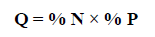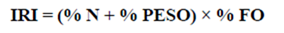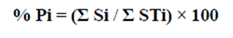INTRODUCTION
Fish live an environment with which they are in permanent interaction and in which any variation or change will affect their development (Romano, 1996; Gamboa et al., 2008). The trophic habits of fish are susceptible to perturbation caused by variations in the environmental conditions of their surroundings (Ruíz, 2018), which are reflected in the availability of food. For example: decreased water transparency might make it harder to see certain prey items, reducing the ability of predators to feed themselves (Botero, 2004). In addition, the way in which fish feed produces an exchange of materials and energy within the trophic web, thereby sustaining an ecological niche in the ecosystem (Guevara et al., 2007). For this reason, an understanding of trophic ecology provides important information on the dynamics and structure of the community (Cogua et al., 2013), making it possible to mitigate and counter the potential impacts to which a community might be susceptible (Amezcua and Portillo, 2010).
Fish of the Paralichthyidae family are lefteye flounders and generally found in coastal environments (Robertson and Allen, 2015), usually on ocean and estuarine bottoms (Lopez et al., 2014), where juvenile flounders encounter appropriate conditions to feed and grow (Flores et al., 2013). Estuaries are places in which fresh and salt water meet. They have distinct physico-chemical and biological characteristics (Acha and Mianzan, 2003) and are highly productive, generating detritus, nutrients and organisms that are available to neighboring ecosystems, including those of the continental shelf (Arancibia, 1987). In addition, estuaries may be directly affected by urban development, since they are found in coastal zones affected by multiple stress factors, including industrialization and overfishing (Marcovecchio and Freije, 2013). These factors of anthropic origin, along with others that are natural, such as river dynamics, are capable of conditioning the environmental dynamic by producing variations in salinity and nutrient levels, temperature and organic materials, which may in turn influence the biological processes of species that depend on, or that relate to, these ecosystems (Sánchez-Gil et al., 2008).
The trophic ecology of C. gilberti Jenkins and Evermann, 1889 (Robertson and Allen, 2015b) has been little studied. However, the species is abundant in tropical estuaries (Moraes et al., 2009). It has been determined that the diet of the Paralichthyidae consists primarily of crustaceans and fish (Guedes et al., 2004; Marques et al., 2009; Santos, 2014). Similarly, the principal food of the genus Citharichthys is crustaceans, followed by polychaetes and fish (Guedes et al., 2004). Finally, the diet of C. gilberti in particular has been recorded to consist of crustaceans (shrimp/crabs), mobile benthic worms and bony fish (Robertson and Allen, 2015b). C. gilberti, is classified as a permanent resident of the Buenaventura Bay estuary (Molina et al., 2020). It is not considered to have commercial value but is a frequent by-catch of commercial trawling. The objective of the current research was to identify the trophic habits of C. gilberti and the influence exerted on its trophic dynamics by seasonal environmental variations in the bay and by different locations within it.
STUDY AREA
Buenaventura Bay is located on Colombia’s Pacific coast in the department of Valle del Cauca . It is one of the wettest places on the planet, with an annual rainfall of 6,980 mm, and high temperatures (Cantera and Blanco, 2001). Precipitation in the estuary is bimodal, with the highest levels recorded in September and October and the lowest in February and March (Otero et al., 2007). Two principal climatic periods have been identified: a dry season between January and June, with monthly rainfall < 500 mm and a rainy season between July and December, with average rainfall > 500 mm (Molina et al., 2020). Two rivers, the Dagua and the Anchicayá, drain into the bay conferring on it the status of estuary and resulting in distinct levels of salinity, fresher in the coastal areas and saltier further out to sea (Pérez, 2007). In common with other coastal areas of the world, the port of Buenaventura has experienced population growth, with increased demand for basic needs such as fresh water, housing and food increasing the levels of anthropic pressure (Correa and Zamora, 2017; Martinez et al., 2019; Vásquez et al., 2021).
MATERIALS AND METHODS
The species samples were collected using artisanal trawl methods using glass fiber boats with 15 HP motors, with a net gauge of 25.4 mm. Trawl speed was between 3.1 and 4.0 km/h. This type of fishing is not selective and is used traditionally to capture shrimp (Salazar, 2014). Sampling was carried out in two distinct areas of the bay. The Inner Estuary, with sample points A1 (3° 50′ 32,36″ - 3° 50′ 56,29″ N; 77° 06′ 33,29″ - 77° 07′ 09,50″ W) and A2 (3° 50′ 22,15″ - 3° 52′ 00,51″ N; 77° 07′ 08,82″ - 77° 09′ 14,00″ W) comprises the inner part of the estuary and is influenced by the waters of the River Dagua; it experiences the highest levels of anthropic pressure, due to its proximity to the urban center. For its part, the External Estuary, with sample points A3 (3° 48′ 50,69″ - 3° 49′ 14,51″ N; 77° 08′ 46,41″ - 77° 09′ 24,74″ W) and A4 (3° 50′ 21,99″ - 3° 50′ 46,35″ N; 77° 09′ 03,18″ - 77° 09′ 35,92″ W), is more influenced by the sea (Figure 1). It is important to bear in mind that the entire area experiences the constant passage of ships and periodic dredging activities.
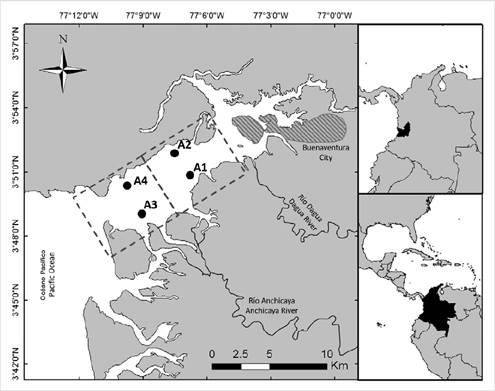
Figure 1 Research areas within Buenaventura Bay. The dotted lines represent the two sampling zones, the Internal Estuary (IE) and the External Estuary (EE). The points represent the sampling sites.
Three trawls were carried out at each sampling point, each of which lasted 10 min: the individuals collected were stored in cold boxes, on ice. Samples of environmental water variables (salinity, dissolved oxygen, transparency, pH and temperature) were taken at each station using a multiparametric meter (Thermo Scientific Orion Five Stars Probe) at a depth of 50 cm, with the exception of water transparency, which was measured using a Secchi disk. The estuary may be considered a well-mixed system, because the difference between salinity at the bottom and the surface is less than two (Otero, 2005).
The samples were taken in 2015 and 2016 during the dry season (first semester, March and June) and the rainy season (second semester, September and November). The flounders were separated from the rest of the catch in the laboratory before being identified taxonomically using the method described by Fischer et al. (1995), Nelson (2006), Robertson and Allen (2015), and Froese and Pauly (2017). The stomachs were extracted and preserved in formaldehyde at 4 % and the stomach contents were then ascertained and separated into broad groups using a stereoscope (Flores et al., 2013; Robertson and Allen, 2015). The prey items were classified and counted, and their dry weights determined by drying in an oven at 70 °C. Permanova samples were established using similarity matrices, constructed on the basis of Euclidian distances of untransformed data for each of the environmental parameters - dissolved oxygen (mg/l), temperature (°C), pH, transparency (cm) and salinity - in order to evaluate significant differences between the seasons (rainy/dry) and the different sampling sectors (internal: A1-A2 and external: A3-A4) (Clarke, 1993; Clarke et al., 2014).
Existing research on feeding habits differs markedly in the aspects taken into account when considering the minimum number of samples required to describe the diet of a species satisfactorily. However, given the difficulty of identifying each item found in the stomach contents, there is a degree of consensus in the literature that analyzing prey items by group produces representative results (Shark and Lucia, 2008; Belleggia, 2009; Garay et al., 2018;). All the analyses described below were conducted by grouping prey items in six trophic groups, as follows: nematodes, mollusks (bivalves and squid), crustaceans (shrimp larvae, Squillidae and Penaeidae shrimps, crabs and isopods), eggs, fish and polychaetes.
Trophic dynamics were analyzed using frequency of occurrence (% F), percentage weight of each prey item (% W) and percent number (% N) for the total number of individuals of each prey item divided by the total number of individuals. These three percentages are commonly used in trophic studies of fish (Sá et al., 2006; Navia et al., 2014; Garay et al., 2018 ). However, taken alone, none provides a complete analysis because very numerous, small, items might obscure the significance of large, scarce, items. In addition, a gravimetric analysis might exaggerate the importance of a heavy organism. For this reason, several indicators should be analyzed together in order to represent the trophic processes in the most complete way possible (Duque et al., 1996; Labbé and Arana, 2001; Ortaz et al., 2006 ). The following indicators were used for the purpose:
1) Feed coefficient (Q), which makes it possible to evaluate relations, granting the same relative importance to the number and weight of prey items. The categories used are preferential prey items (Q > 200), secondary prey items (20 < Q < 200) and accidental prey items (Q < 20) (Hureau, 1970).
2) Index of relative importance (IRI), used to evaluate relations between different prey items. The categories used are preferential prey items (20000 > IRI > 200), secondary prey items (200 > IRI > 20) and accidental prey items (20 > IRI > 0) (Pinkas et al., 1971).
An emptiness index was also used, corresponding to the proportion of empty stomachs found in the sample examined (Molinero and Flos, 1992).
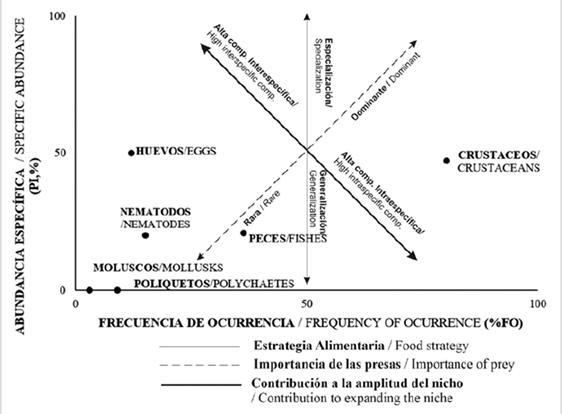
The analysis of feeding strategy was carried out using the graphic method proposed by Amundsen et al., (1996), a process consisting in representing specific abundance % Pi (y) using a Cartesian plane, as follows:
where, Si = number of stomachs that contain prey item i as sole food source, STi = total of stomachs in which prey item i is present
Additionally, the frequency of the occurrence % F (x) of the prey item was determined. Using this approach it was possible to interpret the importance of each prey item, feeding strategy (generalist or specialist) and the intra- and inter-specific contribution to the size of the amplitude of the trophic niche (Ferriz and Iwaszkiw, 2014). The graphic interpetation method proposed by Sánchez et al. (2013) was used for this purpose.
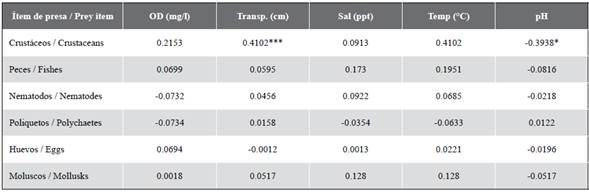
To analyze of the influence of spatio-temporal variables in trophic habits, a comparison by season and sampling sector was carried out of the dietary composition of C. gilberti, based on the databases of prey weights and number of prey items and of the descriptors % W, % N, and Q in order to identify significant differences, using Permanova (p(PERM) = 0.05) analysis. The % F and IRI databases were used to complement this comparison. Finally, Spearman rank correlations (Ortega et al., 2009; Martinez et al., 2019) were used to determine the influence of physico-chemical variables on the trophic habits of the species.
RESULTS
Environmental dynamics
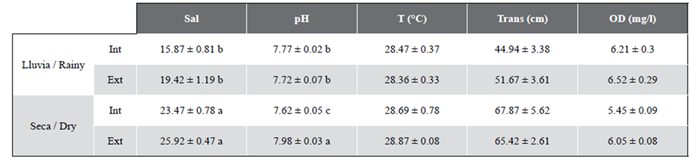
Salinity varied significantly (p(PERM) = 0.0003) between the dry and rainy seasons (Table 1). In both periods, the values for salinity were lower in the inner sector than the external. In the rainy season, salinity values were lower than in the dry season. Salinity values were higher during the rainy season in the external sector (19.42 ± 1.19) and lower in the internal sector (15.87 ± 0.81). The same tendency occurred in the dry season, when salinity was lower in the internal sector (23.47 ± 0.78) than the external (25.92 ± 0.47). In addition, the variable pH showed significant differences (p(PERM) = 0.0332) in the interaction period/sector (Table 1). Average pH in the external sector during the rainy season was 7.72 ± 0.07 and for the internal sector 7.77 ± 0.02. However, the opposite was the case during the dry season, during which average pH in the internal sector was 7.62 ± 0.05, lower than in the external sector, where it was measured as 7.98 ± 0.03.
No significant spatial or temporal differences were observed in temperature (p(PERM) > 0.05). There was a tendency for temperatures to be lower during the rainy season and higher in the dry period, with an average in the external sector in the rainy season of 28.36 ± 0.33 ºC and of 28.47 ± 0.37 ºC in the internal sector. In the dry season average temperatures in the internal sector were 28.69 ± 0.78 ºC compared to 28.87 ± 0.08 ºC in the external (Table 1). On the other hand, waters tended to be more transparent in the dry than in the rainy season, though no significant differences were found (p(PERM) < 0.05). During the rainy season, average transparency for the external sector was 51.67 ± 3.61 cm compared to 44.94 ± 3.38 cm - a little less - for the internal sector. However, the opposite was the case in the dry season, as transparency was a little greater in the internal sector (67.87 ± 5.62 cm) than the external (65.42 ± 2.61 cm) (Table 1). In terms of dissolved oxygen, the tendency was for levels to be higher in the rainy than in the dry season. The average for the external sector during the former was 6.52 ± 0.29 mg/l compared to the slightly lower reading of 6.21 ± 0.30 mg/l for the internal sector. The same occurred in terms of salinity, as during the dry season salt levels were a little lower in the internal sector (5.45 ± 0.09 mg/l) than the external (6.05 ± 0.08 mg/l). The differences found, however, were not significant (Table 1).
Table 1 Environmental variables (means ± standard error) by season (dry and rainy) and sampling sector (internal (Int) and external (Ext)). Salinity (Sal), pH, temperature (T), transparency (Trans) and dissolved oxygen (DO). The results of the post hoc tests and the Permanova pair-wise test, are represented with different letters when there are significant differences (p (PERM) < 0.05), a being greater than b.
Stomach contents and spatiotemporal influence
Of the 92 C. gilberti stomachs examined, the contents of 71.7 % were identifiable. Of these, 17.3 % contained detritus and 10.9 % had no contents (emptiness index). Twelve varieties of prey were found: squid, fish, crabs, Squillidae and Penaeidae shrimps, shrimp and crab larvae, isopods, bivalves, nematodes, polychaetes and eggs.
C. gilberti feeds principally on crustaceans and fish (Table 2). These were the most significant prey items found in its overall diet, categorized according to feed coefficient (Q) and index of relative importance (IRI) as principal or preferential prey. According to these indices, nematodes and polychaetes were secondary prey, while eggs and mollusks might be either secondary or accidental prey (Table 2).
Table 2 Composition of the diet of C. gilberti overall, by sector, sampling area, and by season. Prey descriptors: prey weight (P), prey number (n), weight percentage (% W), number percentage (% N), frequency of occurrence (% F), index of relative importance (IRI) and feed coefficient (Q). The results of the Permanova post hoc tests pair-wise test, are represented with different letters when significant differences occur (p (PERM) < 0.05), a being greater than b. The prey categories were established using the following scales: For Q = preferential prey (Q > 200), secondary prey (20 < Q < 200) and accidental prey (Q < 20) For IRI = main prey (20000 > IRI > 200), secondary prey (200 > IRI > 20) and accidental prey (20 > IRI > 0).
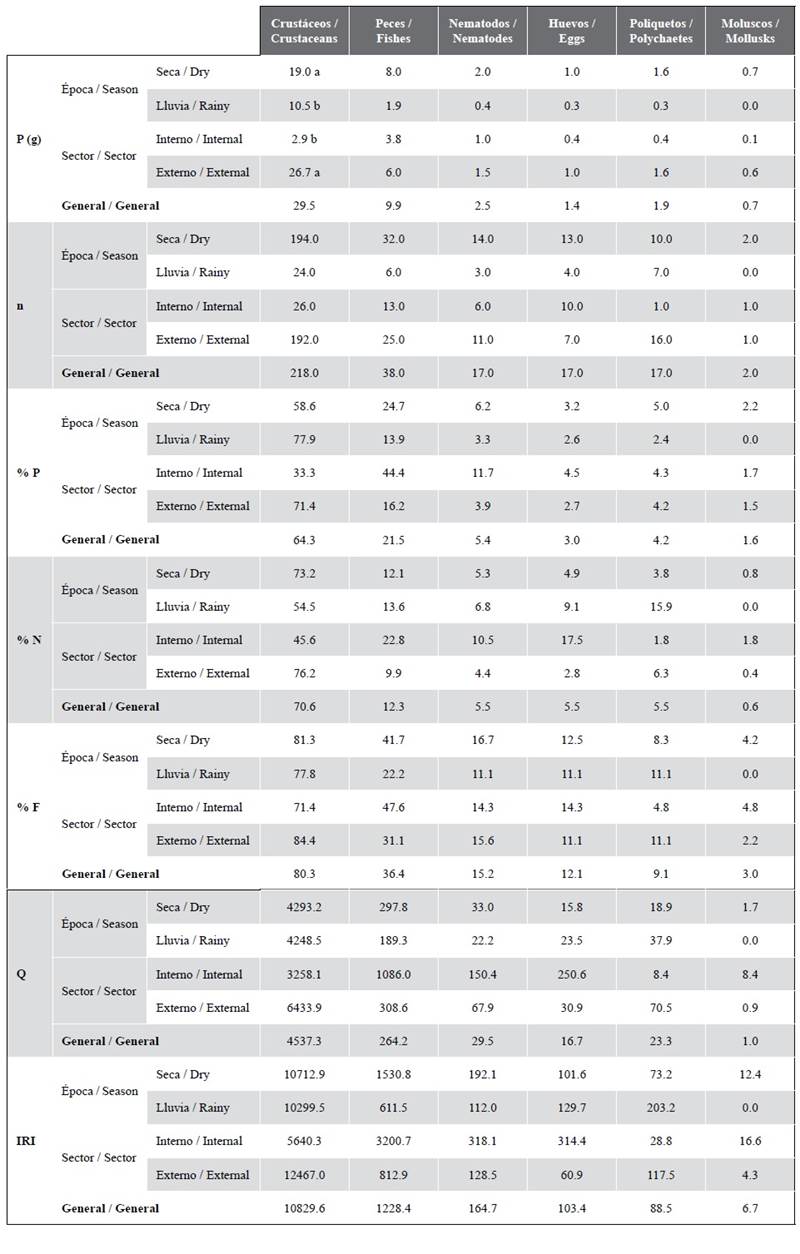
It may be observed that there is a tendency in the case of crustaceans for the values of each descriptor to be higher in the dry season and in the external sector. By contrast, note that consumption of fish was greater by weight in the internal sector (Table 2). Significant differences were found in the weight of total catch between seasons (p(PERM)˂0.05), which was heavier in the dry season. A significant difference was also found in the weight of the crustacean catch between seasons and sample sites (p(PERM)˂0.05), which was greater in the dry season (19 g) and in the external sector (10.5 g) (Table 2). Fish and other trophic categories did not display any significant variation (n, % W, % N, and Q) by season or by location (p(PERM) > 0.05).
Feeding strategy
It was established that C. gilberti follows a generalist feeding strategy (Figure 2). Similarly, it was demonstrated that crustaceans and fish were significant as the dominant prey. Since crustaceans were identified as the principal prey item, it is suggested that the species has a broad niche and displays intra-specific competition. However, in terms of the less significant prey in the habitat, C. gilberti engages in inter-specific competition. Finally, it should be stressed that the prey item fish (second, with the highest IRI and Q %) is second in dominance after crustaceans, ahead of eggs, nematodes, mollusks and polychaetes.
Influence of physico-chemical variables on trophic habits
The correlation analysis of the prey items and environmental variables showed that the weight (% W) of crustaceans was positively correlated with water transparency and negatively with pH. Fish and other food items showed no significant correlations with the environmental variables (Table 3).
DISCUSSION
Trophic habits and feeding strategies
The analysis of the stomach contents of C. gilberti showed that it is a generalist feeder that preys preferentially on crustaceans and fish. These results coincide with those recorded for the family Paralichthyidae and the genus Citharichthys in previous research (Vargas et al., 1999; Flores et al., 2013), which also found polychaetes to be important prey items. In this sense, some studies have shown that smaller flounder species, such as that examined in this case, are principally characterized as eaters of crabs (Labbé and Arana, 2001; Amezcua and Portillo, 2010) in contrast to larger species, which include an equal or greater percentage of fish among their principal prey (Latour et al., 2008).
The importance of crustaceans as the dominant prey items of C. gilberti might be related to their abundance at particular times and at certain locations in Buenaventura Bay. Crustaceans from the families Portunidae, Penaeidae and Squillidae have been found to be characteristic of the estuary, Callinectes arcuatus being the most abundant (Gamboa et al., 2018). In the Colombian Pacific, C. arcuatus is an important fisheries resource (Álvarez, 2015). It is for this reason important to note that changes in the crustacean population in the Bay might affect the ecology of C. gilberti. In addition, the importance of fish in its diet might be associated with the unwavering availability of this prey item in the estuary. It has been recorded that in Buenaventura Bay the majority of the most abundant fish species are permanent residents of the estuary, with a year-round presence in all zones (Molina et al., 2020), with the result that they are available to be consumed at all times.
On the other hand, C. gilberti is marked by high levels of intra-specific competition for crustaceans (the dominant prey). Other flounder species are found in Buenaventura Bay, in addition to C. gilberti, including Achirus mazatlanus and A. klunzingeri (Molina et al., 2020), whose diet, like that of C. gilberti is also based on crustaceans and fish (Tafurt et al., 2021). This increases competition levels, creating a situation in which resources might have to be shared. On the other hand, in common with other flounder species, C. gilberti displays a generalist feeding strategy (Marques et al., 2009; Tafurt et al., 2021), which enables it to adapt and survive easily to the changing availability of resources that characterizes its environment (Flores et al., 2013). In contrast to this, it has been found that flounder species may exhibit a degree of specialization in their diets depending on their surroundings (Guaratuba et al., 1998) and that local adaptations might exist that permit individuals to optimize the use of resources and survive in ecosystems characterized by large environmental variations. For example, in their research Marques et al. (2009) suggest that the flounder S. micrurum (of the Paralichthyidae family) adjusts its dietary composition according to the availability of species in the benthic community. It is thus suggested that C. gilberti is a generalist with a preference for crustaceans, when available, and that it feeds constantly on fish, which thereby forms the basis of its regular diet, as may be seen from the results of the feed coefficients, where fish were the principle prey item when spatial and seasonal differences in their consumption were not factored in.
The tendency observed in the case of crustaceans - that values for all the descriptors used in this study (P, N, % FO, % IRI y Q) are higher in the dry season and in the external sector - coincides with the results of Gamboa et al. (2018), who found that crustaceans were more abundant and rich in variety during the dry season and in the external sector. Raedemaecker et al. (2011) mention that the feeding behavior of flounders responds fundamentally to the availability of resources and the hunting ability of each species. For their part, Flores et al. (2013) conclude that, although flounders tend to feed on the abundant and available resources found in their surroundings, the analysis of their prey does not suggest a response to environmental conditions. Thus, it might be said that while the spatiotemporal environmental variables condition the availability of resources for the species, its feeding strategy adapts to these limiting factors, increasing its trophic spectrum and making it harder to uncover patterns or tendencies that link the fish’s trophic habits to environmental variables (Molina et al., 2020). On the other hand, estuarine species have been observed to possess the ability to display selectivity, actively seeking out and consuming their favorite prey even though these may at times be scarce (Duque y Acero, 2006). This could explain why crustaceans are the favorite prey item despite the fact they are not always the most abundant, given that they are actively sought out.
Influence of physico-chemical variables on trophic habits
In the case of the environmental parameters, a positive correspondence was found between crustaceans and water transparency. In the habitats of generalist fish, one of the principal factors associated with crustacean abundance is high transparency levels, as these favor shrimp development (Pereira et al., 2004). In Buenaventura Bay, the highest levels of transparency registered during the dry season appear to be associated with a greater presence of medium and large sediments (Molina, 2020) as these tend to precipitate more readily, which can increase transparency. Accordingly, it is possible to infer that transparency is a factor that influences the availability of food to C. gilberti. It has been postulated that the principal agents responsible for transporting sediments to Buenaventura Bay are the rivers, streams gullies and lagoons that drain into it, while other processes occur in the bay itself that increase sedimentation and turbidity, such as mangrove clearance, dredging and construction in the port area (Invemar, 2015).
Turning to pH, a clear and significant negative correspondence was found between this variable and crustaceans, which may be associated with the quantity of dissolved substances - especially nutrients - that affect estuarine fish and macroinvertebrates, affecting their abundance and their availability as prey (Day et al., 1989; Duque et al., 2020). It has also been found that the Colombian Pacific is experiencing a long-term tendency to reduced pH levels in coastal areas (Rojas y Pabón, 2015), a phenomenon related to factors such as the absorption of atmospheric CO2 by the oceans. Additionally, it should be borne in mind that - in contrast to what happens in the open sea - variations of pH in ecosystems are difficult to assign merely to global phenomena, as they respond also to local processes occurring along the coastline and inland, such as fresh water flows, sediments, organic materials and pollutants (Feely et al., 2010). Finally, the prey item fish did not display significant differences either by season or location. It is a prey item of constant availability, according to Molina et al. (2020) permanently present in the in the estuary regardless of prevailing environmental conditions at any given time. This suggests that both crustaceans and fish constitute an important part of the diet of C. gilberti, complementing each other and demonstrating the ability of the species to adapt to its environment.
CONCLUSIONS
In Buenaventura Bay, C. gilberti is a generalist carnivore that feeds primarily on crustaceans and fish. Several spatiotemporal variations affect the consumption of crustaceans, the most important of which were the dry season and location in the external sector of the estuary. On the other hand, fish were an important food source in the internal sector when the number of crustaceans was lower. Nematodes and polychaetes bwere secondary prey items and eggs and mollusks were either secondary or accidental prey whose consumption disaplayed no spatiotemporal variation. Crustaceans were ingested principally in clearer waters with lower pH readings, whereas fish - though eaten less frequently - were a constant prey item regardless of season or location, and independently of environmental variables. This study was able to establish the trophic habits of C. gilberti, and constitutes a scientific contribution to understanding of the dynamics and structures of estuarine communities. It is of particular importance given that documented research into some fish species in the Pacific are rare. The authors suggest complementing the information presented here with data from other geographical areas and on other ontogenetic trophic variations, as other studies have shown that flounders may exhibit different behaviors according to their ontogenetic status.











 text in
text in