INTRODUCTION
Euphausiids are exclusively marine crustaceans that feed on particulate matter, phytoplankton and microzooplankton (Cleary et al. 2018; Kohlbach et al. 2019). Some of the species that make up this taxonomic group fulfill a relevant ecological function in the Antarctic pelagic food web because they are prey for fish (Hudson et al., 2014), seabirds (Rogers et al., 2012; Rombolá et al., 2012; Santora et al., 2017), sea lions (Yamanaka, 1983) and Balaenopteridae whales (Stone and Hamner, 1988; Nowacek et al., 2011; Murase et al., 2002), shortening the energy transfer process from primary production to higher trophic levels. Also, these organisms make large vertical displacements in the water column, for which they are considered key elements in the biological pump and even a fundamental element in the benthopelagic coupling process in the Antarctic region (Schmidt et al., 2011; Conroy et al., 2020).
In the Antarctic Peninsula, euphausiids in their larval and juvenile stages together with copepods and salps are one of the three main groups that make up zooplankton (Schnack-Schiel and Mujica, 1994), although some species of euphausiids during their adult stage are considered micronektonic organisms. This is a taxonomic group that has been widely studied in the Antarctic region, particularly Euphausia superba due to its high abundance in the area (Piatkowski, 1985; Hosie et al., 1988; Cavan et al., 2019). For the Gerlache Strait, knowledge of euphausiids includes geographical distribution, vertical migratory behavior, population size structure (Nordhausen, 1994a; Zhou et al. 1994; Lawson et al. 2008; Wiebe et al. 2011; Cleary et al. 2016), herbivore and respiration metabolic rates, their contribution to carbon flux (Huntley and Brinton, 1991; Hernández-León et al. 2000; 2001; 2013; Reiss et al. 2017) as well as the relationship between the rates of recruitment and abundance with ENSO events of origin in the tropical Pacific (Loeb et al. 2009).
The Gerlache Strait, located in the northwestern part of the Antarctic Peninsula, presents geomorphology and bathymetry that promotes the development of particular circulation patterns in the north and south, which result in different hydrographic conditions (Rodríguez et al., 2002; Zhou et al., 2002; 2006; Torres-Parra et al., 2020). Considering that the conditions of temperature and salinity largely determine the structure and composition of the planktonic community in a locality (Rodríguez et al., 2002; Leonori et al., 2017), it is expected that the species that make up the set of Epipelagic euphausiids in the Gerlache Strait exhibit a particular trend of spatial variation, which would be related to the thermal and saline conditions of each sector of the Strait, in such a way that the structure of the set of euphausiids will be different between these sectors.
To test this hypothesis, aspects related to the spatial ecology of the set of epipelagic euphausiids in the Gerlache Strait were evaluated during the austral summer of 2015 within the framework of the first scientific expedition from Colombia to the Antarctic - ”Caldas Expedition”, addressing the following research questions: 1. Is the taxonomic composition of the group of euphausiids present in the epipelagic zone in the north and south of the Gerlache Strait similar? and 2. Is the spatial distribution of the abundance of euphausiids homogeneous in the epipelagic zone of the Gerlache Strait during the study period?
STUDY AREA
The Gerlache Strait is located in the northwestern sector of the Antarctic Peninsula, delimited by the Palmer Archipelago in which two island formations stand out, the islands of Antwerp and Brabant (Figure 1).
The strait is 180 km long and between 8 and 60 km wide, with a depth of 300 m in the extreme south and 1000 m in the extreme north (García et al., 2002). The surface circulation in the Gerlache Strait flows to the northeast with velocities greater than 30 cm s-1 feeding the Bransfield Strait Current (Zhou et al., 2002). A dynamic coastal anticyclonic gyre system develops in this region due to a large number of shallow bays along the strait (Zhou et al., 2002). During the austral summer of 2015, the thermal and saline structure in the superficial part of the water column showed different conditions in each of the entrances to the strait, at the entrance to the north, thermal stratification conditions prevailed with homogeneous salinity, while the South entrance showed greater mixing and low salinity in the first 30 m (Giraldo et al., 2019; Torres-Parra et al., 2020).
MATERIALS AND METHODS
The zooplankton samples were obtained during the Caldas Expedition aboard the ship “ARC 20 de Julio” of the Colombian National Navy. Sampling was carried out from January 17 to 22, 2015, at 20 oceanographic stations (Figure 1). Continuous vertical recording of temperature, conductivity, and depth was performed at each station using a Seabird® CTD profiler (SBE 19plus SeaCat profiler CTD and SBE 25plus Sealogger CTD). The horizontal distribution of temperature and salinity was analyzed at standard depths (1, 10, 50, and 100 m) to infer the thermohaline conditions in the study area, using the kriging interpolation method of the Surfer® program.
Surface tows were made to collect zooplankton at each sampling station using a simple conical net of 0.6 m in diameter and 200 µm mesh with a Hydrobios® flowmeter installed in the center of the mouth, to estimate the volume of filtered water. The samples were fixed in formalin at a 4 % concentration, buffered with saturated sodium borate. All tows were carried out under daylight conditions. The samples were labeled and analyzed in the laboratory of the Research group in Oceanographic Sciences of the Universidad del Valle. In the laboratory, the sample was divided into four equal parts using a Motoda-type plankton subsampler, ¼ of the zooplankton sample was used to estimate the dry biomass. From the remaining – fraction, the euphausiids were separated, identified, counted, and classified by stage of development, following the taxonomic keys of Antezana et al. (1976), Baker et al. (1990), Gibbons et al. (1999) and Brinton et al. (2000). All counts were standardized (individuals 1000 m-3) based on the volume of filtered water.
To quantify the dry biomass of the zooplankton from each sampling station, the constant dry weight method was used (Postel et al., 2000), concentrating the sample on a previously dried and weighed cellulose filter, being brought to constant weight in an oven at 60 ° C for 24 h, to be subsequently weighed on a 0.0001 g precision analytical balance. Weight records were multiplied by 1.25 to adjust for tissue loss in formaldehyde (Hopkins, 1971). To quantify the biomass contribution of the different species of euphausiids identified in the study area, the average weight of the adult individuals of each species (excluding juveniles) was established and multiplied by their abundance, establishing the contribution percentage of each species to the total biomass of zooplankton.
The analysis of the conditions of temperature, salinity, and the set of euphausiids in the North and South sectors of the strait was carried out as comparison units according to the geomorphological and oceanographic characterization described by Zhou et al. (2002) and Giraldo et al. (2019). T-student tests were carried out after checking the assumptions of normality and homogeneity of variances with the data transformed with the box-cox transformation to establish whether there were differences between temperature and salinity in the North and South sectors of the Gerlache Strait. To evaluate differences in the abundance of euphausiids between the two sectors of the Strait, a non-parametric Mann-Whitney analysis was carried out, and a non-parametric Spearman correlation analysis was carried out to evaluate the correlation between the abundance of euphausiids as a function of temperature. and salinity at the evaluated depths. These statistical analyzes were performed with Statistica 7.
A multidimensional non-metric scaling analysis (nMDS) was carried out from a similarity matrix established with the Bray Curtis algorithm, to infer the spatial variation of the set of euphausiids comparing the North and South sectors. Also, the difference between the sets was evaluated using a Similarity analysis (Anosim) and the contribution of each species to the similarity of the sets of each sector and the dissimilarity between the sectors of the strait was established using a percentage similarity analysis (SIMPER) (Clarke, 1993; Clarke and Ainsworth, 1993; Clarke and Warwick, 1994; 2001). All community analyzes were conducted using the Primer 6 program (Clarke and Gorley, 2006).
RESULTS
The temperature of the first 100 m of depth had significant differences between the North and South sectors of the strait (Table 1). The superficial stratum and at 10 m depth of the North sector presented higher temperature values than the South sector, while between 50 - 100 m depth the temperature was lower in the North sector (Figure 2). Salinity was homogeneous in both sectors for most depths except in the superficial stratum where the lowest salinities were recorded in the eastern region of the North sector (Figure 3). The salinity at 1 m depth was significantly different between the North and South sectors (Table 1).
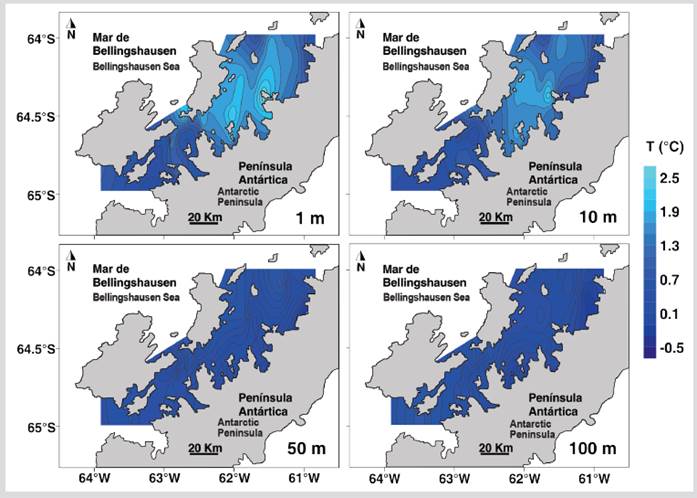
Figure 2 Distribution of sea temperature at depths of 1, 10, 50, and 100 m during the austral summer of 2015 in the Gerlache Strait, Antarctic Peninsula.
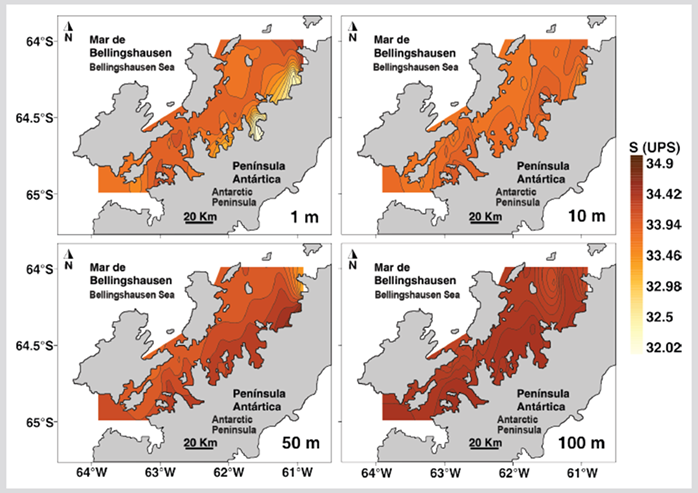
Figure 3 Salinity distribution at depths 1, 10, 50, and 100 m during the austral summer of 2015 in the Gerlache Strait, Peninsula, Antarctica.
Table 1 T-tests to contrast differences between temperature (° C) and salinity (UPS) between the northern and southern sectors of the Gerlache Strait during the austral summer of 2015.
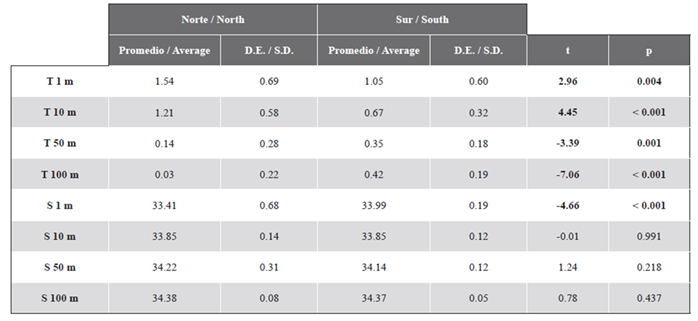
The highest concentrations of zooplankton biomass were recorded at the entrance to Wilhelmina Bay (station 12 = 2246.87 mg 100 m-3) and between Lion Island and Antwerp Island (station 17 = 1194.50 mg 100 m-3) (Figure 4). The concentration of zooplankton biomass was between one to two orders of magnitude lower in the rest of the study area (5.32 - 697.24 mg 100 m-3) (Figure 4).
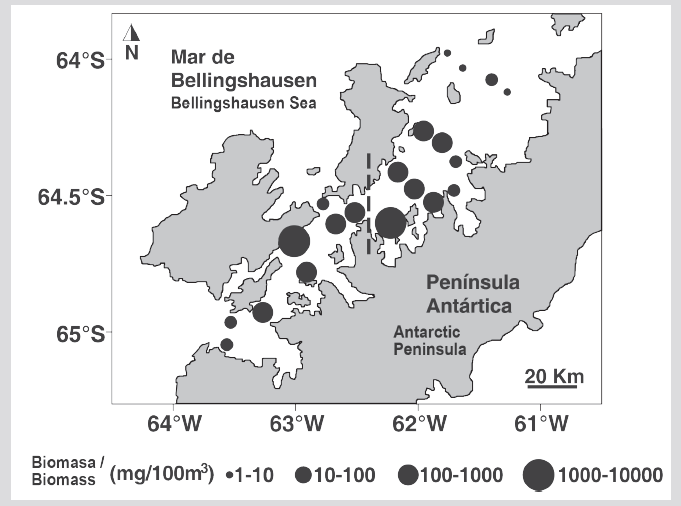
Figure 4 Distribution of zooplankton biomass (mg 100 m-3) estimated during the Austral summer of 2015 in the Gerlache Strait, Antarctic Peninsula.
Euphausiids were captured in 70 % of the sampling stations, identifying four species: Euphausia crystallorophias Holt and Tattersall 1906 (79.7 %), Euphausia superba Dana 1850 (11.4 %), Thysanoessa sp. (7.6 %), and Thysanoessa macrura Sars 1883 (1.3 %). The highest abundance of euphausiids was E. crystallorophias recorded at station 12 at the entrance to Wilhelmina Bay (818 ind 1000 m-3) (Figures 5 and 6). All the individuals present in the samples corresponded to juveniles, males, and females. The larvae were not captured. The male-female ratio of E. crystallorophias was 48.5 % males and 51.5 % females, for E. superba 68.7 % males and 31.3 % females, for Thysanoessa sp. 65.3 % males and 34.7 % females, and T. macrura all individuals were female.
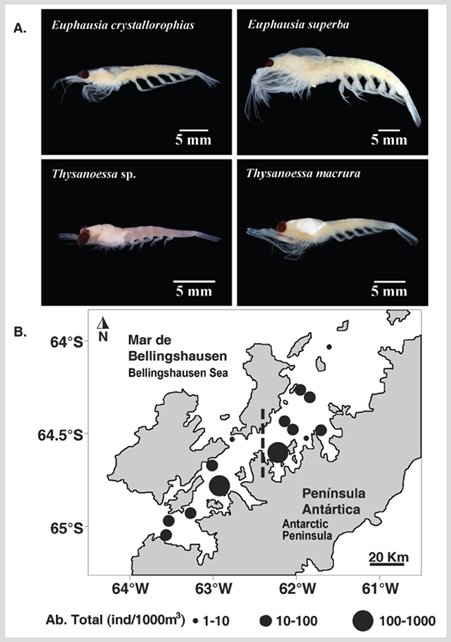
Figure 5 A. Photographs of the species collected. B. Distribution of the total abundance of euphausiids (ind 1000 m-3) during the austral summer of 2015 in the Gerlache Strait, Antarctic Peninsula.
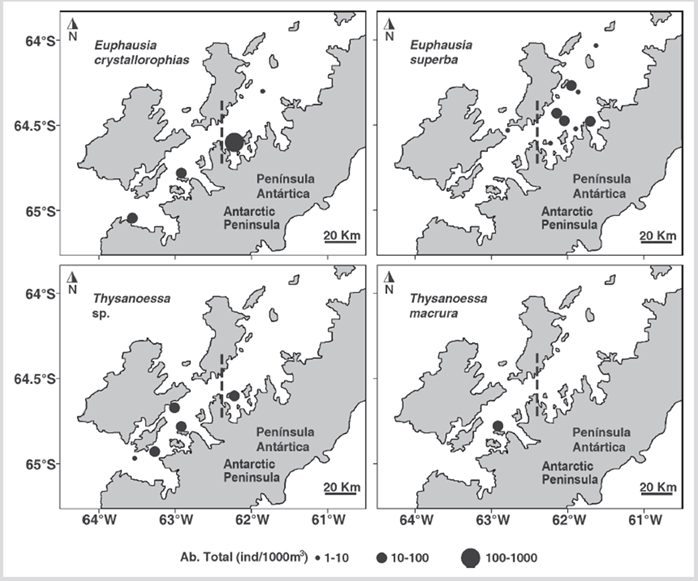
Figure 6 Distribution of the abundance of euphausiids by species (ind 1000 m-3) during the austral summer of 2015 in the Gerlache Strait, Antarctic Peninsula.
The abundance of the species did not have statistically significant differences between the North and South sectors (Mann-Whitney, U = 749, p = 0.852). However, E. crystallorophias was more abundant at station 12 located in the North sector. Furthermore, the catches of E. superba were made mainly in the North sector while the species of the genus Thysanoessa were almost exclusively recorded in the South sector, except for station 12 (Figure 6). No significant relationship was found between the abundance of any of the species with temperature or salinity, except E. superba positively correlated with surface temperature (R = 0.57, p < 0.05) and 10 m depth (R = 0.64, p < 0.05).
Euphausia crystallorophias had a contribution of zooplankton biomass equivalent to 32.1 %, E. superba 12.7 %, T. macrura 0.4 %, and Thysanoessa sp. 0.3 %. Regarding the North and South sectors, E. crystallorophias represented 50.1 % of the zooplankton biomass in the North sector and 6.7 % in the South sector. Euphausia superba represented 20.2 % in the North sector and 2.1 % in the South sector. Thysanoessa sp. it represented 0.2 % in the North sector and 0.4 % in the South sector. T. macrura was only recorded in the southern sector of the Gerlache Strait, representing 0.9 % of the zooplankton biomass.
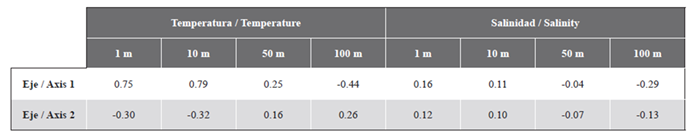
The group of euphausiid species in the Gerlache Strait showed two significantly different groups (Anosim, R = 0.52, p = 0.005), one group made up of most of the stations in the southern sector of the strait and the other with the stations of the North sector (Figure 7). For the two-dimensional solution obtained by the nMDS, the Spearman correlations were only high and positive between the temperature at 1 and 10 m and axis 1 (Table 2). The Simper analysis suggested that the dissimilarity between the North and South sectors was 89.19 %. Euphausia superba (41.0 %), E. crystallorophias (32.4 %), and Thysanoessa sp. (24.6 %) were the species that contributed most of the dissimilarity between the sectors, while E. superba was the species that most contributed to the similarity of the North sector (99.8 %) and Thysanoessa sp. the one that had the greatest contribution to the similarity of the South sector (89.7 %).
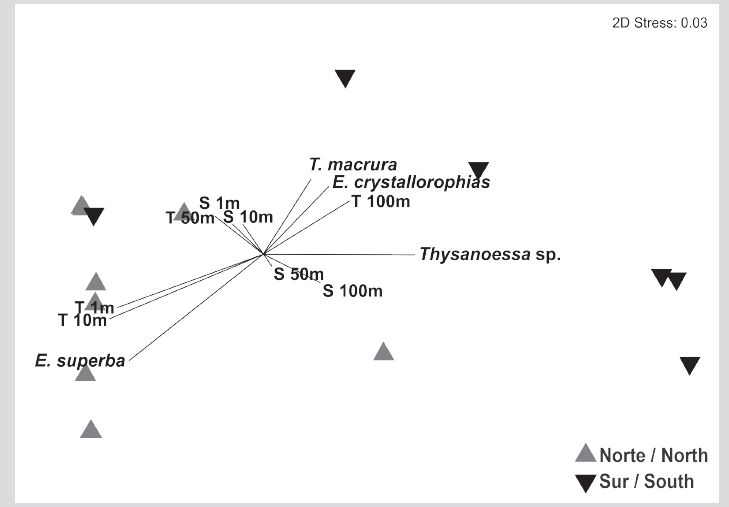
Figure 7 Multidimensional non-metric analysis using the Bray Curtis algorithm, based on the abundance matrix of euphausiid species recorded during the southern summer of 2015 in the Gerlache Strait, Antarctic Peninsula.
DISCUSSION
Four species of euphausiids were recorded in the epipelagic zone of the Gerlache Strait during the austral summer of 2015. This low specific richness coincides with previous studies in Bahía Margarita, south of the Gerlache Strait (Marrari et al., 2011; Parker et al., 2011), where they reported T. macrura, E. crystallorophias, and E. superba. For the Scottish Sea, Ward et al. (2004) recorded E. superba, T. macrura, and T. vicina, species that had previously been reported for the Gerlache Strait during winter (Nordhause 1994a).
In Antarctic neritic waters, Euphausia crystallorophias is the most common euphausiid species (Mauchline and Fisher, 1969; Thomas and Green 1988; Pakhomov and Perissinotto 1996; Sala et al., 2002; La et al., 2015). This is a species with a broad feeding spectrum, including diatoms, bacteria, organic debris, and algae that grow under ice (Mauchline and Fisher, 1969; Pachamov and Perissinotto, 1996; Melnikov and Spiridonov, 1996; Ju and Harvey, 2004; Lee et al., 2013) and that reaches average densities between 450 to 1400 ind 100 m-3 (Everson, 1987; Pakhomov et al., 1998). The highest abundance of E. crystallorophias in the summer of 2015 was 818 ind 1000 m-3 in the central area of the Gerlache Strait, being the species that dominated in number and biomass contribution the set of euphausiids in the study area, similar to as reported by Daly and Zimmerman (2004) and Marrari et al., (2011) for the western sector of the Antarctic Peninsula. Furthermore, the abundance recorded in the present study was greater than that which had been reported for the neritic zone of the Bellingshausen Sea (Margarita Bay) by Siegel and Harm (1996).
Euphausia superba is perhaps the most widely studied euphausiid species in the world (Nicol, 2006; Siegel, 2016). This species is endemic to the Antarctic Ocean, not only is it the largest euphausiid (adults: 42-65 mm) or the most abundant in the Antarctic Ocean, but it is also a key species in the biogeochemical flow of carbon and iron and the trophic dynamics of the Antarctic pelagic ecosystem (Everson, 2000; Nowacek et al., 2011; Gleiber et al., 2012; Trathan and Hill, 2016; Schmidt et al., 2016). E. superba has sustained extensive international fishing activity for 50 years (Nicole et al., 2012; Nicole and Foster, 2016). Although this species mainly inhabits the Antarctic oceanic environment (Siegel and Watkins, 2016), the Gerlache Strait has been reported as one of the most abundant areas in the Antarctic peninsula (Huntley and Brinton, 1991), probably associated with the circulation pattern local that favors the residence time of the larvae in a protected environment with high productivity (Varela et al., 2002; Zhou et al., 2002, 2006; Jiang et al., 2013), as has also been reported in the Strait of Bransfield (Hofmann and Murphy, 2004). However, most of the large swarms of E. superba are distributed in oceanic waters, approaching coastal areas during the summer (Atkinson et al., 2008; Tarling et al., 2009).
Thysanoessa macrura is the euphausiid species with the most consistency in its spatial distribution in the Antarctic Ocean, and it can outnumber E. superba swarms (Kittel and Stepnik, 1983; Daly and Macaulay, 1988; Nordhausen et al., 1994a). Thysanoessa macrura performs short vertical migrations in the day-night cycle, concentrating in the epipelagic zone of the water column (Loeb and Shulenberger, 1987; Lancraft et al., 1989; Nordhausen, 1992), in the Gerlache Strait this species has been reported at depths up to 120 m (Nordhausen et al., 1994b).
Around 60 degrees south Thysanoessa vicina and T. macrura show an overlap in the geographic distribution range, so it is possible that the type of Thysanoessa sp. recorded in this study corresponds to one of these two species considering that they are morphologically similar, and their traditional taxonomic identification depends on diagnostic characters such as the body size of adults, which for T. vicina is approximately 16 mm and for T. macrura it is about 30 mm. According to Thiriot-Quiévreux et al. (1998), these species have a different number of chromosomes, so it is recommended to implement karyotype analysis to establish with total certainty the potential presence of T. vicina in the Gerlache Strait.
In the present study, no larvae of any species were captured. T. macrura spawns at the end of winter and by the summer season the individuals will already correspond to juveniles (Wallis et al., 2018). However, E. crystallorophias and E. superba have the spawning season during the summer (Zhou et al., 2002), so the absence of initial stages of development of these two species during the sampling period may be a consequence of atypical climatic conditions registered in the Antarctic Peninsula during the first semester of 2015, which delayed the entry of conditions characteristic of summer in the region (Blunden and Arndt, 2016).
The sex ratios recorded in the present study were different for each of the species. In the case of E. superba, it has been reported that males grow faster than females (Kawaguchi et al., 2007), which could explain the higher rate of males compared to females registered in this study. For E. crystallorophias the sex ratio was close to 1, which coincides with that recorded by Pakhomov and Perissinoto (1996), who reported this same ratio for this species in different neritic environments of the Antarctic continent during summer. For T. macrura, only females were recorded, possibly due to the few individuals that were captured of this species. For this species dominance of females has been reported, reaching up to 75 % of the adult population during summer (Nordhausen, 1992; Haraldsson and Siegel, 2014).
Few studies have specifically shown a relationship between temperature or salinity and the abundance of euphausiids in Antarctica (Weber et al., 1986; Trathan et al., 2003; Lee et al., 2013 Leonori et al., 2017). On a spatial scale of 1 - 100 km, euphausiid aggregations may depend on local bathymetric conditions, for example, in areas such as the continental slope or within the Gerlache Strait, the highest concentrations of euphausiids will be the result of the effect of the topographic characteristics added to the local circulation pattern that favors local retention processes (Zhou et al., 2002, 2006; Jiang et al., 2013). This could be one of the reasons why the highest abundances of euphausiids were recorded in station 12 of the present study, due to their location in a protected area and with low speed in the currents that form in the area (Zhou et al., 2002; Torres-Parra et al., 2020).
The thermohaline conditions in the epipelagic zone of the Gerlache Strait during the austral summer of 2015 were similar to those reported during the austral summer of 1995 (Rodríguez et al., 2002), with higher temperatures in the stratum of 1 - 10 m than in the 50-100 m stratum, while in the southern sector the temperature was similar in the first 100 m depth. The low surface salinity recorded in the northern sector of the Gerlache Strait, particularly near Charlotte Bay and Hughes Bay of the northern sector of the Danco coast, is probably due to the contribution of meltwater, a condition that is frequent during the austral summer in the coastal areas of the Antarctic continent (Smith and Klinck, 2002; Eveleth et al. 2017), although the climatic conditions during the sampling period promoted conditions of low thaw rate (Blunden and Arndt, 2016).
The distinction of the set of epipelagic euphausiids in the two sectors of the Gerlache Strait could be influenced by different trophic resources in each sector. Changes in temperature and salinity in the water column can have important effects on the dominant type of phytoplankton (Mendes et al., 2018). Rodríguez et al., (2002) reported that the southern sector of the Gerlache Strait was dominated by ultraflagellates while in the northern sector it was dominated by microplanktonic diatoms. Likewise, Giraldo et al. (2019), reported differences in the size of the copepods present in the northern and southern sectors of the strait, with adult copepods in the northern sector being an order of magnitude smaller than those in the southern sector of the Gerlache Strait. This condition could be modulating the structure and composition of the group of euphausiids in this locality, considering also that E. superba has presented a preference for small copepods (Atkinson and Snÿder, 1997), which could explain the exclusive presence of this species in the North sector of the Gerlache Strait











 texto en
texto en