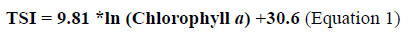INTRODUCTION
The socio-economic development of coastal cities produces a significant negative impact on the quality of the resources and services offered by ecosystems. In particular, bays and coastal lagoons constitute conflicted ecosystems since they act as waste receptors, which makes them very vulnerable, given that they are subjected to a high anthropic pressure and an accelerated pace of deterioration (Gómez, 2013). At the same time, these ecosystems play a relevant role in constituting key elements of the local landscape. They support touristic and recreational activities, as well as the development of numerous coast-dependent industries and activities related with communication, tourism, and maritime trade.
Eutrophication is one of the most serious problems faced by aquatic ecosystems, and it is understood as the natural or anthropogenic process consisting of enriching water with nutrients at such a pace that it cannot be compensated by total mineralization. In this way, the decomposition of the excess organic matter produces a gradual decrease in dissolved oxygen, with implications for the trophic web and ecosystem health. Eutrophic waters have a high productivity and biomass content on all trophic levels, thus favoring the proliferation of algae; deep waters become oxygen-poor, and there is an intense growth in aquatic plants (Arce, 2007). The above has a negative impact on ecosystem services, thus affecting humanity’s different uses for them.
Among the collateral effects of eutrophication, Harmful Algal Blooms (HAB) can be mentioned. These are natural, multicausal events that are increasingly being reported worldwide in surface waters (Carmichael, 2001; Tomlinson et al., 2006; Anderson et al., 2008; Alawadi, 2010; Verity, 2010; Calandrino and Paerl, 2011), whose diversity and impact represent a challenge for the management of threatened coastal resources (Anderson, 2004).
Phytoplanktonic organisms that cause HAB include dinoflagellates, cyanobacteria, diatoms and other phytoplankton groups (prymnesiophyta and raphidophyta) with less relevance. There are generally those that produce toxins and therefore may contaminate seafood or produce fish mortality, and those that do not produce them but have other harmful effects, such as organism mortality by anoxia, fish mortality by physical damage to their gills or other organs, and production of mucilage or other metabolites that affect the environmental quality (Carreto et al., 2007). Most species are associated with non-toxic events, whereas a few dozen produce toxins (Anderson, 2004).
In Cuba, HAB constitute a problem that requires further study, although they have been research subject in eastern Cuba since 2001 in several ecosystems, among which Santiago de Cuba and Guantánamo bays stand out (Gómez et al., 2001, 2006, 2007, 2009, 2014; Gómez, 2007; CITMA, 2011), as well as the San Juan river (Echavarría et al., 2002), water reservoirs to drinking water supply (Gómez et al., 2010, 2011; Rodríguez-Tito et al 2017), and, since 2011, the Baconao Lagoon.
The Baconao Lagoon is a tropical, coastal-littoral lagoon located on the southwestern coast of Cuba; separated from the sea by a coastal barrier and a narrow channel with a high siltation level due to constant anthropic and natural disturbances. These characteristics impose dynamics on the ecosystem which affect the water residence time and, therefore, the gas and nutrient gradient, primary productivity, and total organic production. This scenario favors eutrophication, with a tendency for the proliferation of phytoplankton species, which find the ideal trophic, light, and temperature conditions in these waters. HAB have repeatedly occurred in this ecosystem since 2011, together with water discoloration and fish kills in 2011, 2012, 2014, and 2017, along with the identification of the presence of toxic dinoflagellates in all cases (Gómez et al. unpublished data). The recurring presence of risk indicators cause this ecosystem to need a control and monitoring system that allows taking effective measures, along with the development of an early culture through the perception, which allows for an integrated understanding of the risk (Gómez, 2013). The development of this research starts with a report involving the massive fish kills in the Baconao Lagoon in May of 2017. In light of this situation, the Delegation of the Ministry of Science, Technology, and Environment (CITMA) in Santiago de Cuba decides to summon the Flora y Fauna enterprise, belonging to the Ministry of Agriculture, in charge of managing the ecosystem; as well as the Ecotoxicology and Environmental Services Laboratory (LESA), belonging to the National Center for Applied Electromagnetism (CNEA), a science and technological innovation entity (ECIT), associated with Universidad de Oriente, Santiago de Cuba, Cuba, with experience in HAB characterization and management.
The general objective of this study consists of establishing the scientific and methodological basis for the implementation of a risk management protocol for the contamination with marine phycotoxins, which is adapted to the ecosystem’s features by establishing methodological guidelines for its generalization. The following are considered as specific objectives: 1) to characterize the risk situation that cause the event, 2) to define the specific procedure for decision making, and 3) defining the necessary methodology for the implementation of the protocol.
AREA OF STUDY
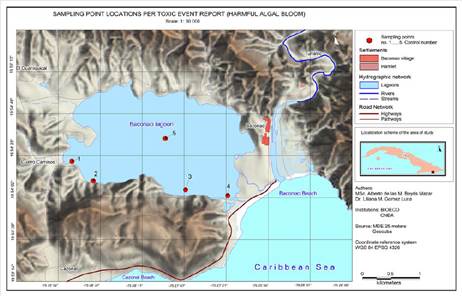
The Baconao Lagoon is located in the municipality of Santiago de Cuba, about sixty kilometers away from the city (Latitude: 19, 9097°, Longitude: -75, 4667°) (Figure 1). It is a semi-closed ecosystem with an approximate surface area of 4 km², which is influenced by the Baconao river and the tides influx, with a visible spatial heterogeneity and a mean depth of 6 m. The lagoon is located at the boundary of the biosphere reservation with the same name, in the Sierra Maestra mountain range, and it belongs to category 2 according to the UICN’s Category System. It is classified as a Protected Area of Managed Resources (CNAP, 2004).
The lagoon is a fragile biotope, vulnerable to extreme events such as hurricanes, droughts, abundant rains, and increases in solar irradiation due to vegetation cover loss, which not only alters its physicochemical parameters, but also the ecological interactions of the aquatic habitats. This lagoon holds a significant biological diversity, with prominent elements such as the surrounding mangroves, affected by environmental variables and anthropic pressure, which is why they have been selected as objects of conservation (Figueredo and Acosta, 2008). The degree of anthropic-related effects that the mangroves in the area have received is fundamentally due to the dumping of solid and liquid wastes produced by the nearby communities and touristic activities, with implications for the physiological processes of this ecosystem. Similarly, the loss of habitat, which is evidenced by the reduction of the occupied area, is the threat that most affects the mangroves, which is fundamentally associated with logging activities to turn the sites into tourist zones (Figueredo and Acosta, 2008), among other uses, where a decrease in the total surface of the lagoon is observed.
Among the main uses of the Baconao Lagoon’s ecosystem are recreational activities related to walks and sightseeing, as well as preservation activities, agriculture, and the breeding and reproduction of the dolphin Tursiops truncatus (an introduced species), which adds another conflict to the use of the ecosystem, with implications for management plans. There is also a breeding of crocodiles (Crocodylus acutus).
MATERIALS AND METHODS
Elaboration and implementation of the risk management protocol
The methodological reference used for the elaboration of the risk management protocol for the contamination with phycotoxins is the one established by Gómez et al. (2012) (Figure 2), based on which a general work scheme is elaborated, which is presented as the result of this research. The protocol includes primary and secondary indicators that, although they cannot substitute the detection of toxins, they constitute imperative complementary analyses, valuable for the implementation of any management alternative based on the precautionary principle, mainly in those contexts where the toxins detection is not viable. It is important to consider both indicators, given that not all events caused by harmful algae are related to the development of large biomass accumulations capable of producing a water discoloration; there are species that are harmful even at very low concentrations (Carreto et al., 2007). HAB can reach concentrations of the order of 106 cellL-1, and even higher. However, it has been reported that a toxic episode may affect cellular concentrations of the order of 102 cellL-1 (Sar et al., 2002), which justifies the need to implement the risk management protocol in case of the presence of potentially toxic species.
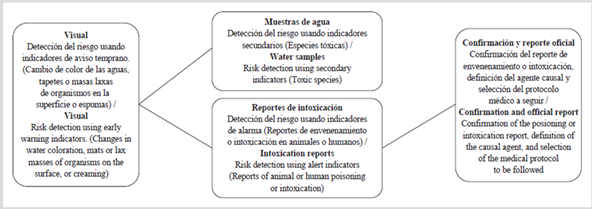
Figure 2 General protocol for the integrated risk management for the contamination with phycotoxins (Gómez et al., 2012; Gómez, 2013).
Methodology for sampling and qualitative analysis
For the risk characterization analyses, samples obtained by third parties were first analyzed (CITMA specialists and Flora y Fauna) at the moment of the first notice to the Ecotoxicology and Environmental Services Laboratory (LESA). These samples were taken directly in borosilicate bottles without preserving for fresh observations. In the lab, qualitative analyses were performed (identification of phytoplankton species and dinoflagellate cysts) to elaborate an immediacy report and guarantee the implementation of the first phase of the protocol.
Quantitative analyses were later performed (phytoplankton count and an analysis of the physicochemical parameters of the water) to characterize the risk situation from samples taken by experts at specific sampling points, which were established based on an exploratory trip to the ecosystem. They were located where changes in coloration or fish kills were reported, including reference points from other studies conducted in the lagoon for sectorial interests, which considered criteria related to the particular uses and potential dumping. A sampling point located on the vertical line of the deepest part of the water mass is included (Point 5, Figure 1).
Five sampling points are established: 1. El Fanguito 1; 2. El Fanguito 2; 3. Patana; 4. Channel, and 5. Lagoon center. Other samplings were incorporated in the following days, according to the evolution of the HAB, the presence of foams, or zones with intense discoloration. Additionally, biological samples (gills) were analyzed, which belonged to fish kills collected on the shores of the lagoon, as evidence of the risk situation.
Sampling was conducted during the morning hours (8:00-9:00 am). It was carried out directly within the first 30 cm of the water column at each of the sampling points, taking three integrated samples (De la Cruz, 1984; Venrick, 1995). To support the phytoplankton identification, qualitative sampling was performed by means of a net with a 20 µm mesh sieve, a 24 cm diameter, and a 1 m depth, with a PVC 250 mL collector with mesh-coated lateral windows. The depth of the sampling point was determined beforehand with a YSU Pro DSS probe (USA), in order to avoid the removal of silt during sampling. The net was vertically dragged along the water column until a visible filtration was obtained.
During the entirety of the sampling process, the applicable protective measures were used (boots, gloves, face masks, and goggles), considering the potential risk. The samples were taken in sterilized borosilicate vials with 250-500 mL capacity and screw caps, leaving a gap between the surface of the sample and the cap or closure to allow for gaseous exchange, as is suggested by different phytoplankton sampling protocols (FWE et al., 2005, Greenberg et al., 2008). After being conveniently labeled and fixed with a Lugol solution (maintaining at least an unfixed sample per point), the samples were transported to the lab in complete darkness, observing the relevant aseptic measures so as not to cause their contamination, and proceeding immediately to their count and analysis. Every manipulation of the samples was performed with latex gloves, in compliance with the corresponding medical sanitary and biosafety measures.
Identification of phytoplankton species
Observations were performed on fresh and fixed samples (neutral Lugol at 1 %) using a Motic B professional vertical optical microscope (Germany). Concentrated samples were observed from resuspension in 1 mL of a 20 mL filtration, carried out with the aid of 25 mm and 0.2 µm cellulose acetate filter membranes, besides the ones taken with the net. These analyses included samples previously incubated during seven days after being enriched in an f2 medium, where continuous daylight conditions were maintained using a Philips 40 W DayLight (TLT 40 W/54 RS), at an intensity of 58.59 mE m-2s-1 and a temperature of 20.12 ± 2 °C. Two to three drops of each sample were placed on a slide for their observation under the optical microscope, observing and taking photographs of at least five panoramic replicas per sample, as well as individual photographs by means of a Panasonic DMC-LC50 digital camera (3.2 Mpixs with a 3X optical zoom and a 9X digital zoom).
During these observations, the present micro-algae were identified, till specie when it was possible, using several dichotomic keys and taxonomic criteria (Bold and Wynne, 1978; Biagini, 1980; Dillard, 1999; Faust and Gulledge, 2002; Wher and Sheath, 2003), as well as online searches on different websites: Infoseek (Japan), the MIT phytoplankton species gallery (USA), and the online AlgaeBase and Algaterra databases (Yasumoto et al., 1980; Balech, 1988; Vasconcelos, 2001; Adolf et al., 2006; Brand, 2006; Badylak et al., 2007; Comas, 2007; Guiry and Guiry, 2014).
As a complement, Lugol staining and observation of dinoflagellate thecal structures by means of staining with Calcofluor White (Fritz and Triemer, 1985) were conducted; the observations were made with an inverted epifluorescence microscope (Zeiss, Axiovert A1, Germany). The length and width of the cells in fresh or fixed preparations were measured with a previously calibrated lens, in the case of the Motic B microscope, or by using the Zeiss Auxiovision software (Zeiss, Germany) for the inverted microscope.
Qualitative analysis of dinoflagellate cysts
Dinoflagellate cysts are identified both in the water column and the sediment, in order to understand the dynamics and recurrence of this phenomenon. Sediment samples were taken in shallow areas with less than 2 m in depth at points 1, 2, and 3 with a light OCT gravity probe (Matsuoka and Fukuyo, 2000). Three samples were collected per point, for a total of nine. Each sample was fragmented using the upper 2 cm of the surface silt, which were packed in a plastic container using neutral formalin at 10 %. These sampling points were selected for the analysis of sediments since, given the ecosystem’s dynamics, they have the highest sedimentation rates.
Cleaning and sieving of the cysts were performed following the technique described by and Matsuoka and Fukuyo (2000). For the identification of the cysts, direct observation was conducted, considering aspects such as the shape of the main body, ornamentation, structure, processes, and wall color, according to Matsuoka and Fukuyo’s criteria (2000).
Methodology to analyze the evolution of algal blooms
In addition to considering the quantitative and qualitative analyses, interviews were carried out with volunteers and decision makers who visited the ecosystem during the harmful event. These interviews were based on open-ended questions oriented towards the evolution time, general and first evidences. As a complement, laboratory confirmatory analyses were performed to be able to describe the facts with the corresponding evidence. The primary indicators and the meteorological data from 15-20 days before and during the event were considered for this analysis.
Methodology for phytoplankton count
The total phytoplankton counting (cellmL-1) was carried out using a Neubauer improved hematological counting chamber (2.5×10-4 µL). The samples were fixed with Lugol, performing the corresponding dilutions when necessary. Additionally, a differential cyanobacteria count was performed. In case of algal bloom occurrence, the dominant species counting was conducted (greater than 50 %).
Analysis of biological samples
Gills from six fish between 5 and 19 cm (n = 12) were analyzed, performing observations in fresh directly from histological preparations of gill tissue, secretion smear, and water filtration from three successive washes of the gill tissue, with the objective of looking for evidence of the presence of potentially toxic phytoplankton species cells and mucus. A Motic B professional upright optical microscope (Germany) and a stereoscope were used (URA Technic, Zuzi; CE).
Physicochemical parameters
Physicochemical parameters were determined in situ within the first 50 cm of the water column using a multiparametric YSI Pro DSS measuring device (USA): temperature (°C), dissolved oxygen (mgL-1), salinity, pH, conductivity (µScm-1). Using a Mettler Toledo oxygen meter (Germany), dissolved oxygen determinations (mgL-1) were conducted on water samples taken by third parties and those taken at the different points during the monitoring of the event, as well as conductivity (μScm-1), pH, and salinity by means of a pH-meter and a Mettler Toledo conductivity measuring device. Additionally, chlorophyll a concentration was determined (mg.m-3) with an Aquaflor portable digital fluorometer (USA), performing the relevant adjustments with a correction factor for chlorophyll a in vivo.
Calculation of trophic indexes
From the chlorophyll a values, the Trophic State Index (TSI) was calculated according to the OCDE (1982) (Equation 1).
From this index the aquatic habitat is classified according to its trophic state, considering different criteria (OCDE, 1982, Vollenweider and Kerekes, 1982; Contreras-Espinosa et al., 1994).
RESULTS
General decision-making procedure: methodological aspects for its implementation
The general decision-making procedure and the subsequent implementation of the risk management protocol for the occurrence of cyanobacteria and cyanotoxin contamination in the Baconao Lagoon is presented in figure 3, along with the necessary methodological details for its implementation. Visual inspection was deemed to be the first activity, carried out jointly between the key actors of the Ecosystem, led by the CITMA (Ministry of Science, Technology, and Environment), considering their social commitment.
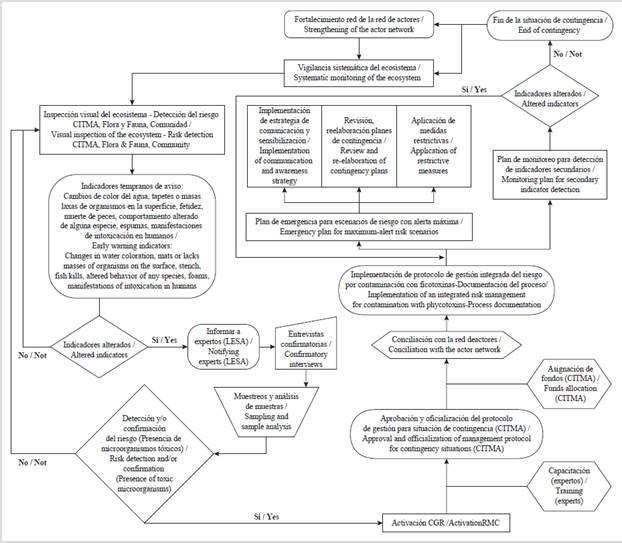
Figure 3 General decision-making protocol and implementation of an integrated risk management protocol for phycotoxins contamination in the Baconao Lagoon.
Since the analysis of the primary or early warning indicators was positive, the protocol was immediately implemented, involving the primary health care staff from the early actions. The implementation consists of four fundamental sub-processes: the application of restrictive measures; the review and redrafting of contingency plans; the implementation of a communication and awareness strategy, focused on mitigating impacts on the ecosystem; and the design and implementation of a monitoring plan for the detection of secondary indicators.
The early warning indicators predefined to activate the decision-making protocol in the face of phycotoxins contamination were changes in the color of the water, the presence of mats, stains, or lax masses of organisms on the surface; stench; fish kills, altered behavior of some species in the ecosystem (fish, mammals, birds); the presence of foams; nocturnal luminescence, and/or alterations in the health status of the population that has settled in the ecosystem or uses it. The secondary indicators define the existence of a contingency and the extent of the risk situation, which, if maintained, the protocol must still be activated. Otherwise, systematic vigilance of the ecosystem must be maintained, with the subsequent strengthening of the actor network.
The creation of a work group for emergent environmental situations is a necessary initiative in light of the growing impact of drastic fluctuations on the climate. This group or commission created for risk management (RMC) must harmonically integrate the actor network and the specialists involved in the short and long term to guarantee the sustainability of the proposed initiatives. The RMC must involve, strengthen, and consolidate the actor network, preforming training, conciliations, strategic alliances, as well as contributing to raising the awareness of key actors and decision makers. Training in aspects related to risk management is relevant within the framework of the new environmental scenario. In this context, implementing the current environmental regulations and good practices constitutes a mandatory and necessary exercise, so that the environmental cost of human activities is minimal.
Protocol activation
On May 29, 2017, the decision-making protocol is activated, in response to a warning regarding changes in the ecosystem’s primary indicators (change in water coloration and the fish kills) on the shores and the surface of the water. This was notified by the Flora y Fauna enterprise to CITMA.
Risk confirmation
To confirm the risk, confirmatory interviews were conducted, as well as the analysis of the first samples delivered by CITMA and Flora y Fauna to LESA. The risk is confirmed through the following early warning indicators: brown-reddish and greenish coloration occupying approximately 30 % of the ecosystem; fish kills of several species and sizes on the surface and shores of the lagoon; a strong stench; altered features of the sediment, with zones colored with intense green or green-brown, the smell of sulfur, and a stench; the presence of foams and lax masses floating on the surface of the water.
As additional information, through observation and interviews with the veterinarian in charge of dolphin breeding, a discrete change in the behavior of these animals was verified, which seemed apathetic and rather inactive, which is why they were intensively monitored. They stuck to the margin opposite to the sampling point during almost the whole event.
Key actors involved
Three key actors participated in the first visual inspection: CITMA, Flora y Fauna, and members of the communities near the ecosystem. Immediately after the inspection, other actors were involved: LESA, given their expertise in HAB, and the Baconao Aquarium (belonging to Flora y Fauna), for their interests in the ecosystem, specifically regarding the breeding and maintenance of dolphins in captivity within the area.
After risk confirmation, the RMC is created, which warns the key actors about the risk situation (executives of gastronomic establishments, community leaders, fishermen, tourist guides, the PNR (National Revolutionary Police), doctors in charge of primary healthcare, employees of private businesses, and legal persons participating in the formal and informal market chain of the ecosystem’s resources). During the awareness process, the executives and environmental management specialists of the businesses located in the vicinity of the ecosystem were considered, as well as the entities that use it directly or indirectly.
The executives of these businesses were called upon to:
Evaluate alternatives for the development of their productive process in the case of risk situations;
Update the risk management plans, including the risk of contamination by phycotoxins;
Review the contingency to include mitigation actions in case of emergencies related to HAB and their impacts;
Document the situations arising from the risk events in incident records;
Implement environmental education and/or training programs related to the presence of phycotoxins;
Implement a communication strategy that allows to rapidly inform those involved in case of a risk situation;
Perform innovative analyses to guarantee the quality and safety of the offered product, considering specific indicators of the risk derived from phycotoxins presence.
Early strategic actions
Starting from the confirmation of the presence of potentially toxic organisms, early strategic actions are developed, among which the elaboration of an immediate official report (in less than 1 h) was considered to declare the existence of imminent risk. This report, in addition to verifying the primary indicators, seeks to confirm the presence of potentially toxic species or the existence of excessive growth of any phytoplanktonic species.
Immediately after the confirmation of the risk, the RMC was activated, to which the experts and CITMA present the report for subsequent intensive training, considering key elements that will favorably contribute to the implementation of the management protocol, once the funds were approved and allocated. In this regard, CITMA’s leadership was relevant, given that their governmental mandate is to watch over the environmental quality of ecosystems.
The RMC established an integrated risk management system, through alliances and reconciliations, this guaranteeing the systematic monitoring of the ecosystem. The celerity of the strategic actions is what will ultimately ensure the effectiveness of the risk management situation.
Implementation of the process
For this phase, the four previously described key subprocesses are considered, with the design and implementation of a monitoring plan being relevant, which is led by experts, with the participation of the RMC and other key actors. Participative sampling is considered with prior training of those involved.
The sampling for monitoring was conducted every three days during 24 consecutive days, considering the preestablished warning indicators. In parallel, a visual inspection of the ecosystem was performed (Figure 4). On the 25th day, declaring the end of the contingency was considering, in accordance with the results of the performed the monitoring. From then on, weekly visual inspections were maintained during two months, and it was recommended that monthly monitoring be performed, considering the possible recurrence of the risk situation.
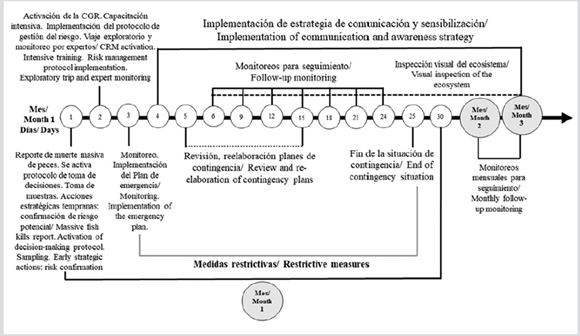
Figure 4 Timeline of the actions taken for the implementation of the risk management protocol for phycotoxins contamination in the Baconao Lagoon.
During this stage, the emergency plan for risk scenarios involving maximum alert was elaborated. Uses of the ecosystem were restricted, such as navigation, bathing, fishing, and recreation, in order to prevent not only direct exposure, but also exposure to sprays in the vicinity of the ecosystem. All this, for 30 days. The aquatic habitat’s contingency plans were reviewed and redrafted, while coordinating with the Baconao Aquarium, thus establishing the necessary measures in the case of dolphin evacuation. Actions related with the monitoring of animal behavior were included.
Finally, during this stage, the implementation of a communication and awareness strategy was considered to mitigate the impact on the ecosystem, thus guaranteeing systematic reports to the community and local authorities, as well as contact with key actors and direct communication with decision makers and community leaders. During the time of the protocol implementation, the meteorological forecast was considered for the decision-making process, in close coordination with INSMET (the National Meteorological Institute) in Santiago de Cuba. In addition to CITMA’s leadership, creating alliances with experts was relevant to promoting adequate dynamics in the decision-making process, which constituted a key success factor in implementing the risk management protocol.
Identification of phytoplankton species
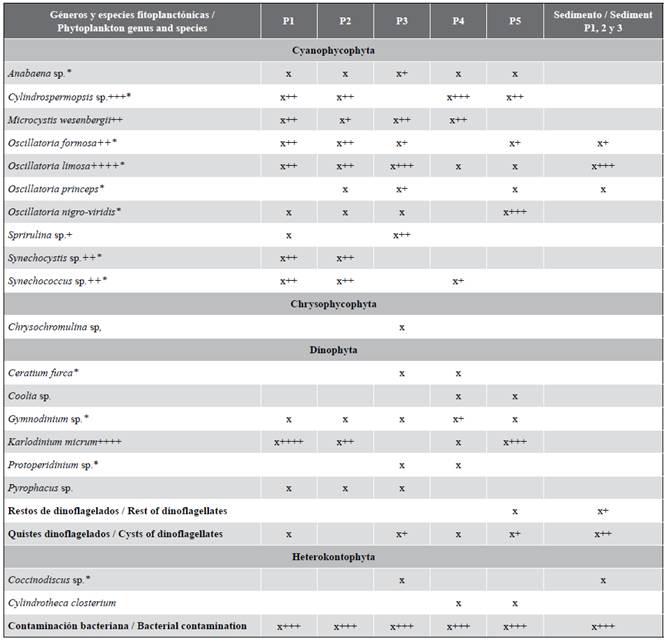
The analyses allow stating that the analyzed samples are positive for the presence of dinoflagellates and cyanobacteria, with abundant cysts at some sampling point. A total of 19 phytoplankton species were identified in the entirety of the aquatic habitat, which include 10 cyanobacteria species, seven of which were potentially toxic; and six dinoflagellate species, one of which is an ichthyotoxin producer: Karlodinium micrum and another one associated with the production of ciguatoxins or Ciguatera Fish Poison (CFP) (Coolia sp.). Two heterokontophyte species were also detected, one of which is associated with the production of CFP, as well as one Chrysophyta (Table 1).
It is important to point out that, 12 of the 19 identified species, are recurrent (63 %) and mostly cyanobacteria. Similarly, genera Microcystis and Karlodinium are also recurrent.
Biological sample analysis results
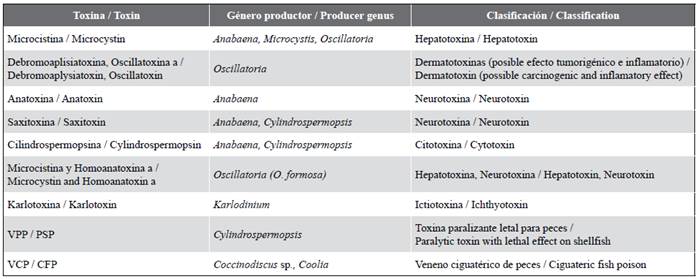
In the gill samples, the presence of mobile Karlodinium micrum cells (n = 10) could be confirmed, as well as the presence of Oscillatoria nigro-viridis (n = 4), O. limosa (n = 8), Cylindrospermopsis sp. (n = 10), and Microcystis wesenbergii (n = 7). Although the presence of potentially toxic species could explain the fish kills, it is important to point out that the presence of abundant mucus is confirmed, which could be evidence of death by asphyxiation. In table 2, potential toxins are presented according with the phytoplankton species present in the ecosystem (Cronberg,1999; Minillo et al., 2000; Vasconcelos, 2001; Moreno et al., 2003; Aboal, 2005; Cameán et al., 2005; Delgado, 2005; Jos et al., 2005; Adolf et al., 2006; CDCP, 2006, Cronberg y Annadotter, 2006; Delgado et al., 2006; Andrinolo et al., 2007; Carrasco et al., 2007; Anderson et al., 2008; Da Silva, 2009; Faust, 2009; Moraes, 2009; Golubic et al., 2010).
Table 2 Toxins potentially present in the Baconao Lagoon according to the list of phytoplankton species and their classification with regards to their possible effects.
During the microscopical analyses of the water samples, cellular dinoflagellate cell rests were also observed. On the other hand, abundant brown-reddish contents with an oily appearance were observed, as well as abundant micro-particulate matter with an intense brown-reddish coloration.
Description of the evolution of the algal bloom based on evidence
The event must have manifested at first with a brown discoloration and the prevalence of dinoflagellates and green patches towards the shore in several areas of the lagoon, which confirms the presence of filamentous cyanobacteria in the intertidal zone at points 1 and 2. Dinoflagellate proliferation must have taken place about two weeks before the risk situation report, given that aged cells with abundant cellular contents are observed, as well other lysed cells with cellular material exposition.
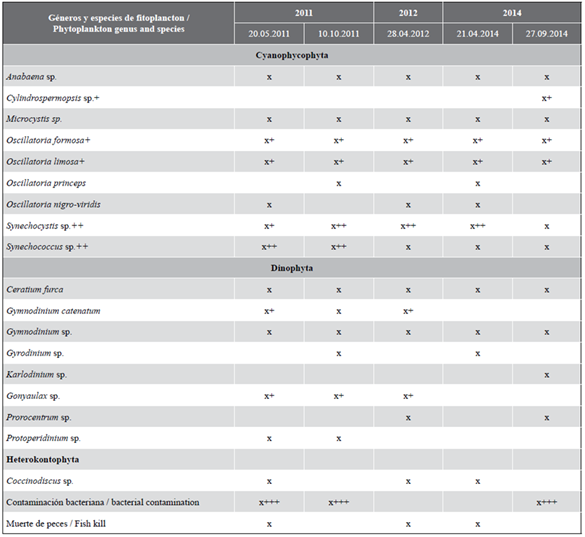
It is important to consider that similar events have occurred in the ecosystem since 2011, which constitutes relevant background for risk characterization analyses. In table 3, the results of previous water discoloration reports in the ecosystem obtained by LESA are presented along with the toxic organisms involved.
This background has conditioned the systematic analysis of the lagoon due to fundamentally sectorial interests, and it plays an important role in understanding the dynamics and/or occurrence of these events in the lagoon.
Previous records indicate the presence of at least 18 phytoplankton species in the ecosystem, out of which at least 12 have toxic potential. Six species are also present in previous blooms since 2011: Oscillatoria formosa. O. limosa, Synechocystis sp., Synechococcus sp., Ceratium furca, and Gymnodinium sp.
Relevant species due to their abundance and toxicity
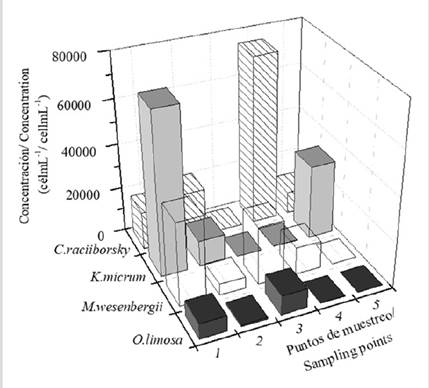
Four species stand out for their abundance and toxicity: Cylindrospermopsis raciiborsky, Microcystis wesenbergii, Karlodinium micrum, and Oscillatoria limosa. Their concentration at different sampling points is shown in Figure 5.
These species form multispecific blooms throughout the aquatic habitat, with variations among the different sampling points. The concentration of C. raciiborsky varied between the different points with values between 10 × 103 and 75 × 103cellmL-1. This species was not detected at point 3 (Figure 5). After 24 days, its presence at the site of highest concentration (point 4) decreased to 120 cellmL-1.
Karlodinium micrum appeared with its highest concentration at points 1 and 5 (33 × 103 and 72 × 103cellmL-1, respectively) (Figure 5), with a minimum concentration of 93 cellmL-1 at point 4. It was not detected at point 3. After 24 days, its presence at point 1 decreased to 50 cellmL-1.
Regarding M. wesenbergii (Figure 6A), deformed cells are observed in the ecosystem, probably due to the effect of salinity, which may indicate a fluvial contribution. Its concentration varied among the different sampling points between 5 × 103 and 37 × 103 cellmL-1, with point 1 having the highest concentration. It was not detected at point 5. After 24 days, its presence at point 1 decreased to 11 cellmL-1.

Figure 6 A. Images of identified species; B. Dinoflagellate cysts. A1. Cyanobacteria: Oscillatoria limosa and Spirulina subsalsa. A2. Coccinodiscus sp. A3. Microcystis wesenbergii. A4. Pyrophacus sp. A5. Ceratium furca. A6. Karlodinium micrum (normal appearance above, Lugol-stained K. micrum below). A7. Dinoflagellate cell rest. B1. Cyst compatible with Gymnodinum catenatum. B2. Cyst compatible with Protoperidinium conicum. B4. Cyst compatible with Alexandrium affine. B3, B5-6 Other cysts.
Oscillatoria limosa cells (Figure 6A) have abundant gas vesicles at most points. These bubbles have a granular appearance, which is typical of floating masses or mats. It appears that point be with its highest concentration (8.6× 103cellmL-1), and its lowest concentration was detected at point 5 (93 cellmL-1). After 24 days, its concentration at point 3 was of 22 cellmL-1.
At points 1 and 2, these four species form a mixed bloom with Synechocystis sp., Synechococcus sp., and O. formosa, with K. micrum being the most abundant species at point 1, whereas C. raciiborsky constitutes the majority at point 2.
At point 3, M. wesenbergii and O. limosa formed a bloom with S, subsalsa, with the first being prevalent; whereas, at point 4, all four species were present, but only C. raciiborsky and M. wesenbergii formed a bloom with Gymnodinium sp. and Synechococcus sp. The prevalence of C. raciiborsky was noteworthy.
At point 5, a bloom was identified with the presence of three out of the four species: K. micrum, C. raciiborsky and O. limosa with O. formosa and Oscillatoria nigro-viridis, with the prevalence of K. micrum.
Sediment analysis results
In the analyzed sediment samples, Oscillatoria species were mostly (O. limosa, O. formosa, and O. princeps). Isolated Coccinodiscus sp. cells were also observed, as well as dinoflagellate cells rests and cysts.
At least six different types of cysts were observed both in the sediment and the water column:
Cysts with distinctive characteristics, such as a spherical shape, a lack of ornaments, the dark brown coloration of its wall, and its fine reticulated appearance, which match those described for Gymnodinium catenatum.
Abundant ornamented cysts with a wall and spinate processes in the shape of pointy thorns, as well as antero-posterior compression. These match Protoperidinium conicum (Matsuoka and Fukuyo, 2000).
Ellipsoidal cysts with ornaments and spinate elongated processes, with bifurcated tips and a light brown wall.
Spherical cysts without ornaments, a colorless wall, and a flat surface, which matches Alexandrium affine.
Spherical cysts with more than one layer, a dark brown coloration, and an apical archeopyl with fine processes in the shape of small-sized needles on the ornamented wall.
Cysts with more than one layer, a pale-yellow wall, a spherical shape, and small spinate processes (Figure 6B).
Physicochemical analysis of the water column
The results of the analyses are presented in table 4. The dissolved oxygen values (DO) in the aquatic habitat were generally low. Points 2, 3, and 4 were the most affected, with values of 1.25 ± 0.01, 1.44 ± 0.01, and 1.34 ± 0.00 mgL-1, respectively. These values were significantly higher than those obtained at points 1 and 5. The maximum detected values are below limits stated by the regulations for fishing interest water bodies NC 25:1999, which is why it is inferred that the water quality ranges from dubious (2-5 mgL-1) to bad (˂ 2 mgL-1).
Table 4 Results of in situ physicochemical analyses. Mean values and standard deviations are presented. Different letters indicate significant differences (p = 0.05).
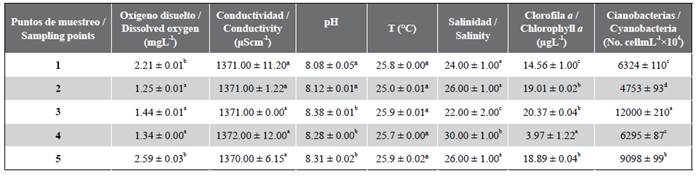
The pH values had little variation, and may remain within the range of 8-8.40, which is adequate for these water bodies. The water temperature had values between 25 and 25.9 °C. Salinity values remained between 22 and 30 ups throughout the ecosystem, with its minimum values at point 3, which could be related with specific dumping; whereas the maximum values were recorded at point 4.
According to the results obtained at points 2, 3, and 5, the chlorophyll concentration does not vary significantly. However, the cellular cyanobacteria concentration differs significantly at each point, with 3 being the most affected, it has the highest levels of chlorophyll a and cyanobacteria (p = 0.05), followed by point 5. A lax mass was detected at point 3 with a greenish coloration and large dimensions, as well as low dissolved oxygen values. Points 3 and 5 have been the most affected in comparison with regard to the analyzed parameters. The lowest chlorophyll a were recorded at point 4 (3.97 ± 1.22 µgL-1) (p = 0.05).
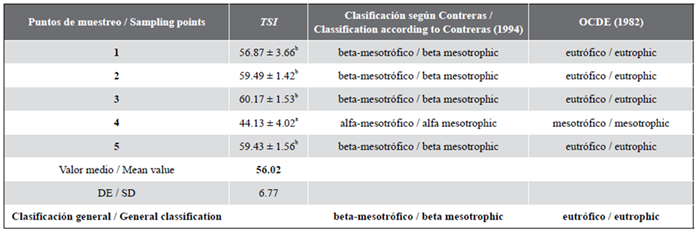
Evaluation of the trophic conditions of the aquatic habitat
In table 5, the TSI values and the classification of the trophic state of the ecosystem are presented considering different criteria (OCDE, 1982; Vollenweider y Kerekes, 1982; Contreras-Espinosa et al., 1994). The point with the lowest TSI values was point 4. All values were high at all sampled points (p = 0.05), which allows to classify the ecosystem as eutrophic.
DISCUSSION
General diagnosis
The increasing deterioration of coastal ecosystems involves the quality of their waters, biodiversity, and landscape (both submerged and emerged). In general, this compromises the services, which generates use conflicts, in addition to the direct impact on aesthetic values, fishing resources, infrastructure, and built heritage (Gómez, 2013).
The Baconao Lagoon is an ecosystem of interest for local tourism that must be systematically observed to prevent negative undesired impacts on human health. HAB affect the sea life of this ecosystem; one of the alert indicators is fish kills. Due to the uses of the ecosystem (tourism, recreation), these events constitute a risk both human health and the ecosystem itself, which is related to anoxic conditions, stench, and the presence of foams.
The evidence allows stating that the ecosystem is a coastal eutrophic lagoon at risk by contamination with phycotoxins, which is associated with the presence of species that cause HAB, fundamentally cyanobacteria and dinoflagellates, highlighting the presence of four relevant, potentially toxic, HAB-causing species, which is undoubtedly related to the identified risk situation:
Cylindrospermopsis raciiborsky, an invasive, cosmopolitan, halotolerant species with a preference for anoxic and phosphorus-rich environments; it is also a cylindrospermopsin producer, which is associated with fish kills by paralysis, given that it contains Paralyzing Shellfish Poison (PSP), as well as neo-saxitoxins and saxitoxins (Antunes, 2015; EOL, 2017).
Microcystis wesenbergii, a potentially toxic, microcystin-producer cosmopolitan species (Boyer et al., 2004; Beresovsky et al., 2006; Belykh et al., 2011, 2013, Bittencourt-Oliveira et al., 2011) that grows in eutrophic ecosystems and is associated with algal blooms (Cronberg y Annadotter, 2006).
Karlodinium micrum, a mobile small-sized dinoflagellate, a common phytoplankton species in coastal ecosystems. It is usually present in low concentrations (102-103 cellmL-1), but it is able to form algal blooms (104-105 cellmL-1). Its presence is associated with fish kills due to the production of ichthyotoxins and cytotoxins, generally called karlotoxins (Adolf et al., 2007).
Oscillatoria limosa, a toxigenic, benthic species that produces microcystin (Cronberg and Annadotter, 2006)
In spatial terms, the whole lagoon has a high-risk potential for the occurrence of HAB, specifically along the margin where sampling points were established, given that, according to ecosystem dynamics, it is the most vulnerable, as they are backwater areas with a slow water exchange. However, it is important to highlight the heterogeneous behavior of species at the different sampling points, thus forming diverse blooms. On the other hand, evidence indicates that dinoflagellates form recurrent blooms in the lagoon, which contributes to primary productivity, which is why they constitute an important component of the plankton.
Considering the meteorological reports, this ecosystem was disturbed by the influence of rainwater after the first 20 days of April with some calm periods, which may have favored the risk event, in addition to the high temperatures and the high evapotranspiration rates that characterize the region.
In 2017, rainfalls were above normal throughout the country, thus constituting the fifth rainiest year in the last 57 years. This behavior was associated with the accumulated rain reported in the central and eastern regions of Cuba, which constituted the sixth and fifth rainiest years since 1961 (INSMET, 2017a). In the second ten-day period of April, the abundant rainfall stood out throughout the country. On the other hand, April and May reported temperature values above the historic mean, with alternative minimum values from the second half of April up until; while temperature values above 31 °C were also reported for the last decade of night (INSMET, 2017b; Fonseca-Rivero, 2018).
The high temperatures reported during 2017, with particularly warm condition during the periods of rain, correspond to the tendency towards warming observed in Cuba in recent years and the global long-term warming tendency (Fonseca-Rivero, 2018), which undoubtedly favors risk events due to the proliferation of phytoplankton species (Paerl et al., 2011).
Although the ecosystem is a typical coastal lagoon dominated by coastal-marine conditions it appears that, due to the close marine relations and the channel conditions, there is significant pluvial and fluvial influence on the lagoon’s process dynamics, whereupon a 3-2 % variation in salinity was determined, which confers oligohaline characteristics on the ecosystem.
This research allows confirming a contingency situation in light of the presence of a multi-specific algal bloom almost all over the ecosystem, with several potentially toxic microorganisms involved. The main evidence for this is as follows: fish kills, a high cyanobacteria concentration, the presence of lax greenish masses on the surface of the lagoon, the presence of several toxic microorganisms associated with the production of ichthyotoxins, ciguateric and shellfish paralytic poison, stench, low dissolved oxygen values, and a high chlorophyll a concentration.
At some point, there must have been a transition from a red or brown tide to a green one with toxic and non-toxic cyanobacteria species, the latter being more potentially lethal when combining its toxic action with the anoxic conditions of the ecosystem, which may justify the presence of mucus in fish gills, which was verified in all analyzed samples.
By comparing the performed analyses, the HAB may have caused the fish kills, according to the report, which may be related to abrupt changes in weather conditions, with pluvial and fluvial contributions which favored the proliferation of phytoplankton species. The fish kills may have been caused by the effect of toxins and/or by asphyxiation, given that, even though the presence of ichthyotoxin species was corroborated, abundant mucus secretion was also detected in the gills of the analyzed fish. The ingestion of dead fish by the dolphins may have caused the change in their behavior.
It is worth highlighting the presence of a dust cloud from the Sahara Desert over the Caribbean region from March 16, 2017, which persisted until the end of April, as it was verified after the analysis of satellite images and information bulletins of National Meteorological Institute (INSMET, 2017a). This fine-particulate dust is loaded with iron and has a reddish coloration, which is compatible with the traces of red micro and nano particulate matter observed, which is why a synergy due to the water fertilization by the presence of Saharan dust is not discarded (Walsh and Steidinger, 2001; Dorta et al.; 2002; Sellner et al., 2003; Langlois et al. 2012), along with the conditioning weather factors that jointly allowed the accelerated growth of the phytoplankton species present in the ecosystem, which is eutrophic per se. All this, together with the temperature values recorded in the ecosystem, which was above 25 °C, thus favoring the proliferation of phytoplankton species and cyanobacteria (Kosten et al., 2012; Rosso and Giannuzzi, 2015; Yan et al., 2017; Nalley et al., 2018).
The lowest chlorophyll a recorded at points 1 and 4 may be related, at point 1, with two nearby streams (Figure 1) and, at point 4, with the proximity to the inflow of water from the sea. It is important to point out that the chlorophyll a concentration values in the water column range from small amounts (exceptionally 0.01 mgm-3) to amounts nearing 100 mgm-3, even though the specific maximum concentrations that exceed said limits have been obtained in some ecosystems (e.g. 126 mgm-3) (Contreras-Espinosa et al., 19994).
The mechanisms that may have been related with the occurrence of the HAB could have been the changes in weather conditions, the ecosystem’s own dynamics, and the direct losses by herbivorism, confirmed through the presence of the abundant and diverse zooplankton.
On the implementation of the risk management protocol
The proposed risk management protocol for phycotoxins contamination was focused on mitigating and preventing negative impacts. Its primary objective was to protect human health, considering the existence of tourism activities and fish, shellfish, and Portunidae illegal fishery in the ecosystem, as well as strengthening the actor network, with sustainable alternatives based upon the creation of a workgroup that included executives, technicians in charge of monitoring the risk event, and scientific personnel or experts in HAB (interdisciplinary team), where the presence of entities such as the government was relevant. It was represented by CITMA, those responsible to the area (Flora y Fauna), the community, and sanitary authorities.
The inter-sectorial strategic alliance between CITMA, Flora y Fauna, and the University (LESA) constituted a key factor for the successful implementation of the protocol, which allowed for the joint and rapid action by all parts with the leadership of CITMA. The approval of the protocol by local authorities and the elaboration of a timely communication system also constitute success factors. On the other hand, it was relevant to involve the primary and secondary health care personnel to be able to manage the risk situation and its potential impact on neighboring communities. In general, the actions taken contributed to the knowledge of the ecosystem in terms of its sustainable management, as well as to improving, among the key actors, the perception of the risk by the presence of phycotoxins.
The first stage of protocol was characterized by the visual detection of risk through primary indicators (level 1), as well as alert indicators such as toxicity reports (level 2). Coordination with the actor network was essential in this stage, thus guaranteeing the communication flow of the results of the risk characterization.
Considering that these events may be recurrent in the ecosystem, it is necessary to implement control tools supported by the applicable regulations, a matter that, as of now, is not well established at the national level regarding the risk by HAB, which may have great negative environmental, economic, and social impacts (Anderson, 2009, Anderson et al., 2012).
In the documented case, no diseases are reported in humans, which constitute a favorable aspect of the management protocol implemented. However, medical preparation and assistance must be guaranteed in case of intoxication due to this type of toxins.
The basic parameters that complement the systematic monitoring must be of a physical, chemical, and biological nature, both qualitative and quantitative. Similarly, meteorological variables must be considered since the risk scenario must be observed in its entirety, also taking into account that this kind of event is multicausal (Anderson et al., 1998. 2002; Ajani et al., 2001; Burkholder et al., 2008; Burns, 2008; Adolf et al., 2009). The dynamics of the harmful and/or toxic episodes varies depending on many factors, not only on the hydrographical and topographical features of the area, but also on ecological and biological aspects of the organisms involved, as well as the social-environmental conditions of this setting.
Among the physical factors it is relevant to consider the meteorological data of the study area: climatic variables such as winds, rainfall, sunstroke hours, and the average irradiance level, as well as water temperature, turbidity, tidal regime, and even the presence of Saharan dust (Sellner, 1997; Walsh and Steidinger, 2001, Sellner et al., 2003, Thomazeau et al., 2010; Rijkenberg et al., 2011), since, when atmospheric parameters are conditioned, the rainfall regime, thunderstorms, and the cyclogenesis and evolution of tropical cyclones are modulated (López et al., 2015). Among the chemical indicators, the pigments concentration, especially chlorophyll a, as well as salinity and nutrient concentrations, namely nitrates, phosphates, ammonia, iron, and silicates. Biological analyses must be focused on phytoplankton and phytobenthos, considering qualitative and quantitative aspects.
Documentation is a key process during the implementation of the risk management protocol. In this case, it allowed for a detailed description of the episode, complemented by the use of specific instruments such as surveys, interviews, focus groups, among others, to be able to understand dynamics and estimate the duration of the phenomenon from its initial establishment, as well as its perception by the users of the ecosystem.
When the presence of potentially toxic organisms is confirmed, restrictive measures on the uses of the ecosystem must be immediately established. It is necessary to interrupt any use that may generate collateral damage to people or animals or compromises the balance and services provided by the ecosystem. This constituted an essential element to prevent human intoxication.
Hereafter, some important recommendations for coastal-dependent entities are presented in light of the new environmental scenario and the risk due to the phycotoxin contamination, specifically for coastal ecosystems related to touristic activities, fishing, and/or recreational or leisure activities:
The search for early indicators that warn about contamination of the ecosystem with potentially toxic species is imperative.
Monitoring and control programs must be established for each aquatic habitat regarding this kind of event or any risk situation.
A hydromorphological description of the aquatic ecosystem and a general diagnosis of its uses and conflicts must complement the risk analyses.
Some species produce potent toxins that may be accumulated by filtering marine organisms, especially by bivalve mollusks. Gastropods, crabs, and certain fish are capable of trophically accumulating toxins. Their ingestion has originated and is the cause of very serious human intoxications which may lead to death in some cases (Carreto et al., 2007). Therefore, the systematic risk analyses and timely control measures must be guaranteed to prevent negative impacts.
Decision-makers must be directly advised by experts; only experts can confirm the total remission of algal bloom and the end of the emergency phase.
When establishing regulations, the importance of indicators for risk detection from early stages through visual supervision must be considered.
Training and communication must be a part of specific strategies that need to be developed with a focus on sustainability as a function of ecosystem management.
To complement the above, a decision-making chart for cases of phycotoxins contamination must be considered, which must state that the toxic species may belong to six different groups that differ in their morphological, physiological, and ecological characteristics (Okaichi, 2003). Taking into account that the most abundant microorganisms in the ecosystem in terms of diversity and total density were cyanobacteria; a decision-making matrix is presented for the alert level in case of cyanobacteria blooms, which complements the risk management protocol (Table 6).
Table 6 Decision chart for the alert level in case of planktonic cyanobacterial blooms modified to coastal lagoons (Gómez et al., 2012).

In all cases, it is suggested, if the necessary resources are available, that a vector analysis be performed (shellfish, fish, bivalves), as well as the toxin profile of the water’s phytoplankton biomass, toxicity tests (such as the alkaline phosphatase test), and even the isolation of species and the establishment of monoalgal cultures for study. These aspects have also been previously suggested (Sar et al., 2002). The chart includes the monitoring stage and must be implemented by decision-makers at different levels. For each action level, three conditions are established: A, B, and C, with the latter being a transition to the next level.
Table 6. Decision chart for the alert level in case of planktonic cyanobacterial blooms modified to coastal lagoons (Gómez et al., 2012).
The balance between the risk analyses and the cost-benefit relationship will always be present in entities operating in contaminated ecosystems, specifically in those where there is previous documentation of recurring algal blooms or toxic outbreaks. However, it must be understood that operating under risk conditions implies compromise from key actors and decision-makers, given that it may imply severe damage to human health, in addition to the progressive deterioration of the ecosystem up to the total unfeasibility of its services, of which there are already documented cases at the global level (Murugan et al., 2013).
CONCLUSIONS
The Baconao Lagoon is a eutrophic ecosystem at latent risk of HAB. The presence of a mixed HAB involving toxic cyanobacteria dinoflagellates conditioned the massive fish kills, in response to which a decision-making procedure and a risk management protocol focused on mitigating and preventing negative impacts on marine species and neighboring communities were immediately implemented.
Immediate actions were developed, among which the following stand out: the creation of the multi-sectorial and interdisciplinary risk management commission, the restriction of ecosystem uses, communication with all actors, the review and re-elaboration of the contingency plans of entities with interests on the aquatic habitat, and the development of an intensive monitoring plan that allowed the participation of experts in their direct interaction with the decision-makers.
Coordinated action between key actors and CITMA’s leadership guaranteed the successful implementation of the protocol for a contingency situation. The implemented protocol and the decision matrix for the alert level in case of planktonic cyanobacterial blooms modified for coastal lagoons may be applied on any ecosystem under these risk conditions.
In ecosystems where HAB are recurring events, monitoring must be systematic, which is achieved through the strengthening of the actor network; the alliance between key actors facilitates exchange and permanent cooperation, as well as rapid action during contingency situations, along with community participation.











 text in
text in