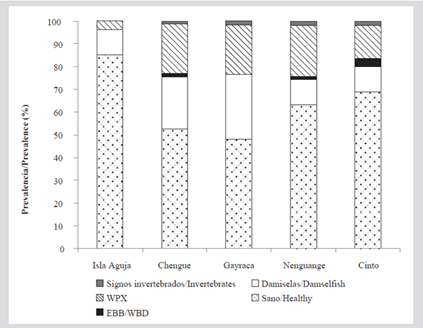
A total of 595 colonies were evaluated, 58 % were healthy, 20 % with WPX lesions, 19 % with damsel territories, and 3 % with signs of predation by worms, snails, and EBB. On Aguja Island, the highest number of healthy colonies (85 %) was observed, being less within the bays and oscillating between 48 % in Gayraca Bay and 69 % in Chengue Bay. A lower frequency of affectations was also observed in Aguja Island with 10 % of the colonies with damsel territories and 3 % with WPX, while in the four bays signs of predation by invertebrates were observed with low frequency (between 1.2 and 1,9%). EBB was observed in Chengue, Nenguange, and Cinto with less than 4 % frequency, while more than 10 % of the colonies presented damsel territories and WPX disease (Figure 10).
Acropora cervicornis cover
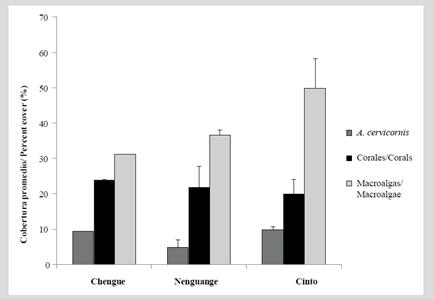
The total average coverage of A. cervicornis of the TNNP of the previously described FACs, plus the additional formations located in the Bay of Chengue and Cinto, was 8.0 ± 1.6 %. Macroalgae covered 39.1 ± 4.9 % and corals 21.9 ± 5.0 % of the substrate (Figure 11). The only FAC in which it was possible to compare the coverage with respect to 2001 was in Nenguange (7C), where a reduction of 69.6 % for A. cervicornis and 51.2 % for macroalgae was found, while in other corals there was found an increase of 62 %, constituted by the species Agaricia tenuifolia, Pseudodiploria strigosa, Madracis decactis, Millepora complanata, Montastraea cavernosa and Siderastrea siderea with more than 1 % coverage. In the other two FACs in Nenguange (6C and 9C) only dead skeletons were recognizable. The new formations for the Bays of Cinto and Chengue presented the live cover of A. cerviconis with 9.6 and 9.4 % respectively, 19.9 and 24.0 % of other corals and 49.7 and 31.2 % of macroalgae.
Population structure of Acropora cervicornis
Only 48 colonies were measured, which had sizes between 23 and 890 cm2. The only FAC found among those registered by Garzón-Ferreira et al. (2004) was the 7C in the Bay of Nenguange, mainly composed of small size colonies (47.0 %, n = 8); the rest were large colonies (53.0 %, n = 9). In this bay a restoration initiative for this species has been developing; however, these colonies were not taken into account because they came from fragmentation and were in the process of transplantation. The new formations evidenced at Cinto and Chengue consisted of large colonies at Chengue, and colonies with a broader size distribution at Cinto (Figure 11). The Kruskal-Wallis analysis did not show significant differences in the size of the colonies among bays (H2 = 3.43, P = 0.179).
The sizes of the logarithmically transformed colonies were distributed in seven class intervals, between 1.36 and 3.04, and were normally distributed in the three bays (Table 1). Nenguange and Cinto had a wide size distribution, while Chengue had colonies of intermediate size (Figure 12). The negative bias in Chengue and Nenguange with g1 values between -0.028 and -0.146 indicates the predominance of large colonies. For Cinto Bay, the bias was positive (g1 = 0.140), which indicates the predominance of small colonies. The coefficient of variation was higher in Nenguange (24.1 %) and lower in Chengue (8.8 %) and the kurtosis presented negative values in the three bays (Table 1).
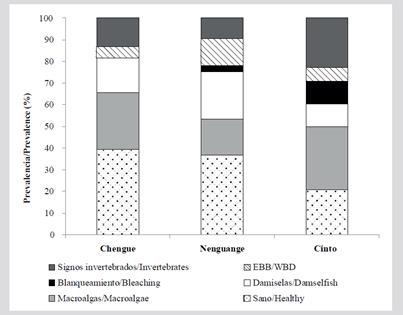
Acropora cervicornis status
A total of 159 living colonies were evaluated in Bays of Chengue (n = 38), Cinto (n = 48) and Nenguange (n = 73). Of these, 33 % were in good health, 23 % had macroalgae, 17 % had damsel territories, and 14 % showed signs of predation by invertebrates. Finally, 13 % of the colonies presented EBB or bleaching. By bays, Cinto had the lowest percentage of colonies in good health (21 %), as well as the highest prevalence of colonies with macroalgae (29 %) and with signs of predation by invertebrates (23 %). The Nenguange Bay was the one that presented the greatest involvement of the EBB (12 %) and together with Cinto they presented whitening, but this was not observed in Chengue (Figure 13).
DISCUSSION
The mortality of Acropora species throughout the Caribbean was recorded since the early 1980s. To date, few studies have documented the progressive recovery or loss of populations of these species. Therefore, the study carried out by Garzón-Ferreira et al. (2004) constitutes a reference that allows, together with this study, to know comparatively the change that they have suffered and the current state of the populations of Acropora palmata and A. cervicornis in the TNNP.
Although the estimation of the areas was carried out with different methods, aerial photography allows estimating the area, and like any method, there is always a margin of error regarding the size that an ecosystem occupies; However, an approximation of the potential areas of the very good resolution was obtained, mainly of the A. palmata formations, as they are shallow habitats. Selgrath et al. (2016) recommend the use of remote sensors when better precision is desired. In addition, Ekebom and Enkkilä (2003) mention how the resolution of aerial images provides sufficient information for reliable identification of the habitat. For A. palmata, Garzón-Ferreira et al. (2004) recorded an area of 119 319 m2 and for A. cervicornis of 1200 m2. The data in this study show that these formations have suffered a reduction of 30 % and 99.3 %, respectively.
Although there has been a general reduction in the area occupied by A. palmata, this species continues to cover a significant area in the TNNP. The reduction is due to the decrease in the size of 14 formations and the fact that six were not found in the 2001 evaluation (Garzón-Ferreira et al. 2004). However, nine formations showed an increase in their area, which added to the inclusion of the 20P formation in Nenguange (which was not taken into account in 2001), and two new formations (one in Chengue and the other in Aguja Island), they gave rise to a total area of almost 85 000 m2 of A. palmata in the TNNP compared to the 119 319 m2 estimated in 2001 (Garzón-Ferreira et al. 2004). Although a reduction in coverage was found in some individual FAPs, the trend in most formations was upward, with an overall coverage from 17.0 % in 2001 to almost 20.0 % in this study. Likewise, the macroalgae that covered 80 % of the substrate went to 50 %. Vega-Sequeda et al. (2008) also registered lower macroalgae coverage: 32.4 % in Gayraca and 67.4 % in Cinto. Corals increased by 12.8 % with respect to that reported by Garzón-Ferreira (2004), and their composition, taking into account the species with greater coverage, has remained stable because both M. complanata (3.5 and 7.9 %), S. siderea (2.5 and 5.0 %) and P. strigosa (1.2 and 8.6 %) continue to be the species that remain the main components of FAP. However, it would have been expected that M. complanata, having short larval periods (Lewis, 1989), or Porites astreoides, with a self-fertilization strategy (Doropoulus et al. 2015), would have continued to colonize the available substrate, as has been seen in reef ridges of Cuba (Caballero-Aragón, 2020), but in the TNNP both species have been reduced.
The frequency of large colonies (> 10 000 cm2) of A. palmata in the TNNP between 25.0 and 57.3 % is high compared to other studies that have addressed the distribution of length frequencies at the regional level. This is very important, taking into account that the three-dimensional structure of the colonies of this species brings complexity and ecological functionality to the ecosystem. For example, it is expected that these colonies have a high reproductive potential, since they are fertile from 1000 cm2 (Soong and Lang, 1992), and in the TNNP 79.8 % had sizes greater than 1000 cm2. Although distinguishing new recruits is difficult, small colonies were observed colonizing available spaces. Also, the normal distribution of the data transformed to logarithm, and the variables of skewness and negative kurtosis show a good state of the population, with a possible faster transition of length classes as a result of its rapid growth. Knowlton (2001) mentioned that for a population to recover, it needs to be dominated by healthy colonies characterized by a wide size-frequency distribution, as was the trend found in this study.
The result of large sizes can also be due to the disturbance regime to which the reefs are subjected. Gardner et al. (2005), who modeled the information of 286 reefs throughout the Caribbean between 1980 and 2001, found how 177 sites experienced the impact of hurricanes with a reduction of coverage in average of 17 %. This leads to the fragmentation of colonies and the generation of small colonies. Vardi et al. (2012) evaluated the populations of A. palmata in the Florida Keys between 2004 and 2010, observing the reduction in coverage, colonies of sizes between 100 and 900 cm2 (≈ 32 %), and dominance of small colonies < 100 cm2 (56 %). Although the passage of hurricanes along the continental coast of the Colombia Caribbean is very infrequent (Ortíz-Royero, 2007), in Santa Marta there have been hurricanes like Matthew in October 2016. Although this generated the breakdown of colonies, such as was observed in Aguja Island, there were no coverage data or the length-frequency distribution that would allow evaluating the effects of this event. However, the data from this study show the resilience of this population of A. palmata, since the assessment in 2018 shows large colonies and a quick recovery.
Regarding the condition of the A. palmata populations, its affectation by the common predators described for the species in the Caribbean stands out (Knowlton et al., 1990). Although the formations are in good health, a significant proportion of colonies show the establishment of damselfish (Stegastes spp.) Territories, evidenced by the lesions in the form of “chimneys” on the surface of the coral tissue (Kramer et al., 2008). According to Ceccarelli et al. (2005), this may be negative, given that territorial fish (damsels) exclude herbivorous browsing fish, and in their absence increases the biomass of macroalgae with a consequent decrease in coral tissue. Although this study showed fewer macroalgae coverage, it is advisable to continue carrying out studies evaluating this effect on the TNNP FAP.
WPX disease has been postulated as one of the main causes of Acropora population loss in the Caribbean (Bruckner and Bruckner, 1997; Rodríguez-Martínez et al., 2001; Patterson et al., 2002). In the TNNP several colonies presented the disease; however, these lesions affected < 10 % of the living coral tissue, and during the study its reduction and in some cases its disappearance was observed, which coincides with Rogers and Muller (2012) who observed how A. palmata recovers from lesions of WPX in a time of 1.7 months. The colonies of the TNNP are influenced by the decrease in temperature in the period of upwelling between December to April (Bayraktarov et al., 2014b) and as proposed by Muller and van Woesik (2014), the severity, frequency, and recovery of this pathology are related to thermal anomalies, which poses a favorable scenario, since these temperature drops can give it an advantage in population health.
In contrast to A. palmata, the situation is dire for A. cervicornis. For this species, although 12 formations had been registered and not all with living cover, the criteria for its definition was given by the presence of recognizable skeletons. To date, only one formation of this type has been established with a reduction in 83 % of the area. Despite this, two different formations were located in Cinto Bay and the presence of isolated colonies in Chengue. In Nenguange, initiatives are carried out by the National Natural Parks Unit of Colombia to repopulate the species; however, survivorship records are required to provide information on the success of the program. The reduction in coverage was also significant. The only site where it was quantified was in Nenguange, which went from 40.5 % (Garzón-Ferreira et al., 2004) to 4.8 %, while the new formations in Chengue and Cinto did not reach 10 %. The trend of reduction of macroalgae and increase of corals was similar to that described in A. palmata and these formations were also composed of M. complanata, Orbicella annularis, Colpophillia natans, Diploria labyrinthiformis, and P. strigosa, being species commonly present in the formations. evaluated by Garzón-Ferreira et al. (2004).
The number of colonies found was reduced in the three bays where A. cervicornis was found, represented by large colonies and no evidence of recruits. In general, for the entire Caribbean, this species has shown signs of further deterioration and significant losses have been evidenced by bleaching events and diseases. In the Santa Marta region, Rodríguez-Ramírez et al. (2008) in the TNNP in 2003 mentioned an incidence of bleaching of 2.9 % and in Chengue Bay the National Coral Reef Monitoring System (SIMAC) in 2010 recorded about 18 % of bleached corals. The temperature has been attributed as the main cause of deterioration; however, Romero-Rodríguez et al. (2014) incorporated different environmental variables in data series between 1998 and 2010, and found that in addition to temperature, continental discharges influence bleaching. For 2005 (between September and November) and 2010 (August, November, and December), bleaching was recorded. Although there is no evidence of deterioration of the species in 2005, the time series shows how a formation in good condition in 2009 passed in 2010 to present whitening and in April 2011 to practically disappear. These records are evidence of deterioration, however, many more combined effects have led to population declines.
When the condition of A. cervicornis was evaluated, the average of colonies with territories of Stegastes spp. was 17 % and with macroalgae 22 %. This may be the result of the territorial behavior of damselfish, which excludes the browsing reef herbivores (Ceccarelli et al., 2005), and which increases macroalgae (Hoey and Bellwood, 2010). Mejía-Niño and Garzón-Ferrerira (2003) already showed in the TNNP how from the evaluated alga-coral interactions, leafy algae predominated, causing physical damage by abrasion; however, there are no direct evaluations in this regard that can give indications if these interactions are in the causes that have compromised these populations. In this regard, Schopmeyer and Lirman (2015) documented the prevalence of damselfish and the negative effects of algal grasses on A. cervicornis along with the Florida Keys. The results showed how the occupation by fish was the main cause of mortality (34.6 %) in those colonies evaluated with signs of stress, compared to diseases (1.6 %), algae/sponge coating (5.6 %), or predation by corallivorous invertebrates (7.9 %). The presence of the snail Coralliophila erosa and other invertebrates, as well as the resulting injuries, were evident in this study (14.5 %) and, although Schopmeyer and Lirman (2015) obtained similar results and suggest that the decrease in snail predation is due to the presence of damselfish, it is necessary to evaluate this in the medium and long term to obtain evidence on the impact on population health. The reported predators contribute to the population decline of A. cervicornis, which, added to its low coverage, demonstrates the critical condition of this species in the TNNP.
In general, although EBB was not such an evident condition, its manifestation may be due to the increase in pathogens that may be harbored in macroalgae as demonstrated by Sweet et al. (2013). These authors suggest that algae and corals host different prokaryotic groups and that potentially pathogenic groups for corals reside within the microbiome of algae. However, Nugues et al. (2004) mentioned that there must be physical stress as a prerequisite for there to be a transmission of pathogens between algae and coral. During this study, the temperature was not recorded, and, since the TNNP area is subject to the seasonal dynamics of upwelling of relatively cold waters (Bayraktarov et al., 2014b), the periodic measurement of some variables is required, which for the critical case of A. cervicornis, can be taken into account in restoration programs and allow decisions to be made that can help with its maintenance.











 text in
text in