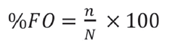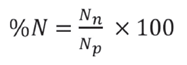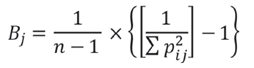INTRODUCTION
The Magdalena River Estuary Delta System Ramsar Site of Ciénaga Grande de Santa Marta Lagoon Complex (CGSM LC) is home to high concentrations of both resident and migratory waterfowl, which is why it has been categorized as an area of importance for bird conservation. (Ruiz-Guerra et al., 2008; BirdLife International, 2018). There are two national protected areas administered by National Natural Parks of Colombia: the Vía Parque Isla de Salamanca (VIPIS) and the Ciénaga Grande de Santa Marta Flora and Fauna Sanctuary (CGSM FFS).
The CGSM LC has been affected since the late 1960s by human actions both in its interior and in its tributaries, generating a hydrological imbalance in the system, the loss of mangrove forest, and the reduction of fish, mollusk, and crustacean populations; which play a fundamental role in the functioning of the ecosystem and sustaining artisanal fishery, on which different local populations depend (Botero and Salzwedel, 1999; Sánchez-Ramírez and Rueda, 1999; Lorenz and Serafy, 2006). Although water rehabilitation works have been carried out, conditions continue to appear within the system (Invemar, 2019) This situation is perceived by local inhabitants and by different actors linked to the system as the cause of a decrease in approximately half of the ecosystem services of the wetland, which warrants greater conservation efforts (Vilardy et al., 2012; Invemar, 2019).
Trophic ecology studies are crucial for the conservation of threatened ecosystems because they provide important information on the functional role of species and their habitats (Bó et al., 2007). Among the most important aspects of trophic ecology is the dietary composition, the partition of trophic resources, and the breadth of the food niche, since they allow increasing knowledge about the trophic interactions of a predator with its prey and its implications for conservation and preservation of species and the ecosystem (López-Calleja, 1995; Bó et al., 2007). These aspects may vary with the abundance of prey, habitats, and/or geographic sites and are modeled with the optimal foraging theory, which predicts an increase in niche width and prey diversity versus a reduction in the availability of their most optimal prey, and greater specificity by increasing their availability (Valdovinos et al., 2010; Román-Palacios and Román-Valencia, 2015). In cormorants, trophic ecology studies provide the basis for understanding their role in aquatic ecosystems, their influence on the fish community, and their interaction with fishing populations (Dias et al., 2012).
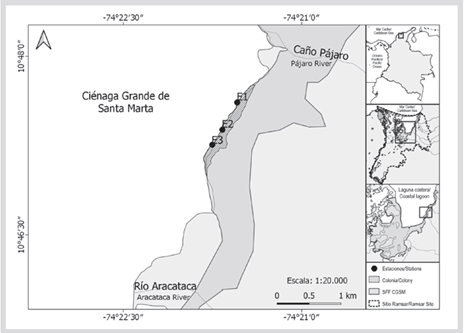
The main reproductive colony of the Neotropical cormorant (Nannopterum brasilianus) from Colombia is located in the CGSM LC, northwest of the CGSM FFS (Figure 1). It is estimated that this colony can exceed 30 000 individuals (Ruiz-Guerra et al., 2008; Ruiz-Guerra et al., 2012). Previous trophic studies have determined that N. brasilianus is a generalist and piscivorous bird, which feeds by diving in marine, freshwater, and estuarine environments (Gil de Weir et al., 2011; Conde-Tinco and Iannacone, 2013). This species eliminates the parts of the prey that it cannot digest, regurgitating pellets generally once a day, just before dawn or going out to forage (Orta, 1992; Zijlstra and Van-Eerden, 1995).
The cormorant has high plasticity that allows it to adapt to the availability of prey, observing cases such as Dos Patos Lake (Brazil) where it is a generalist who presents a breadth of specialist niche during part of the year, a condition that Barquete et al. (2018) called “temporal specialization”. To date, benthic or mesopelagic bony fishes of the Ariidae, Engraulidae, Sciaenidae, Mugilidae, and Gerreidae families have been recognized as part of the diet of the Neotropical cormorant (Barquete et al., 2008; Muñoz et al., 2008; Gil de Weir et al., 2011). The only antecedent of the N. brasilianus diet in the CGSM LC was made by Hennig (1997), whose study coincided with the rehabilitation actions in the lagoon complex in 1995-1999 and the massive fish death events of 1994-2000.
Knowledge about the trophic ecology of N. brasilianus within the CGSM LC is important considering its population size and its role as a fish predator which could give it an influence on the structure and function of wetlands (Smith and Smith, 2001; Begon et al., 2005). The present study aims to provide information on the trophic ecology of N. brasilianus during its nesting period in the CGSM LC in 2017. For this, the taxonomic composition of the diet, the breadth of the food niche, the richness of prey, the trophic diversity, and prey fairness; by minimally invasive pellet analysis. The results obtained were analyzed in the context of previous studies carried out in Latin America.
STUDY AREA
The reproductive colony of N. brasilianus is located in the northeastern sector of the CGSM FFS, between the mouth of the Aracataca River and the mouth of Caño Pájaro (Figure 1). The climate of the CGSM LC is tropical arid since the action of the trade winds north of 10 °N is sufficient throughout the year to greatly inhibit precipitation, with average annual temperatures between 27° and 30 °C in the water column and relative humidity between 50-100 % (Andrade-Amaya, 2000; Blanco et al., 2006). The annual rainfall regime varies between 401 and 1321 mm, with an average of 897 mm/year, which is distributed in a unimodal climatic pattern with a period of winds between December and April, and a period of calm, in which rainfall from May to November, with maximum rainfall between September and November (Blanco et al., 2006; García et al., 2013).
MATERIALS AND METHODS
Field phase
The sampling was carried out weekly between May (four weeks) and June (two weeks) of 2017, coinciding with the reproductive period of N. brasilianus in that year. Three sampling stations were visited between 9 and 11 a.m., previously selected for their accessibility and availability of material to collect, avoiding stressing the birds as proposed by Olmos et al. (2000) and Petraci et al. (2009). The pellet collection was performed manually from mangrove branches, leaves and roots, with the effort of two collectors for 30-35 min per site. The pellets were stored dry in hermetically sealed bags, externally labeled with adhesive seals and internally with parchment paper labels, and were refrigerated for less than two hours until they were taken to the laboratory for processing.
Laboratory phase
The pellets were washed with 70 % ethanol, disintegrated with the help of forceps and lancets to separate the bone remains such as jaws, otoliths, vertebrae, and other remains of the material. The bone structures found were washed and stored in 70 % ethanol (Olmos et al., 2000). The bony fish otoliths were used for the identification of the fish component down to the lowest possible taxonomic level using different identification guides (Abilhoa and Correa, 1992; Volpedo and Echeverría, 2000; Hernández-García et al., 2004; Martínez et al., 2007; Espino-Barr et al., 2013) and the Anàlisi de FORmes d’Otòlits (AFORO) and COSS-BRASIL (Lombarte et al., 2006; Rossi-Wongtschowski et al., 2016). The otolith characterization work by Rossi-Wongtschowski et al. (2016) was used for the description of the sagitta-type otoliths. The description of asteriscus-type otoliths was based on Assis (2003) and for lapillus-type otoliths it was based on Assis (2005). For descriptions of the otoliths, a Leica M205A stereomicroscope was used and they were photographed with an integrated Leica DFC450 camera.
Analysis of data
To evaluate the representativeness or completeness of the sample, estimated as the sampling coverage according to Chao et al. (2014), the estimator (Ĉind). This analysis was carried out using the iNEXT statistical package, in the RStudio V.1.3.959 programming environment (Hsieh et al., 2016; RStudio, 2020). For this, each pellet was taken as the sampling unit, assuming that each bird generally throws one pellet in the morning before foraging (Orta, 1992; Zijlstra and Van-Eerden, 1995). To determine the food composition of the diet, two relative measures of prey quantification were used, detailed by Hureau (1970) and Hyslop (1980) for analysis of stomach contents:
(1) Frequency of Occurrence (%FO): expresses the percentage of occurrence of each of the dams (Hyslop, 1980) and is calculated as:
Where,
n is the number of pellets in which prey i was present, and
N is the total number of pellets observed.
(2) Numerical Frequency (%N): explains the numerical frequency of each prey present in relation to the total number of individuals of all prey and is calculated by:
Where,
N n is the number of individuals from prey i found in the pellet j, and
N p is the total number of individuals from all prey in pellets.
On the other hand, to calculate the breadth of the trophic niche, the Standardized Levins index (B j ) was used, based on the measurement of uniformity in the individuals consumed. The values of B j oscillate between 0 and 1, with values higher than 0.6 typical of generalist species, while lower values correspond to specialist species (Krebs, 1989). Its equation is:
Where,
n is the number of prey items consumed by the species, and
p is the probability of finding the item (i) in the sample (j).
Diet species richness, trophic diversity, and prey fairness were calculated with Hill numbers. These are a measure of diversity that allows a unified and intuitive interpretation in effective species units based on relative abundance data (Jost, 2006). To estimate the Hill numbers, the model proposed by Chao et al. (2014) was used for incidence frequency data included in the “iNEXT()” function of the iNEXT package (Hsieh et al., 2016). Hill numbers present an exponent q that determines their sensitivity to incidence frequency, where q = 0 is equivalent to species richness, q = 1 to diversity, and q = 2 equitability (Hill , 1973; Chao et al., 2014).
RESULTS
76 pellets were collected, in which remains of fish, mollusks, and crustaceans were found (Figure 2). An average of 4.4 ± 2.8 fish per pellet was calculated (Figure 2). Sample coverage (Ĉind = 0.94) points out that the results are representative of the diet of N. brasilianus for the period May-June 2017, with a confidence interval of 0.95. Thus, the probability of finding a new species if a new pellet were added to the sample is 0.06 (1-Ĉind) (Figure 3).
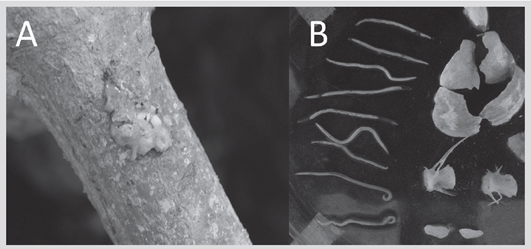
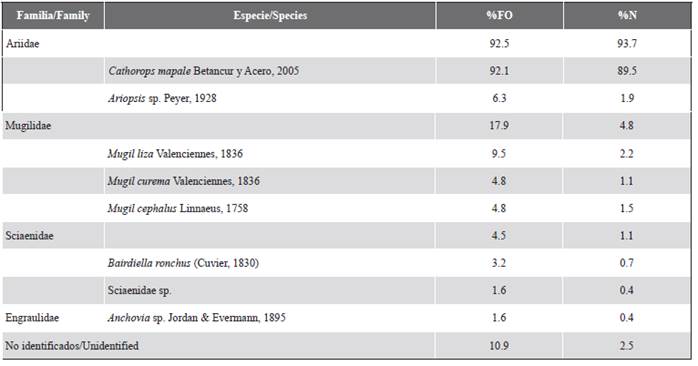
Figure 2 A) Nannopterum brasilianus pellets on a root of Rhizophora mangle, L. B) Hard structures of N. brasilianus prey and parasites that were observed in a laboratory-processed pellet.
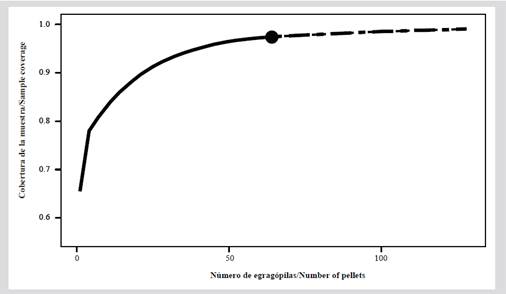
Figure 3 Sampling curve based on the rarefaction (solid lines) and extrapolation (dotted lines) of the coverage of the pellet sampling of Nannopterum brasilianus in the Punta Blanca colony, with 95 % confidence intervals (shaded areas) collected in May and June of 2017.
In the pellets, remains of undigested material from three groups were found: i) crustaceans, represented by an exoskeleton; ii) mollusks, represented by a bivalve valve, and iii) teleost fish, represented by spines, vertebrae, fins, skulls, and otoliths. Of the total of pellets collected, otoliths were found in 64 (84.2 %) of them, and in total, eight morpho-species were identified from the otoliths: five at the species level, two at the genus level, and one at the family level (Figure 4).
Figure 4 Otoliths are found in pellets of Nannopterum brasilianus. (1-2) Cathorops mapale lapillus-type otolith. (3-4) Lapillus-type otolith of Ariopsis sp. (5-6) Sagitta-type otolith of Anchovia sp. (7-8) Mugil liza sagitta-type otolith. (9-10) Sagitta-type otolith of Mugil curema. (11-12) Mugil cephalus sagitta-type otolith. (13-14) Bairdiella bronchus sagittatype otolith. (15-16) Sagitta-type otolith of Sciaenidae sp. The otoliths are arranged with the dorsal edge towards the top of the image, except for images 8 and 10 where the dorsal edge is disposed downward. The anterior region is indicated by the letter (A).
In the frequency of occurrence, the Ariidae family presented the highest values (92.5 %FO), followed by Mugilidae (17.9 %FO). At the species level, Cathorops mapale (Ariidae) stood out for its presence in a large part of pellets (92.1 %FO) (Table 1). Regarding the numerical frequency, a total of 275 prey individuals were observed in the samples. Most belonging to the Ariidae family, followed far behind by Mugilidae and Engraulidae (93.7, 4.9, and 0.4 %N, respectively). The species with the highest numerical frequency was C. mapale (89.5 %N), followed by Mugil liza (Mugilidae) with 2.2 %N. The niche width for Nannopterum brasilianus presented a specialist behaviour of 0.02. While the richness of species in the diet (q = 0) was an effective number of 12 equally frequent species, trophic diversity (q = 1) was 3.9 and fairness (q = 2) was 2.1.
DISCUSSION
The completeness of the samplings is an important parameter when making comparisons between different studies (Chao et al., 2014). However, the researchers reviewed that the diet of N. brasilianus in Latin America does not require this information (Table 2). In this sense, the results obtained in the present study can serve as a baseline for future investigations that wish to evaluate spatial and temporal differences in the trophic ecology of the species with greater certainty, especially when you have limited periods or spatial coverage as in this study.
Table 2 Fish families found in the diet of the Neotropical cormorant in other regions of South America and the Ciénaga Grande de Santa Marta (CGSM). %N: numerical frequency. %FO: frequency of occurrence.
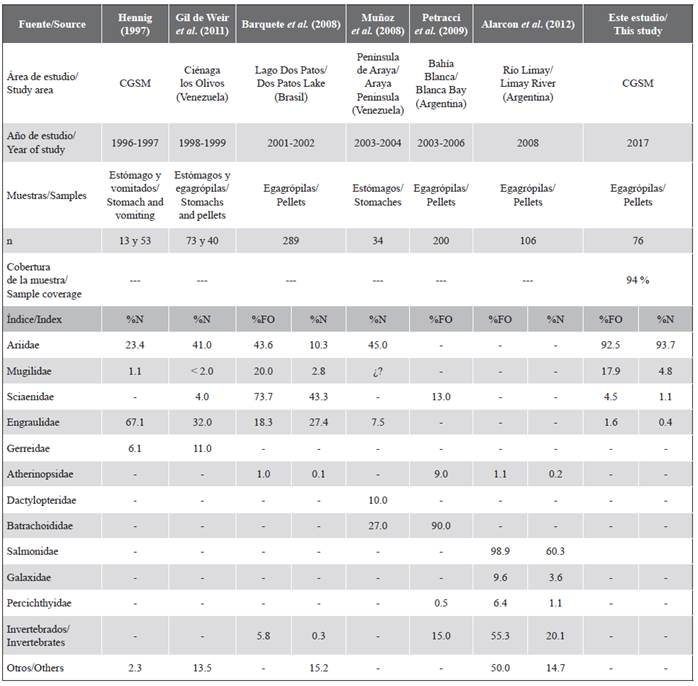
The diet of N. brasilianus was represented by benthic and demersal fish, while mollusks and crustaceans were considered as indirect ingestion, as reported in other studies (Regidor and Terroba, 2001; Barquete et al., 2008; Muñoz et al., 2008; Petracci et al., 2009). The Ariidae family was represented by two species in the cormorant diet, which represented almost all of them both in the frequency of occurrence and in numerical frequency. This family of fish has benthic life habits, ideal for the type of capture by diving, characteristic of cormorants (Mejía-Ladino et al., 2002; Barquete et al., 2008). The results corroborate the importance of this family of fish in the diet of the Neotropical cormorant, reported in other tropical countries such as Brazil and Venezuela (Table 2).
The relatively high record of C. mapale in the diet of the Neotropical cormorant during this study coincided with the high fishing landings recorded for C. mapale in the CGSM LC during May 2017, when it was the second most captured species with a total of 330.5 tons (Invemar, 2020). On the other hand, Hening (1997) reported that the diet of N. brasilianus during the reproduction of the cormorant, in August 1996, was dominated by 92.99 % by individuals belonging to the subfamily Engraulidae (Anchovia clupeoides and Cetengraulis edentulus). However, these species represented less than 1 % of the catches recorded in the CGSM LC during that year. However, Manjarrés et al. (2007) reported that C. edentulus was the most important species in the fishing landings from the marine area adjacent to the CGSM LC, between 1994 and 1998, being especially abundant in 1996 when catches of approximately 484 tons were estimated. This indicates that the individuals of N. brasilianus belonging to the colony located to the northeast of the CGSM FFS can feed in the Caribbean Sea, in search of individuals that have the adequate sizes to be consumed.
It is important to mention that the present study, although it is complementary, is not comparable with that of Hennig (1997). This is mainly because this author analyzed stomachs and vomits during climatic conditions influenced by a warm phase of the El Niño Southern Oscillation (ENSO) event, while in the present study pellets were analyzed during a neutral phase of ENSO (NOAA, 2020). Indeed, for the CL CGSM it has been reported that ENSO affects the abundance of fish and invertebrate species caught by the artisanal fishing fleet (Invemar, 2019).
The numerical frequency of the Mugilidae family in the N. brasilianus diet was more significant than in other studies; however, in terms of occurrence it was similar to Barquete et al. (2008) (Table 2). This corroborates the importance of Mugilidae in the diet of Phalacrocoracidae, where they are recurrent prey (Jahncke and Goya, 1997; Olmos et al., 2000). It is worth mentioning that Hennig (1997) identified only one species of this family (M. incilis) in the cormorant diet, while in the present study three species were identified (M. liza, M. curema, and M. cephalus), whose diets are based on the primary production of the lagurnar complex (debris, diatoms, and inorganic sediments), for which they play an important role in the transfer of energy from the estuarine system to cormorants (Jacot, 1920; Osorio, 1988).
The fish of the Sciaenidae family had a minor contribution to the N. brasilianus diet, indicating sporadic consumption, similar to other studies (Hennig, 1997; Olmos et al., 2000; Gil de Weir et al., 2011). On the contrary, Barquete et al. (2008) found that the Sciaenidae family made the highest contribution in numerical frequency and occurrence, which coincided with the high abundance of Micropogonia furnieri (Desmarest, 1823) in deep waters and sites with greater marine influence (Marín et al., 2013). This is explained because the importance of this family in the cormorant diet is greater in populations that inhabit marine ecosystems (Petracci et al., 2009).
The family Engraulidae had little occurrence and numerical frequency in the diet, in contrast to previous studies that record a greater contribution of this family in the diet of N. brasilianus (Table 2). The decrease in the Cetengraulis edentulus population (Cuvier, 1829), inferred by the decreasing trend of its landings and the capture of juveniles led to it being categorized as near threatened in the region (Duarte et al., 2017), which poses a reduction in its supply as food for the cormorant. Regarding Anchovia clupeoides (Swainson, 1839), Hennig (1997) reports an average consumption length of 14 cm (between 10-20 cm), and the average size of catch by fishermen has been decreasing from 19 cm (total length in 1999) to 12 cm (total length in May and June 2017) (Invemar, 2020). This suggests that the fishing exploitation of A. clupeoides could have affected the availability of prey with palatable sizes for consumption by N. brasilianus.
Based on trophic ecology studies, N. brasilianus has been considered to be a generalist or opportunistic species, which selects its prey based on availability and which takes advantage of the most abundant and easy-to-capture resources (Conde-Tinco and Iannacone, 2013; Ovegård, 2017). The niche width observed in the present study suggests a specialist habit, which is corroborated by the values of trophic diversity and equitability. However, when considering the richness of species in the diet and in accordance with that reported by Hennig (1997), it could be a case of “temporal specialization” as described by Barquete et al. (2008) for the Neotropical cormorant in Dos Patos Lake in southern Brazil and modeled by the optimal foraging theory (Valdovinos et al., 2010). For cormorant species, egg-laying and hatching are known to be asynchronous, and are probably related to the supply of more available food and seem to allow a rapid response to environmental conditions (Siegel-Causey, 1997). This case of the relationship between reproductive success and food supply has been observed in other piscivorous birds, such as the case of pelicans and their reproductive success due to the upwelling events of temporary cold waters that concentrate aggregations of prey fish in months of predictably (Pelecanus occidentalis in Mexico: Hernández-Vázquez et al., 2011; in Panama: Martínez, 1983; in Gorgona: Falk-Fernández, 1993).
CONCLUSIONS
Nannopterum brasilianus presented a specialized feeding habit during its nesting in 2017, but the richness values in the diet and the antecedents suggest that it is a generalist bird and the observed corresponds to an adaptive response modeled because of the availability of dams. However, larger studies are warranted in time and space to corroborate what has been raised.
The use of pellets in the study of the cormorant diet is an informative, minimally invasive, and non-lethal method, but it presents challenges for its optimal implementation in the CGSM LC, such as the absence of otolith records of the potential prey present in the ecosystem, as well as correlation analysis between the size of the otoliths and the sizes of the fish. Since these inputs are not available, it was not possible to identify all the components of the richness of prey, nor to calculate the sizes of the prey consumed.











 text in
text in