Species of Thalassoma are known for their trophic versatility and broad dietary preference, as well as for presenting a generalized foraging behavior in a wide spectrum of reef microhabitats (Fulton et al., 2017, Dunkley et al., 2018). Although several species of this genus are recognized for their cleaning feeding behavior (Arnal et al., 2006; Baliga and Mehta, 2014; Quimbayo et al., 2017), such behavior occurs less frequently in T. lucasamun in the Eastern Tropical Pacific (ETP), a region where it forages meanly on invertebrates (Quimbayo et al., 2017). Most information available for this species focuses on its reproductive patterns, abundance, and distribution (Warner, 1982; Giraldo et al., 2001), but knowledge about their feeding habits is still scarce (Foster, 1987; Robertson and Allen, 2015; Quimbayo et al., 2017). To contribute to the knowledge of the trophic ecology of T. lucasanum in the ETP, we characterized its diet by analyzing the gut contents of individuals captured in La Azufrada coral reef, Gorgona Island, Colombian Pacific.
La Azufrada is the largest (length: 780 m, width: 80-180 m) and most continuous fringing reef of the continental Gorgona Island (2°58′10″ N and 78°11′05″ W), 35 km from the Pacific coast of Colombia (Glynn et al., 1982; Muñoz and Zapata, 2013). The dominant corals are branched stony corals of the genus Pocillopora, with large colonies of massive corals of the genera Pavona and Gardineroseris present in the deep zones (Zapata and Vargas-Ángel, 2003).
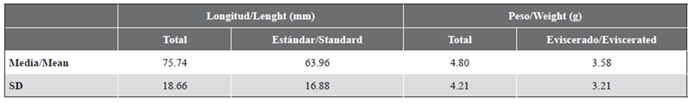
Five T. lucasanum were captured using a trammel net (length: 8m, height: 1.5 m, mesh size: 1.27 cm × 1.90 cm) between 10:00-12:00 h on La Azufrada’s reef flat throughout September 2019. Several attempts were made to capture the fish in the field using different fishing gear (v.g. Hawaiian spear, manual fishing net, trammel net); however, the agility and speed that characterize these fish made it difficult to catch them, resulting in a small sample size. Total and standard length as well as total and eviscerated weight were registered for each individual (Table 1). Then, each individual’s gut was dissected and preserved in 70 % ethanol. Once in the laboratory, all prey or distinguishable fragments of prey were removed of each stomach (including the anterior portion of the intestine), and identified to the lowest taxonomic level possible, counted, and weighed on an analytical balance (dry weight: 60 °C × 24 h) (Hyslop, 1980; Cortés, 1997; Mar-Silva et al., 2014). The digested material with no distinguishable fragments was also sorted and weighed. The vacuity coefficient (%V), frequency of occurrence (%F), percentage by number (%N), percentage by weight (%W) and the index of relative importance (%RI) were calculated for each prey (George and Hadley, 1979; Hyslop, 1980; Fabi et al., 2006). To describe the trophic niche breadth, Levin’s index (Bi) and its standardized form (BA) were calculated (Krebs, 1999).
Table 1 Summary of morphometric measurements of Thalassoma lucasanum collected for gut content analysis. n = 5 individuals. SD: standard deviation.
We registered 20 prey items in the gut contents of T. lucasanum (V%: 0). Because most prey items were highly digested, only one was identified to genus level and four to family level (Table 2). From the unidentified prey, 12 were classified as morphospecies (one ostracod, two bivalves, and nine gastropods), but due to their small size, their individual weight could not be quantified.
Table 2 Diet composition of Thalassoma lucasanum in La Azufrada coral reef, Gorgona Island, Colombian Pacific. Frequency of occurrence (%F), percentage by number (%N) and weight (%W), and index of relative importance (%RI) for each prey.
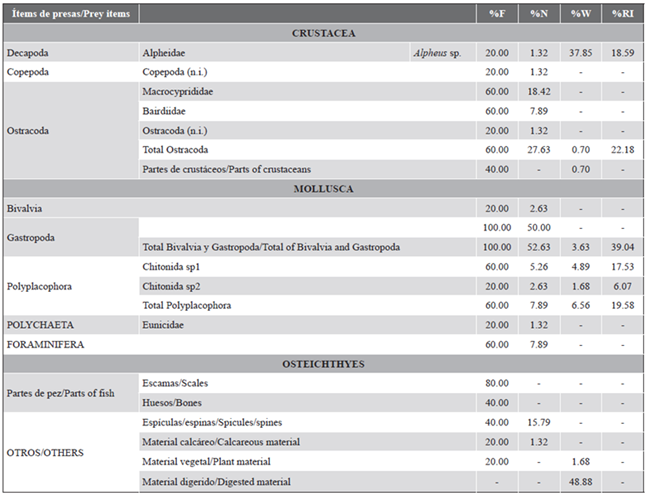
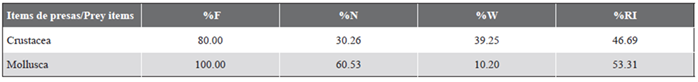
Our results show that Thalassoma lucasanum in La Azufrada coral reef mainly feeds on small benthic invertebrates, including mollusks, crustaceans, and polychaetes (Table 2). Mollusks were the most frequent (F%: 100) and most important prey item in the diet (%RI: 53.31). Among mollusks, gastropods were the most frequent (100 %) and most abundant (50 %) prey, followed by chitons (%F: 60) and bivalves (%F: 20). However, in terms of biomass, the contribution of mollusks was low (%W: 10.20) compared to the crustaceans consumed (%W: 39.25). Crustaceans comprised the second most frequent (80 %) and most important (%RI: 46.69) dietary component, with a highlighted frequency and abundance of ostracods (F%: 60, %N: 27.63), decapods of the genus Alpheus (F%: 20), and copepods (F%: 20) (Table 3).
Table 3 Summary of the diet composition of Thalassoma lucasanum in La Azufrada coral reef, Gorgona Island, Colombian Pacific, listing the main prey items registered. Frequency of occurrence (%F), percentage by number (%N) and weight (%W), and index of relative importance (%RI) for each prey.
Our results are consistent with descriptions made by Robertson and Allen (2015), who documented a varied diet for T. lucasanum based on mollusks, sea urchins, crustaceans, and polychaetes. Among these prey items, mollusks were the main feeding resource of T. lucasanum in La Azufrada coral reef, while other studies have reported small herbivorous crustaceans as its main diet component (Goodson, 1988; Williams, 1991). The preference to prey upon mollusk (soft-bodied and/or hard-shelled) have been documented for other Thalassoma species such as T. duperrey, which is an effective predator of nudibranchs (Gochfeld and Aeby, 1997), and T. lutescens, whose molar-shaped pharyngeal teeth allow it to grind up shells and hard-bodied organisms like chitons (Gushima et al., 1991). Due to the high preference for mollusks shown by T. lucasanum in La Azufrada, its trophic niche breadth was narrow (Bi: 1.20; BA: 0.01), which suggest a specialized diet for this species (Krebs, 1999). However, these results contrast with those reported for other species of the genus Thalassoma, such as T. bifasciatum, considered as a generalist forager that feeds mainly on the benthic substrate and that also presents a facultative cleaning feeding behavior (Dunkley et al., 2018). It is possible that the preference for mollusks found in T. lucasanum in the present study is due to the high availability of this resource, related to the high diversity and abundance of mollusks that inhabit the reef substrates (Robertson, 1970; Hadfield, 1976; Ramesh et al., 1996). In branched corals of genus Pocillopora, more than 100 mollusks species have been reported (Austin et al., 1980; Ramesh et al., 1996), comprising one of the most abundant groups in pocilloporid corals, along with decapods (Alvarado and Vargas-Castillo, 2012). According to Fulton et al. (2017), the high trophic versatility that characterizes fish of the genus Thalassoma could facilitate switching their prey towards the most common resource within a given habitat, which might allow these species to rapidly exploit the available resources. However, to determine more accurately the main food sources of T. lucasanum and the breadth of its trophic niche, it is necessary to carry out an analysis of its diet considering a larger sample size.
The diet of T. lucasanum was also comprised by fish scales and bones that were found in 80 % and 40 % of the stomachs analyzed, respectively (Table 2). The presence of fish scales in the gut contents may result from cleaning activities performed as facultative cleaners (Baliga and Mehta, 2014). When T. lucasanum perform cleaning activities, they might feed on fish ectoparasites, although this feeding behavior is rare in T. lucasanum compared to other cleaning species (Quimbayo et al., 2017). Dentition of most wrasses allows them to exploit a wide diversity of prey, from zooplankton to ectoparasites of crustaceans and fish, as well as hard-shelled bivalves and gastropods (Westneat and Alfaro, 2005; Parenti and Randall, 2011). Therefore, it is also possible that small fish comprise T. lucasanum diet, although less frequently. Likewise, the presence of sea urchins’ spines and crushed calcareous material suggests that T. lucasanum feeds on sea urchins and could be ingesting coral pieces while capturing its prey. A molecular approach of stomach contents could provide a more robust analysis to identify highly degraded prey, as well as nutritional information necessary to quantify more accurately the importance of each prey item in this species (Dunkley et al., 2018; Amundsen and Sánchez-Hernández, 2019).
Our study shows that T. lucasanum feed mainly upon benthic invertebrates, mostly hard-shelled mollusks and crustaceans with a strong association with the coral substrate (Figure 1). Due to the small sample size, the results obtained represent an approximation to the diet for the species, contributing to establish the baseline knowledge of its feeding habits in the region. Because T. lucasanum is one of the most abundant fish species in La Azufrada coral reef (Zapata and Morales, 1997; Alzate et al., 2014; Palacios et al., 2020), characterizing its diet provides a better understanding of predation activities and dynamics of food webs in coral reefs in the ETP.
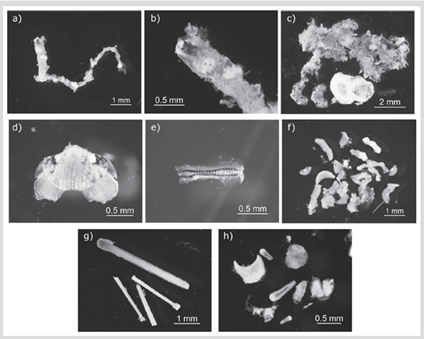
Figure 1 Prey items found in the stomach contents of Thalassoma lucasanum. Polychaeta: A) Eunicidae. B) Jaw detail of Eunicidae. Mollusca: Polyplacophora. C) Plates and tissue of digested Chitonida sp1. D) Plate detail of Chitonida sp1. E) Radula of Chitonida sp1. F) Plates and tissue of digested Chitonida sp2. Others: G) Sea urchin spines. H) Calcareous material











 text in
text in 



