INTRODUCTION
Studies based on the ecology, distribution, composition and biodiversity of Antarctic mesozooplankton have focused intensively on a single species, the krill Euphausia superba, while other species have received less attention (Schnack-Schiel and Hagen, 1994), or it has focused on certain sectors, such as the West region (the Weddell and Ross Seas), with the East area being a little less studied. However, the great political and scientific interest that has been aroused in recent years, directed towards Antarctica with exploration and baseline cruises, has made Antarctic fauna one of the most studied (Clarke, 2008). The North Shetland Islands and the Antarctic Peninsula have been some of the most studied in recent years thanks to their easy access and transit (Griffiths, 2010; Griffiths et al., 2011), but the passage between two islands of the Peninsula, the Anvers and Bravant Islands, known as the Gerlache Strait, is in a state of scarce knowledge, focusing mainly on studies on terrestrial geology (Birkenmajer, 1995, 1999) and on benthic and planktonic biodiversity (Hopkins, 1985; Zmijewska and Yen, 1993; Hernández-León et al., 1999; Hernández-León et al., 2000; Hernández-León et al., 2001, Rodríguez et al., 2002a; Rodríguez et al., 2002b; Giraldo et al., 2019). Regarding the planktonic community, pelagic copepods that inhabit Antarctic waters are the most important zooplankton organisms along with krill and salps in terms of density and biomass (Shreeve et al. al., 2005; Atkinson et al., 2012; Kouwenberg et al., 2014; Corlins et al., 2018; Shreeve et al., 2018), are also the most diverse taxa of zooplankton with about 300 registered species (Razouls et al., 2005-2020), while for hydromedusae there are about 71 species and for siphonophores about 30 species recorded in Antarctic and Subantarctic waters (Lindsay et al., 2014). The distribution of these three taxonomic groups is not clear, especially for the gelatinous ones, due to the limited taxonomic knowledge and the collection methods. Therefore, any sampling effort that is made is valuable information to increase knowledge about the planktonic fauna of the Antarctic seas.
This study is part of the project Biodiversity and oceanographic conditions of the Gerlache Strait “Biogerlache-Antarctica” Phase I, of the Institute for Marine and Coastal Research - Invemar; to characterize the Antarctic fauna of the strait. It is a project that began in 2014, being a participant in the I Scientific Expedition of Colombia to the Antarctic “Caldas Expedition” (2014-2015) and continued to participate in the III Expedition “Admiral Padilla” (2016-2017) and V Expedition “Admiral Campos” (2018-2019). In particular, this manuscript is contributing information to the general objective of the project, focusing on the biodiversity of the zooplankton community, specifically on copepods, hydromedusae and siphonophores collected during the III Expedition (2016-2017) and proposes its distribution pattern in the Gerlache Strait. A taxonomic inventory of the species deposited in the Museum of Marine Natural History of Colombia (MHNMC) of Invemar is presented: 36 lots in the Cnidarians collection, from INV CNI4383 to INV CNI4399 and 8 lots in the Crustacean collection, from INV CRU8995 to INV CRU9002.
STUDY AREA
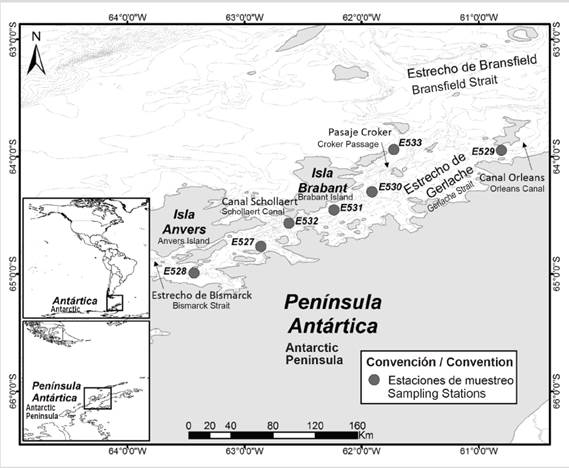
The Gerlache Strait is a channel of approximately 200 km in length, located in the middle of the Antarctic Peninsula and the Anvers and Bravant Islands (Figure 1). The Bismarck Strait and the Schollaert Canal connect it to the Southeast Pacific Ocean and the Orleans Canal and the Croker Passage connect it to the Bransfield Strait (Dragani et al., 2004). It has a depth between 270 and 1200 m, with slopes of 20°. The strait is increasing its depth, in a southwest-northeast direction, finding the greatest depths in the Croker Passage (1200 m), a passage through which the waters of the Bransfield Strait enter, which are a mixture and transition between warm waters and less saline in the Bellingshausen Sea and the cooler and more saline waters of the Weddell Sea (Gordon and Nowlin, 1978; García et al., 2002), this dynamic is confirmed by the local water masses that dominate the area, defined as Transitional Waters with Influence of the Bellingshausen Sea and Transitional Waters with Influence of the Weddell Sea, which change depending on the current, being the isotherm of 1.0 ° C the main way to discriminate between one mass and another during the Austral summer (García et al., 2002).
MATERIALS AND METHODS
Collection of samples
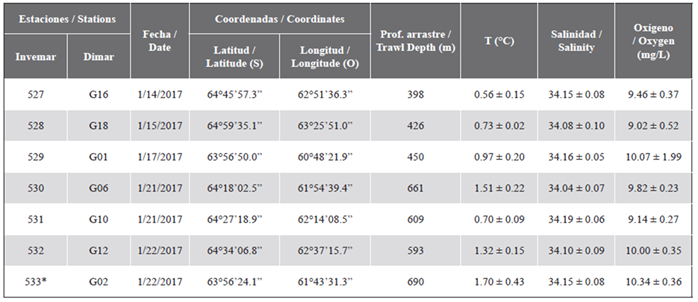
During the III Expedition of Colombia to the Antarctic “Admiral Padilla” aboard the vessel “ARC 20 de Julio”, a zooplankton sampling was carried out in the austral summer 2016-2017, in seven oceanographic stations located along the Strait of Gerlache (Figure 1, Table 1). The numbering of the stations follows the institutional sequence of Invemar projects.
The zooplankton samples were collected by vertical trawling from the surface (0 m) to the bottom, reaching depths ranging between 398 and 690 m (Table 1), using a General Oceanics brand opening-closing system, mechanically activated with messengers; the plankton network (200 µm pore, mouth diameter 60 cm) was equipped with a Hydrobios brand flow meter to know the amount of water filtered by the network and a pressure and temperature data recording sensor with which the exact depth of the lowering of the net was known. The collected samples were narcotized with 10 % magnesium chloride and fixed with a 4 % formalin solution, filtered seawater base (0.45 µm) and neutralized with sodium tetraborate to finally be stored in 500 ml plastic jars.
The samples were transferred to the Museum of Marine Natural History of Colombia, of Invemar, where they were analyzed and entered into the plankton reference collection. The data of temperature, salinity and dissolved oxygen in the water column of each station were taken with a SeaBird CTDO, later the average was determined as a measure of central tendency and the standard error was calculated as a measure of variability (Table 1).
Analysis of collected samples
In the laboratory, only the copepods, hydromedusae and siphonophores were separated in each sample, counted and taxonomically identified down to the species level or the lowest possible level, if the characteristics of the specimen allowed it. With the help of a Leica M205A brand stereomicroscope, the specimens were separated and dissected, with the help of a Zeiss Primo Star brand optical microscope, their specific taxonomic structures were identified, this information was contrasted with current literature to confirm the taxonomic identities of each specimen, both for copepods (Bjorberg, 1981; Nishida, 1985; Bradford-Grieve et al., 1999; Boxshall and Halsey, 2004; Razouls et al., 2005-2019), as well as for hydromedusae and siphonophores (Totton, 1965; Pugh, 1999; Licandro et al., 2017). For the identification of copepods, micro-dissections of morphological structures such as antennae, jaws, maxillae, swimming legs and genital segments were carried out and it was taken into account for the determination of the densities that the specimens were complete, with all their segments and structures, as well as that they were not ecdysis or exuviae and that they were in an advanced adult or juvenile phase (Gaviria and Aranguren, 2003); particularly for siphonophores, the densities were determined taking into account, that they break easily during capture, that the pneumatophore is almost always lost and the nectophores are separated from the nectosome, making their identification and counting carried out from their body parts remnants (nectophores) or the two life phases of these organisms: their polygastric asexual phase (anterior and/or posterior nectophore) and eudoxia sexual phase (bract or bract with gonophore) (Totton, 1965; Pugh, 1999; Panasiuk-Chodnicka et al., 2014; Licandro et al., 2017).
To evaluate the community structure of copepods, siphonophores and hydromedusae, the density was standardized by the volume of filtered water in each station and was expressed in units of ind/1000 m3, for these density values the central tendency measure was used as the standard error. The density data were transformed with the fourth root and subjected to a cluster analysis based on the Bray-Curtis dissimilarity index. Multivariate analyzes were performed, using the analysis of similarity percentages (SIMPER) to determine the species that contribute to the separation between the associations formed; a similarity analysis (one-way ANOSIM) to confirm differences in the structural attributes between the zoning present in the Gerlache Strait and the biota-environment correspondence procedure (BIO-ENV) to define which environmental variables (temperature, salinity and dissolved oxygen) are related to the community structure found in the strait, through Spearman’s correlation coefficient (pw) (a value of pw = 0 would not imply any relationship between the two matrices, while a value of pw = 1 means that there is a strong relationship between them) (Ludwig and Reynolds, 1988; Clarke and Warwick, 2001). These analyzes were carried out with the PRIMER software package (Plymouth Routines in Multivariate Ecological Research) version 5 (Clarke and Ainsworth, 1993; Clarke and Gorley, 2001).
RESULTS
Composition and density of copepods, hydromedusae and siphonophores
In the seven stations sampled, 4100 organisms belonging to 38 species of the phylum Arthropoda (orders Calanoida, n = 32 and Cyclopoida, n = 7) and 10 species of the phylum Cnidaria (Subclasses: Hydroidolina, n = 8 and Trachylina, n = 2) were identified, two arthropods were identified down to the order level and are equivalent to nauplii and three copepodites of the Clausocalanidae, Euchaetidae and Metridinidae families. The six copepods identified up to the genus were in very poor condition to be better identified.
The families with the greatest richness of genera are Aetideidae (n = 5) and Metridinidae (n = 4), both families of copepods were found in more than 50 % of the stations such as Metridia gerlachei, Gaetanus tenuispinosus and Chiridius polaris, other frequent species during the samples were the copepods Triconia antarctica, Oithona similis, Rhincalanus nasutus, Spinocalanus abysalis, Drepanopus forcipatus, the hydromedusa Arctapodema sp. and Solmundella bitentaculata and the polygastric and eudoxia phases of the siphonophores Dimophyes arctica and Diphyes antarctica (Table 2). The stations with the highest species richness are 528 (n = 22), 527, 529 and 532 (n = 20).
Table 2 Taxonomic inventory, distribution, ecology, frequency, density and relative abundance of the species of copepods, siphonophores and hydromedusa found in the Gerlache Strait during the 2016-2017 austral summer expedition. Abbreviations: SA: Subantarctic, A: Antarctic, AS: South Atlantic; E: Epipelagic, Ms: Mesopelagic, B: Bathypelagic, Ab: Abyssopelagic, N: Neritic; Freq: Frequency of appearance (‘+’ represents the frequency of the species in < 25 % of the stations, ‘++’ represents 25-50 % and ‘+++’ > 50 %). Dens: average density individuals/1000 m3, Abun. Rel. (%): Relative abundance is expressed as a percentage. Species contributing 75 % to density are in bold.
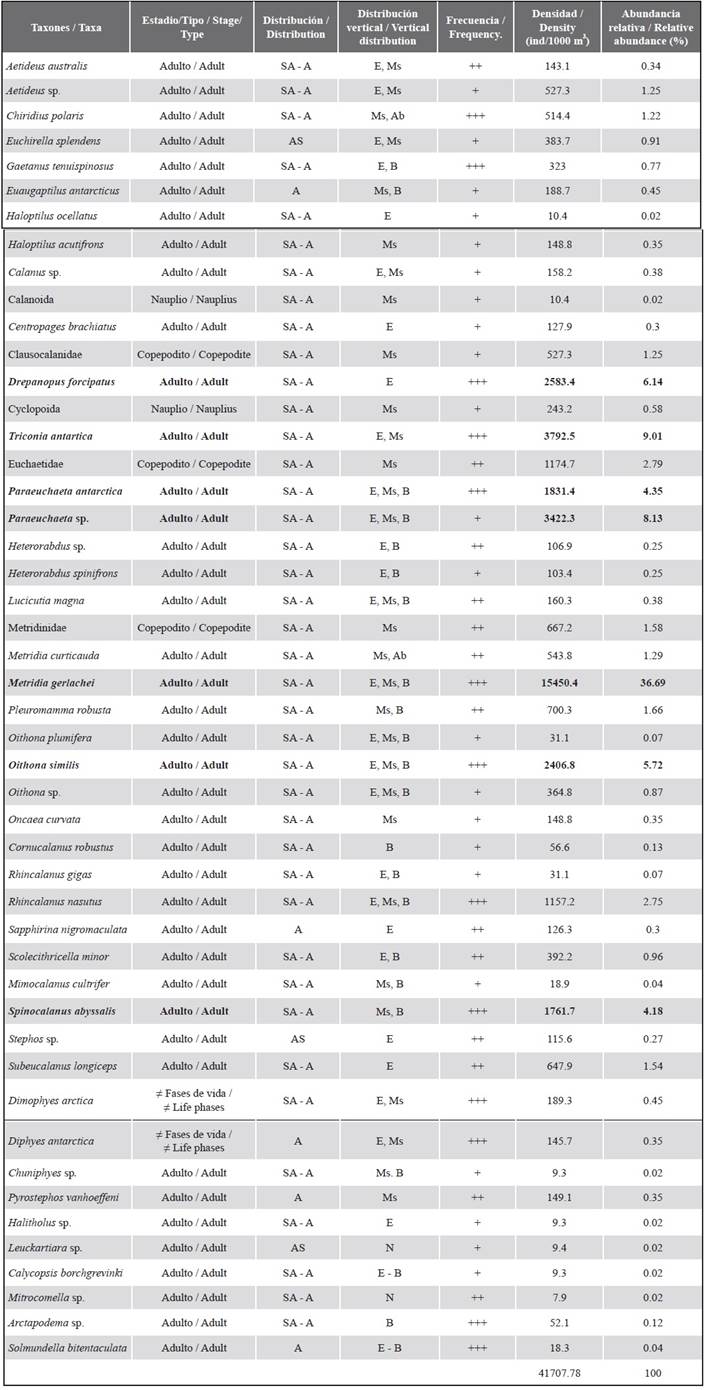
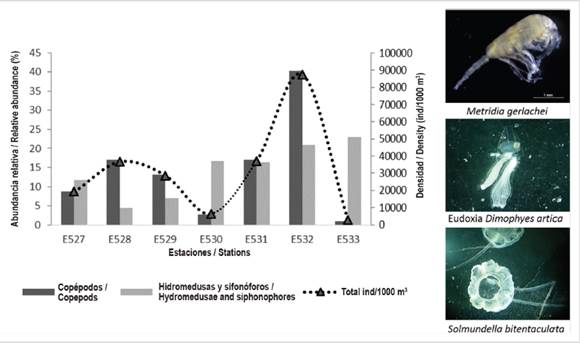
The highest densities occur at station 532 with 87398 ind/1000 m3 ± 2104, followed by station 531 with 37149 ind/1000 m3 ± 953.34; while the station with the lowest densities is 533 with 2811.16 ind/1000 m3 ± 85.92 (Figure 2). The most abundant species is Metridia gerlachei with an average of 15450 ind/1000 m3 ± 5016.2 and Triconia antarctica with an average of 3793 ind/1000 m3 ± 2365.6 (Table 2).
Structure and assembly of species
Two associations were discriminated, defined by the Bray-Curtis dissimilarity dendrogram (Figure 3), the dissimilarity percentages were close to 60 %. One group is characterized by the highest relative abundances of copepods (an entire transect within the Gerlache Strait) and the other group (only two stations E530 and E533) located northwest of the strait (Croker passage) characterized by having the relative abundance and lowest richness of all stations, but with a higher relative abundance of siphonophores and hydromedusae (Figure 2). These associations were statistically differentiated (ANOSIM) based on the fact that the p-value was less than 0.01 and the global R-close to 1 (0.873).
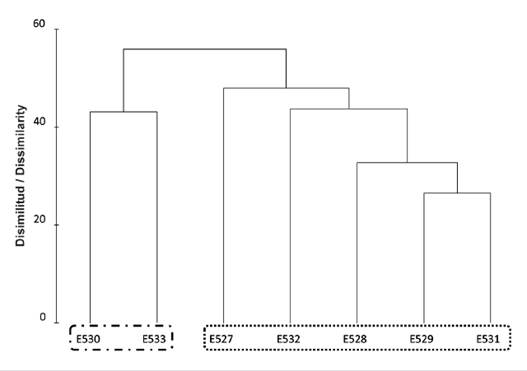
Figure 3 Nonparametric multivariate descriptive analysis of quantitative dissimilarity classification (Cluster) for the samples that were grouped in the Gerlache Strait during the 2016-2017 austral summer expedition. Frame (••••••): Association 1. Frame (• - • - • - •): Association 2.
According to the results of the SIMPER analysis, the group of two stations located in the Croker passage presented five species that contributed to making up the group and that added together are equivalent to 92.71 % similarity, these species are Metridia gerlachei, Triconia antarctica, Spinocalanus abysalis, Dimophyes arctica and Diphyes antartica. For the group made up of the remaining five stations, with a sum of 91.69 % similarity, five species contributed to group the stations, among them were, Metridia gerlachei, Paraeuchaeta antarctica, Oithona similis, Triconia Antarctica and Drepanopus forcipatus. Finally, with the BIOENV analysis and as a statistical method the Spearman rank correlation coefficient, it was found that temperature and salinity factors can have the greatest effect on the structure and composition of copepods, hydromedusae and siphonophores, with a harmonic correlation Spearman’s (ρw) of 0.648.
DISCUSSION
Composition of copepods, hydromedusae and siphonophores
The copepod species Haloptilus acutifrons, Centropages brachiatus, Heterorabdus spinifrons and Oithona plumifera are new records for the Gerlache Strait in waters between 0 and 700 m depth. The remaining 34 species of copepods have been frequently cited and previously reported in the Antarctic region and the Gerlache Strait (Zmijewska and Yen, 1993; Fuentes et al., 2008; Kouwenberg et al., 2014; Cornils et al., 2018; Razouls et al., 2005-2019; Giraldo et al., 2019). There are about 200 species of copepods that inhabit the pelagic environments of Antarctic waters, however, only a few species dominate the Antarctic pelagic assemblage of the Gerlache Strait, among which species belonging to large Calanoids such as Metridia gerlachei, Paraeuchaeta antarctica stand out and Rhincalanus nasutus, small Calanoids such as Spinocalanus abysallis and Drepanopus forcipatus and Cylopoideos such as Oithona similis and Triconia antarctica, this structure has already been described by other authors for Antarctic environments (Razouls et al., 2000; Schnack-Schiel, 2001; Tarling et al., 2017; Cornils et al., 2018; Giraldo et al., 2019) who report that these taxa can add between 70 % and 95 % of the total density and biomass of copepods, their success being possibly attributed to the different life strategies that regulate with seasonal variability, such as the case of M. gerlachei and P. antarctica which are omnivorous copepods that feed during the summer and autumn and later in the winter and spring reproduce by releasing their eggs. While O. similis, D. forcipatus and Triconia antarctica and the other copepods of medium and small sizes are herbivores, preferably feeding on micro-plankton and microzooplankton throughout the year (Nishida, 1985; Schnack-Schiel and Hagen. 1994; Atkinson et al., 1996; Schnack-Schiel, 2001; Giraldo et al., 2019) taking full advantage of seasonal variation and food availability, to increase their reproductive rates and maintain their dominance in Antarctic waters (Atkinson et al., 1996; Alonzo et al., 2000).
When determining the composition of copepods, species inhabiting both epipelagic waters (eg H. ocellatus, C. brachiatus, D. forcipatus, S. longipes) and meso and bathypelagic waters (eg E. antarcticus, P. robusta, M. cultifer, S. gerlachei) and others capable of living throughout the entire water column, such as M. gerlachei, P. antarctica, O. similis and R. nasutus (Yang et al., 2016). For oceanic copepod species that inhabit Antarctic and Subantarctic waters, a homogeneous pattern of distribution can be distinguished, which allows epipelagic and mesopelagic species to have a greater range of distribution in the water column, this pattern gradually changes from southern latitudes to temperate, subtropical and tropical latitudes mainly due to thermohaline circulation and currents that originate in boreal environments (Schnack-Schiel and Mujica 1994; Atkinson., 1999; Yang et al., 2016).
Concerning the species of hydromedusae and siphonophores, although some articles still work on this group of gelatinous animals only up to order (Cabal et al., 2002), the species described here are common for the Antarctic Peninsula (Bellingshausen Sea, Sea of Scotia, Weddell Sea), with siphonophores being the most frequent, such as Pyrostephos vanhoeffeni, considered indigenous to the region (Pugh, 1999) and Dimophyes arctica and Diphyes antarctica, which present the highest densities and a slightly more continental distribution towards the Antarctic Peninsula (Pagès and Gili, 1989; Pugh, 1999, Fuentes et al., 2008; Mackey et al., 2012; Lindsay et al., 2014; Panasiuk-Chodnicka et al., 2014). These two siphonophores were collected in both polygastric and eudoxic forms, the latter being dominant in all stations. The hydromedusae characteristic of the Antarctic region belongs to the orders Narcomedusae (Solmundella bitentaculata) and Trachymedusae (Arctapodema sp.) (Lindsay et al., 2014).
Structure and assembly of species
As reported by Zmijewska and Yen (1993), Calbert et al. (2006) and Giraldo et al. (2019), for the Antarctic summer 2016-2017, there is marked variability in the density of organisms, the highest densities are located mainly in the central and southwest sector of the Gerlache Strait and the lowest densities reported towards the northwest sector, always being Metridia gerlachei the dominant species in terms of density, but with a particular assembly structure that varies between stations, making it possible to find stations with a high presence of siphonophores and hydromedusae and others with a lesser presence of these.
The lower densities northwest of the Gerlache Strait (Croker Passage) are a reflection of a high density of the gelatinous and low density of copepods; this may occur due to the local dynamics of the area such as a system of gentle currents (Zhou et al., 2002; Willmott et al., 2007) or the availability of food. In early summer the small increases in temperature strengthen the growth and development of the siphonophores (Panasiuk-Chodnicka et al., 2014), in addition to being carnivores, they have enough food during the summer to carry out their reproductive processes, which is sustained by the presence of their sexual stages in the area. The presence of the eudoxia sexual phase of Dimophyes arctica with 264.14 ind/1000 m3, in the Croker passage, has already been reported with high densities by Panasiuk-Chodnicka et al., (2014) with 200.82 ind/1000 m3, this siphonophore feeds almost exclusively (66 %) of small copepods (Purcell, 1981), which would explain the low density of copepods at station 533.
The greater diversity and density that occurs in the central and southwestern sector of the Gerlache Strait (stations 532 and 531) can be influenced by the strong currents that enter through the Schollaert channel, towards the Gerlache Strait (Zhou et al., 2002; Willmott et al., 2007), causing a greater exchange of waters of lower temperature and higher salinity that change as they mix with the internal waters of the Gerlache Strait, this together with the thaw that occurs in the Strait during the summer (Gordon and Nowlin, 1978), produces mean sea temperature gradients and provides the water column with the necessary resources for the zooplankton assembly within the strait to be dynamic and highly heterogeneous (Atkinson et al., 1996; Smith et al., 1998; Hernández-León et al., 1999; Cabal, 2002; Marrari et al., 2008; Ducklow et al., 2012; Flores et al., 2014; Giraldo et al., 2019).
Through the BIOENV analysis, the effect that changes in the concentrations of temperature and salinity have on the structure of the community of copepods, hydromedusae and siphonophores (Robinson et al., 2010; Wishner et al., 2018). Much of the spatial variability of the plankton structure is attributed to the physical and chemical changes in the water column produced by these variables, due to the exchange of water from the Bellingshausen Sea through passages with depths of less than 400 m such as the Bismarck Strait, Dallman Bay and the Schollaert Canal (García et al., 2002; Willmott et al., 2007; Flores et al., 2014). Other variations are attributable to biological, behavioral and trophic processes typical of the communities that reside in the area (Folt and Burns, 1999).
CONCLUSIONS
This study provides important information on the biodiversity of the zooplanktonic fauna of the Gerlache Strait, increasing the number of occurrences of copepods, hydromedusae and siphonophores reported for the area in the SCAR-MarBIN and also expanding the range of distribution for the species Haloptilus acutifrons, Centropages brachiatus, Heterorabdus spinifrons and Oithona plumifera.
In the Gerlache Strait, a sectorization of the assembly can be observed, one towards the northeast characterized by having the relative abundance and lowest richness, but with a greater relative abundance of siphonophores and hydromedusae, this due to changes in temperature and availability of food at the beginning of the Antarctic summer, favor their reproduction. The other group located along the strait in a southwesterly direction is characterized by the greater relative abundances of copepods, which are influenced by the exchange of water and nutrients through the Schollaert channel, allowing the assembly to develop, be dynamic and highly heterogeneous.
The distribution patterns of copepods, hydromedusae and siphonophores for the Gerlache Strait require future research, to complement the present set of results since it is necessary to carry out a generalized and standardized sampling of zooplankton in each of the Expeditions from Colombia to Antarctica and a definition of the depth strata of the sampling, to analyze the distribution of this community for the different abiotic and biotic factors that regulate it. In this way, new analyzes could be carried out on the interannual variation of zooplankton during the austral summers and could shed new light on distribution patterns, establish relationships with the oceanography of the area and the role that zooplankton plays in the trophodynamics of the Strait of Gerlache.











 texto en
texto en