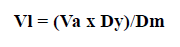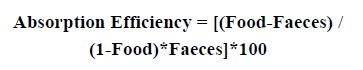INTRODUCTION
The Pacific white shrimp Litopenaeus vannamei is one of the main aquatic species used in coastal aquaculture. This crustacean’s cultivation accounts for about 15 % of the total fishery production and has a global market value of nearly 18 billion USD (FAO, 2017). The Pacific white shrimp has several development stages: larval, post-larval (PL), pre-juvenile, juvenile, and the final adult stage. The PL stage occurs after the larval stages of nauplii, zoea, and mysis. In the zoea and mysis stages, white shrimps are planktonic organisms that eat dead organic matter, algae, and small invertebrates. In commercial aquaculture operations, the food for larval shrimp consists of one or two diatom species, such asChaetoceros gracilisandThalasiosira weissflogii(Kiatmetha et al., 2011), and the concentration of these microalgae in the rearing tank fluctuates around 1 × 105cell/mL (Müller-Feuga et al., 2003). The mysis and post-larval phases are fed with the microcrustacean Artemia salina; however, many hatchery operations replace the live food with artificial food. The PLs produced in hatcheries are transported to rearing facilities under semi-controlled conditions, either in greenhouse ponds or in pre-growth-out ground pools.
Typically, shrimp farming occurs in, or near a mangrove ecosystem. For example, Ecuador, the major shrimp production country of America, has approximately 2,200 km of coastline, with about 75 % of shrimp farming located near a mangroves forest. Yeasts occur in the marine shoreline as a part of a large microbial community (Chi et al., 2012). The use of microorganisms as a potential food source in aquaculture systems has been reviewed by several researchers, who explored the importance of bacteria, yeasts, fungi, and microalgae for maintaining different aquatic animals. The composition of yeast suggests that it can be a reliable alternative food source for marine organisms due to the presence of polymers, polyunsaturated fatty acids, and large amounts of vitamins and minerals (Shelby et al., 2019). The yeast cell wall consists of 57 % β-glucan, 6.6 % oligosaccharides, and 22 % glycoprotein (Meena et al., 2013). Sahlmann et al. (2009), Chi et al. (2010), and Kupetz et al. (2015) reported that yeast has a high nutritional value for fish and shrimp in cultivation conditions. Similarly, Lara-Flores (2003) stated that yeast can effectively replace other source of protein, achieving better fish growth and development. Farzanfar (2006), Sukumaran et al. (2010), and Zheng (2017) reported that feeding yeast to fish and shellfish reduced disease by diminishing the presence of pathogenic bacteria in the intestinal tract. The nutritional value of yeasts makes them appropriate as supplemental food for fish and crustaceans (Pathissery, 2016; Sarlin et al., 2016). For example, Zhao et al. (2017) provided yeast extract mixed with fish oil, phosphorus, and calcium as a protein replacement for fishmeal forL. vannamei. A considerable number of commercial immunostimulant products used in aquaculture are derived from yeasts, which reflects the importance of these microorganisms to this industry (Villamil-Díaz and Martínez-Silva, 2009). Furthermore, due to yeast’s size and nutrient composition, several researchers have proposed using live yeast to bio-enrich prey organisms such as rotifers and brine shrimp (Lavens et al., 1996; Patra and Mohamed, 2003), but this practice is only beginning to be used in aquaculture systems.
Notwithstanding all the positive effects of yeasts, there has been little research on their utility as live food for shrimp cultivation (Villamil-Díaz and Martínez-Silva, 2009). No studies have been conducted to evaluate the ingestion, colonisation, or digestibility of marine yeast in the white shrimp L. vannamei. Moreover, the knowledge about food absorption efficiency in shrimp larvae is restricted to ingestion of microalgae (Urabe, 1991; Evjemo, 2000). Little information is available concerning the performance of shrimp post-larvae sustained exclusively on a yeast diet. The objective of this study was to obtain yeast from mangrove mudflats and test it as a dietary source for the marine white shrimp post-larvae. The use of marine yeast as a food source was investigated by measuring the absorption efficiency of yeast inL. vannameipost-larval stages at different yeast concentrations. The food absorption efficiency, also known as food digestive efficiency or digestibility, is the proportion of organic matter assimilated by an organism (Lucas and Watson, 2002). Hence, for the yeast to be usable for shrimp, it must be assimilated, degrading its organic compounds. Because the food available to shrimp occurs as organic matter, its absorption indicates the shrimp’s ability to absorb its natural compounds. This study evaluates the absorption efficiency of marine yeast by measuring the proportion of organic matter in the yeast (food) and the faeces of the shrimp, where the organic component of the food ingested is assimilated by the process of digestion (Conover, 1966).
MATERIALS AND METHODS
This work includes a sampling stage (mangrove-shrimp area) and an experimental stage (yeast propagation and feeding of PLs). To obtain the yeast, a single sediment sample was taken from a mangrove mudflat in the coastal shrimp farming area near Machala, El Oro province, Ecuador (longitude 9636225 and latitude 620861). The phases of sediment treatment, the maintenance of post-larvae, and the set-up of the experimental tests took place in the aquaculture laboratory of the Agricultural Sciences Faculty of the Technical University of Machala, located approximately 45 km from the sampling point.
Sampling sediments; yeast isolation and culture
Using a sterile spatula, 500 g samples were collected aseptically taken 10 cm from the upper sediment layer of the mangrove forest. The sediment sample was placed in hermetically sealed sterilised plastic containers, kept in cold conditions between 4 to 10oC, and transported to the laboratory immediately after collection. Under fixed conditions in the laboratory, 1 g of sediment was suspended in 10 mL of peptone solution. After that, 1 mL of fresh solution was placed into Saboraud agar culture plates and incubated at 30oC for two days. After the incubation period, when colonies of microorganisms appeared, some of the colony-forming spots were transferred to a test tube containing a concentrated peptone solution. In this way, we obtained a primary yeast stock. The propagation of the yeast proceeded from the 250 mL of stock culture to intermediate stages of 1 and 3 L. The yeasts were cultured in a medium consisting of a solution prepared with seawater filtered through a borosilicate microfibre (0.47 µm). The water was enriched with 0.65 g/L sodium phosphate, 1.0 g/L sodium nitrate, and 10 mL/L molasses. The yeast culture medium was acidified with muriatic acid to a pH of 4.5, the recommended condition for theSaccharomyces cerevisiaestrain (Vieiraet al., 2013). The culture was ready for use when the 5 L of culture reached the exponential growth phase. To ensure the purity of the molasses, the sugarcane was obtained directly from a farm located in the upper lands of the province of El Oro, Ecuador.
A portion of the yeast mass was collected and transferred to a glass fibre filter. Then the yeasts were rinsed with distilled water in a vacuum filtering system. Six samples of the yeast stock were preserved for further organic matter analysis.
Maintenance of Litopenaeus vannamei PLs and experimental design
TheL. vannameipost-larvae (3-4 days old) were obtained from a local commercial laboratory located nearby the testing laboratory at the Technical University of Machala. During the larvae culture operation, the organisms were fed phytoplankton during the zoea phase and a combination ofArtemia salinaand microparticulate diets during the mysis phase (Figure 1).
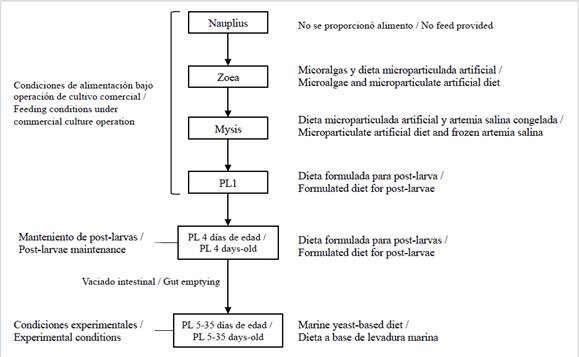
Figure 1 Flowchart of shrimp larviculture adopted in commercial culture operation and maintenance of post-larvae for marine yeast feeding experiments.
During the post-larval stages, the food regime consisted exclusively of formulated food. When 3-4 days old, the PLs were transported to the testing laboratory’s bioassay area at the Faculty of Agricultural Sciences at the Technical University of Machala for the experimental phase. The PLs were acclimatised and maintained in a 500 L culture tank. Following the feeding protocol of commercial laboratory, the PLs were fed ad libitum a diet composed of 100 % formulated food (52 % protein). The PLs were maintained in natural seawater filtered and maintained at an ambient temperature. The water salinity was 26 parts per thousand, and the temperature fluctuated between 26 and 28oC. The PLs were kept at these conditions until 35 days old.
Figure 2 shows the experimental design of this study. At each PL, stage starting from fivedays old, approximately 5,000 PLs were randomly selected from the rearing tank and stocked in a fibreglass aquarium with 60 L sterilised seawater. Before food (yeasts) was placed into the fibreglass aquariums, the post-larvae were kept at rest (no feeding) in 100 % pure water for 12 hours to allow for the total evacuation of the food they had consumed in the maintenance tank. During the rest period, the water was replaced at 100 %, ensuring faecal material removal in the aquariums. Each group of post-larvae was fed differently, according to the design. At each post-larval stage (5, 10, 15, 20, and 35 days old), the organisms were placed in the aquariums for 24 h for yeast feeding exposure, starting with the 5-day-old PLs and repeating at each stage.
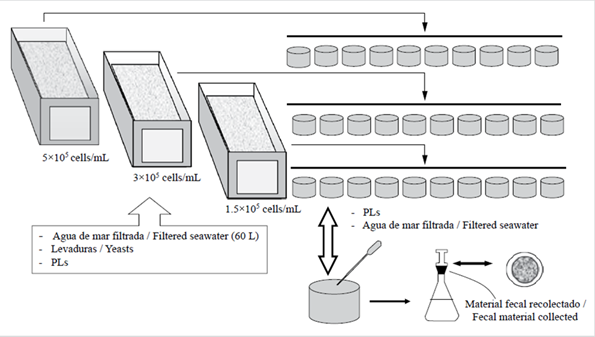
Figure 2 Schematic diagram of the experimental design, showing the three experimental aquariums to stock the Litopenaeus vannamei postlarvae fed for 24 h marine with a yeast-based diet, the 10 containers for the excretion of the post-larvae and the procedure for collecting the fecal material.
The PLs were fed three different concentrations of yeast: 1.5 × 105, 3 × 105, and 5 × 105cells/mL. The yeast density was calculated using a hemocytometer and the Nikon Optiphot microscope. The yeast density was measured every three hours in both the experimental aquaria and the yeast propagation flasks. Constant aeration was provided in each aquarium to maintain the yeasts in suspension in the aquarium’s water column.
The yeasts were added according to the method recommended by the Northeastern Regional Aquaculture Center (1993), adjusting the following formula:
Vl: volume of yeast culture to for feeding post-larvae
Va: volume of the poslarvae aquarium
Dy: target yeast density in the aquarium postlarvae
Dm: density of yeasts in the massive yeast culture
At each phase, the PLs were checked to examine gut content and overall behaviour. The yeasts were continuously fed into the containers by drip injection using a serum physiological kit and adjusting the quantity of yeast culture solution to maintain yeasts’ desired concentration in each experimental phase. For the determination of yeast absorption efficiency at each stage, 10 PL groups were collected after 24 h of feeding. The PLs were collected and transferred to the vessels filled with sterilised seawater. Within approximately one hour, the PLs evacuated their faeces. The faeces were removed from the flasks using a plastic micropipette. The faecal material was placed in small aluminium caps and stored for further analysis.
Determination of yeast absorption efficiency
The absorption efficiency was evaluated by measuring the proportion of organic matter in the yeast (food) and in the shrimp faeces, where the organic component of the food ingested is assimilated (Conover, 1966). The faecal material and the yeast previously collected were separately filtered in a GF/C Whatman fibreglass filter (previously calcinated). The fibreglass filter was rinsed with distilled water while maintaining a vacuum pump filtering system. After filtration, the filters were placed into pre-weighed aluminium caps. The dry weight was determined using a Denver Instrument analytical scale (model X-100), and once the yeast and faecal products were dried in an oven at 60 °C for 48 hours and until constant weight was achieved. After that, the product was transferred to a desiccator to cool for 15 min. The dry matter resulted from the difference between the weight of the filter plus dry product (yeast or faeces) and the weight of the filter. The weight of the inorganic constituents (ashes) was obtained by placing the filters plus the dry material in aluminium caps and burning in a muffle furnace at 450 °C for 4 h. The material was then placed in the desiccator before re-weighing. Finally, the proportion of organic matter was estimated, taking into account the dry weight and the organic content of the food (yeast) and the faeces. The absorption efficiency was calculated using the method of Conover (1966) according to the formula:
where ‘Food’ represents the ratio of organic matter to ash in the food, and ‘Faeces’ represents the ratio of organic matter to ash in the faeces.
Capture and processing of images
Images of the yeast cell, postlarva gut, and faeces were obtained using a Nikon Optiphot microscope fitted with a Plumix Model TMC-7 camera. The images were digitised using an image capture card (ATI All-in-Wonder). The digitised photos were processed using Scion Image 3.0b software. The yeast was measured following the instructions in the program.
Statistical analysis
Statistical analyses were performed using SPSS for Windows. Two-way analysis of variance (ANOVA) with a factorial arrangement was used to determine whether there were significant differences (p< 0.05) between the different concentrations of yeast (1.5 × 105, 3 × 105, and 5 × 105cells/mL) and between the PL age groups. Multiple ranges of Tukey’s test (p< 0.05) were used to identify treatments that could be significantly different.
RESULTS
Yeasts were extracted from the mangrove mudflats around a shrimp farm located in southern Ecuador’s marine coastal zone. Under laboratory conditions the yeast cells were purified in about 10 days. The yeast colonies were recognized by their ivory colour and rounded morphology (Figure 3).
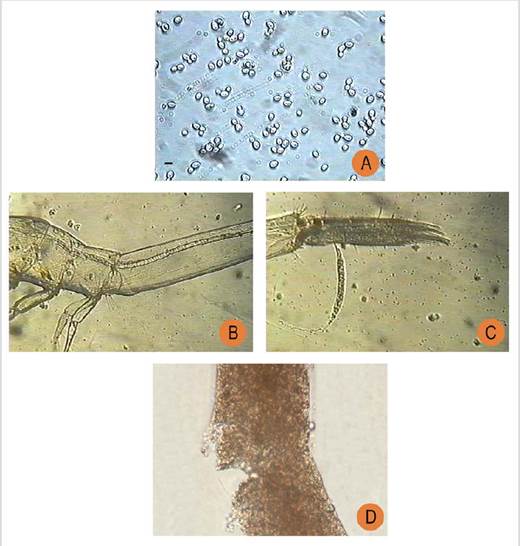
Figure 3 Photomicrographs showing yeast absorption by Litopenaeus vannamei post-larvae during the feeding experiments. (A) Yeast cells; (B) gut of a Litopenaeus vannamei post-larva filled with yeast; (C) the yeast excretion; and (D) the product or faeces (Source: authors collection.)
The yeast cells were proliferated using a molasses (by-product of sugar cane) as the primary growth substrate until reaching an exponential growth phase with a concentration of over 70 × 106 cells/mL. The average size of the yeast was about 3.56 µm with 0.87 ± 0.04 % organic matter content.
During the trials, the microbial colonisation was observed in the gut of the post-larvae, demonstrating that yeast had been ingested and had adhered to the intestinal mucosa of theL. vannamei. It was observed that yeast cells were destroyed by shrimp PLs, suggesting an efficient enzymatic process during the digestion. Importantly, no cannibalism was observed during the yeast feeding experiments.
After the 24-hour feeding period in each treatment, the fecal material consisting of yeasts digested by the postlarvae was collected. In total, 180 samples of faecal material were obtained from L. vannameiPLs to determine the content of organic matter. Figure 4 shows the absorption efficiency of the six different PL stages at the three different yeast concentrations. Overall, the effectiveness of yeast absorption in L. vannameiPLs was 63.71 ± 2.56 %.
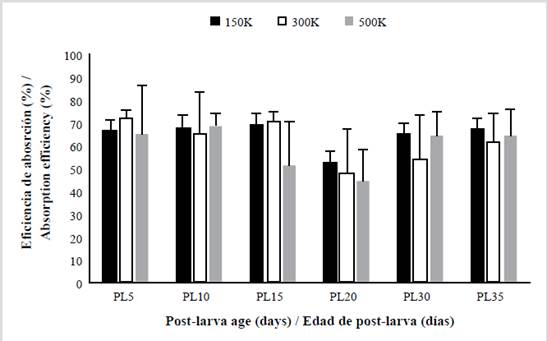
Figure 4 Yeast absorption efficiency (% mean ± SD) in six life stages of the shrimp L. vannamei post-larvae fed with three concentrations of marine yeast. The X axis represent the percentage of absorption efficiency and the Y axis show the post-larvae age.
The analysis of variance (p< 0.05) showed that the yeast absorption efficiency between different concentrations was not significantly different. Similarly, the PL age and yeast concentration level did not have a significant interactive effect on absorption efficiency. The ANOVA test showed that absorption efficiency depended on the PL age. The statistical analysis (Tukey’s test, p< 0.05) identified two distinct groups. The first group consisted of 5-day-old (67.85 ± 10.31 %), 10-day-old (67.22 ± 11.25 %), and 15-day-old PLs (66.84 ± 9.05 %). The second group consisted of 20-day-old (55.29 ± 9.44 %), 30-day-old (63.71 ± 10.65 %), and 35-day-old PLs (61.63 ± 11.43 %). The young post-larvae (5 to 15 days old) had an average absorption efficiency of 67.30 %, which was significantly different from the average of 60.21 % for older post-larvae (20 to 35 days old).
DISCUSSION
Marine yeasts are an essential component of the microbial community in coastal environments, contributing significantly to the balance of mangrove ecosystems and constituting a part of the natural diet of fish, crustaceans, and molluscs. Several yeast species of the genera Candida, Devaryomyces, Saccharomyces, and Schizosaccharomyces exist in mangrove ecosystems (Ahmed et al., 2019). Fish living in marine ecosystems absorb suspended particles and microorganisms, including bacteria, phytoplankton and yeasts, either in suspension or adhered to substrates (Wasieleskyet al., 2006; Gatesoupe, 2007). Therefore, the mangrove mudflats surrounding the tropical shrimp farming area are a vital source of live food for Litopenaeus vannamei post-larvae.
In southern Ecuador, most shrimp farmers use molasses, following different protocols for the activation of bioactive compounds containing yeast and other bacterial strains such as lactobacillus; the use of molasses has increased over the last decade. When producing yeast, shrimp farmers take advantage of using cheap molasses (Martinezet al., 2015), a by-product obtained from sugar cane in tropical countries, as a carbon source. The use of commercial microorganisms and substances added to exogenous feed in all phases of the culture system stimulates beneficial microbial growth and inhibits colonisation by pathogen bacteria (Villamil-Díaz and Martínez-Silva, 2009). In the present study, the yeast species used for the experiments was not identified; however, the acidic environments, the glucose saturation, and the fully aerobic conditions are ideal for the massification of Saccharomyces cerevisiae (Vieira et al., 2011). The 5-, 10-, 15-, 20-, and 35-day-old PLs showed efficient handling and ingestion behaviour during the short-term exposure of yeast feeding. This study demonstrates that shrimp PL are able to absorb the organic components of marine yeast cells, which implies that all biological constituents, such as proteins, organic acids, carbohydrates, fatty acids, and vitamins, are incorporated by the shrimp. When shrimp process and assimilate the yeast’s organic matter, the absorption efficiency measures the organic component of the food assimilated by the organism. Although the method has some limitations, it was practical for the short-term exposure experiments maintaining constant yeast concentration to estimate the absorption efficiency. Measuring absorption efficiency in combination with microscopic observation revealed the interactions of shrimp post-larvae and the role of marine yeast as a food component. In similar experiments, Conover (1996) has suggested that the digestion of food in aquatic organisms lead to the production of nutrient-rich faeces that can be re-ingested by the organism as part of its feeding behaviour. In the present study, other substances, such as excreta and mucus, can be present in the experimental aquarium throughout the short-term feeding. Therefore, the organic matter, including dead or digested yeast, can be re-ingested by the post-larvae.
Concerning yeast concentration, this study found that the yeast assimilation efficiency did not depend on concentration in the range tested (1.5 × 105, 3 × 105, and 5 × 105 cells/mL). Piña et al. (2005) evaluated the development and growth of the zoea larval stage ofL. vannameifed withChaetoceros muelleriand found that the ingestion percentage did not vary with the rations supplied or with the age of the zoea larvae, ranging from 74 % to 86 %. However, they concluded that food ingestion depends on continued food supply. In the present study, when feeding yeast to L. vannamei post-larvae, the intention was to simulate the feeding strategy used in commercial hatcheries, where shrimp larvae are fed phytoplankton such asChaetoceros sp. or Thalassiosira sp. The results suggest that keeping a minimum yeast density of 1.5 × 105 would be advantageous for shrimp post-larvae, even if yeasts are provided only as a complement to other natural or artificial food. Additional research in related crustaceans indicates that food assimilation efficiency decreases as food concentration increases. For example, Evjemo (2000), measuring the carbon content in the food, demonstrated a decrease in assimilation efficiency in several larval stages ofA. rtemia franciscana when the concentration ofIsochrysis galvanaincreased. Earlier, Urabe (1991) reported a decline in the assimilation efficiency forBosmia longistriswhen the concentration ofScenedesmus sp. andChlorella sp. increased. In shrimp larvae culture, the concentration of microalgae provided as food fluctuates around 1.0 × 105cell/mL. Using artificial diets and diatoms, Condreyet al. (1972) reported a range of food absorption efficiency from 55 % to 87 % forPenaeus aztecusandP. setiferus.
Regarding the absorption efficiency and PL age, early post-larval stages ofL. vannameiassimilated the marine yeast more efficiently than older post-larval stages. The absorption efficiency in youngL. vannameipost-larvae (5 to 15 days old) was about 7 % higher than that of older stages (20 to 35 days old) with 20-day-old PLs having the lowest efficiency. Early PL stages appear to be more efficient at digesting yeast than later PL stages. Although it is suggested that 5.23 um is the minimal size for shrimp post-larvae (Gelabert and Pacheco, 2011), the filter-feeding behaviour of the white shrimp appears to be an efficient mechanism for yeasts uptake. The reason for the decrease in absorption efficiency in 20 to 35 days-old PLs could be attributed to a change in feeding behaviour, leading to decreased yeast intake during older stages.
The results, 63.71 ± 2.56 % yeast absorption efficiency forL. vannameipost-larvae agree with previous results reported for other crustaceans and fish species. The use of yeast as a protein source forL. vannameiwas examined by McLean et al. (2006), who reported successful developmental performance. Rumsey et al. (2009) and Zhenminget al. (2006) found that fish digestion of single-cell proteins from marine yeast is generally above 80 %. In comparison with other nutritional products, several researchers confirmed that the protein digestibility of soybean meal forL. vannameifluctuates from about 80 % to 98 % (Zhou et al., 2015; Fang et al., 2016). In other studies, Qui and Davis (2016) examined the use of flash-dried yeast as a supplemental feed and its effects on the growth and digestive capacity ofL. vannamei. Their results show that the apparent dry matter digestibility of soybean meal, fish meal, and flash-dried yeast fluctuated around 75 %, 68 %, and 58 %, respectively. Athitahn and Ramadhas (2000) investigated the food conversion efficiency in the white shrimp Penaeus indicus fed with decomposed mangroves leaves. The authors perceived that the shrimps consumed decomposed leaves and found 87.96 % of assimilation efficiency. The assimilation efficiency was attributed to the high protein content of mangrove leaves. The protein digestibility of flash-dried yeast was significantly lower than that of fish meal and soybean meal. Terrazas and Fierro (2010) also reported that the protein digestibility of fish meal forL. vannameiranged from 62 % to 84 %. Likewise, Brunson et al. (1997) indicated that the protein digestibility of fish meal forPenaeus setiferusis around 75 %. Furthermore, the nutritional value of yeast has been tested by mechanical lipolysed cells or disrupted cells, showing favourable feeding efficiency in fish and shrimp. For example, Shalmannet al. (2019) used heat-inactivated Candida subtilis and investigated its use as a complement for food in Atlantic salmon smolts. Their results showed that fish fed an exclusively yeast-based diet had an efficient growth rate and feed intake. In shrimp culture systems, yeasts are added to the feedstuff, or yeast biomass is inoculated into the culture system. Rivera et al. (2018) extracted the yeastSaccharomyces cerevisiaefrom the mud of a shrimp pond, binding the yeast cells to a carrier. Their results showed that the shrimp strengthened the immune systems of juvenile shrimp L. vannamei. Sarlin and Philip (2016) tested the use of biomass yeast inFenneropenaeus indicus and reported a significant improvement in shrimp growth rate. They found thatCandida sake,C. utilitis, andDebaryomysis hanseniistrains yielded the best results.
The outcomes of the present study support the use of yeast as part of the shrimp diet in the culture ponds, primarily in hatcheries and nursery stage. The shrimp PLs can ingest and digest the yeasts if these microorganisms are present in sufficient quantities in the water column. Although this study does not confirm that post-larvae can be fed exclusively on yeast, it shows that yeast can be used as a portion of the live food for shrimp post-larvae. For example, Liao (1985) recommended the use of live food for convenient and efficient shipping and transportation of post-larvae. Live yeast can be used as feed during PL transportation and acclimation before placement into the breeding system (Lavenset al., 2000). Furthermore, in systems with high organic matter production, such as shrimp nursery culture systems, a microbial feeding approach is necessary to create organic detritus of high nutritional value (Martínez et al., 2015). While yeast becomes a feasible food source for the early stages of shrimp, its use to improve digestion of exogenous food may benefit the food metabolism, enhancing productivity and decreasing adverse effects on coastal ecosystems (Bender and Philips, 2004). Yeast production as part of an integrated mangrove-shrimp farming method can help make aquaculture in tropical countries sustainable by setting standards for ecological interactions (Bushet al.,2013).
Although the present study’s conclusions are limited to the digestion efficiency during the organic assimilation of yeast by the L. vannamei post-larva, this confirms current knowledge about the benefits of marine yeast extracted from mangrove ecosystems. The assimilation of marine yeast by shrimp postlarvae, demonstrated in this study, suggests its use as a potential food alternative to maintain the early stages of shrimp life, contributing to their development and growth. More research is needed to understand other practical advantages of live yeasts and their interactions with the coastal environment when used as a portion of the diet in aquaculture operations.











 text in
text in