INTRODUCTION
The adaptive radiation process of molluscs has been extensive. The divergent evolution of this phylum, as a product of processes of natural selection and mutations acquired throughout a geological history dating back to the Precambrian, 550 million years ago, has led molluscs to successfully colonize ecological niches from the tropics to polar seas (Parkhaev, 2008; Haszprunar and Wanninger, 2012; Castillo-Rodríguez, 2014; Vinther, 2014, 2015; Parkhaev, 2017; MolluscaBase, 2019; Wanninger and Wollesen, 2019). This research, product of Colombia’s first expeditions to Antarctica, has transcended the borders of the tropical seas of the Pacific and Colombian Caribbean to expand the country’s scientific capacity to the seas of the Southern Ocean.
Colombia acceded to the Antarctic Treaty in 1989 and later, in 1991 signed the Protocol on Environmental Protection to the Antarctic Treaty (Secretariat of the Antarctic Treaty, 2018). Faced with this commitment, the “Colombian Antarctic Program” was created, which gave rise to the first Colombian scientific expedition to Antarctica, called “Caldas Expedition” during the austral summer 2014-2015 aboard the ship “ARC 20 de Julio”. The Institute of Marine and Coastal Research “José Benito Vives de Andreis” -Invemar participated in this expedition through a project initially conceived with an oceanographic and climatic approach. In 2015 Invemar continued to implement Colombia’s Antarctic scientific agenda with three work fronts focused on climate change, conservation, and biodiversity. In this way, the institute also participated in the expeditions “Admiral Padilla” (austral summer 2016-2017) and “Admiral Campos” (2018-2019). The last two expeditions mentioned were framed within the project Biodiversity and Oceanographic Conditions of the Gerlache Strait “BioGerlache-Antarctica”, whose objective is the characterization of the Antarctic fauna of the strait and surrounding sites, to create a baseline of biological knowledge of the area, which generates new contributions to the biological inventories of Antarctica and contributes to expanding the information in defining possible conservation areas in the region. This research is one of the products of the expeditions mentioned above and contributes information to the general objective of the project “BioGerlache-Antarctica” with a focus on the diversity of the benthic community of molluscs collected between 5 and 400 m depth.
At a historical level, as a result of different expeditions carried out since the end of the 19th century by pioneering countries in the exploration of the Antarctic continent, works related to the study area and the local malacofauna began to be published (Smith, 1885; Watson, 1886; Pelseneer, 1903; Lamy, 1906; Strebel, 1908; Soot-Ryen, 1951; Powell, 1951; Dell, 1964; Hain, 1990). Other more recent works have a more biogeographic approach (Linse et al., 2006; Aldea and Troncoso, 2008; Aldea et al., 2009; González-Wevar et al., 2017) or taxonomic, based on reviews of specific groups of molluscs that include molecular phylogeny (Oliver and Picken, 1984; Criscione and Ponder, 2013; Urcola and Zelaya, 2017; González-Wevar et al., 2018). In this way, a list of the mollusc taxa identified in the study area (shelled gastropods and bivalves) is presented; its systematics, distribution, and some characters.
STUDY AREA
The sampling stations where the molluscs were collected were located around the islands of the Palmer Archipelago and the South Shetland Islands, in the Gerlache and Bransfield Straits respectively, adjacent to the northwest coast of the Antarctic Peninsula (Southern Ocean, Western Antarctica) (Figure 1). The physical environment on the Western Antarctic Peninsula shelf is strongly influenced by the Antarctic Circumpolar Current that flows along the slope of the Antarctic continental shelf and carries relatively warmer nutrient-rich water than the surrounding water; also by the cold waters that flood the north of the Bransfield Strait from the Weddell Sea and by an extensive network of glaciers and ice shelves; also, due to strong seasonal to interannual variability in the formation of sea ice and air-sea interactions, with significant modulation of climatic phenomena such as ‘El Niño-Southern Oscillation’ and the ‘Southern Annular Mode’ or Antarctic oscillation (Moffat and Meredith, 2018).
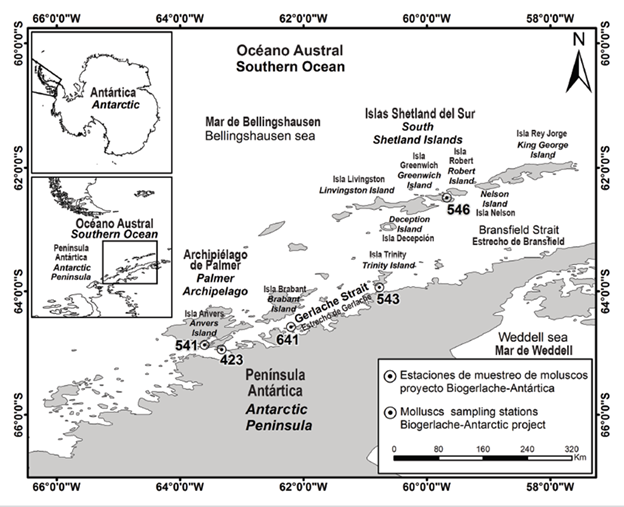
Figure 1 Study area and mollusc collection stations. Prepared in the Information Services Laboratory - Labsis - Invemar.
The Bransfield Strait is a semi-enclosed sea located between the South Shetland Islands archipelago and the coast of the Antarctic Peninsula. The length of the strait is approximately 50,000 km2 and can be divided into three main basins, separated from each other by minimum depths of the submarine floor of less than 1000 m. The western basin of the Bransfield Strait is connected to the neighboring Bellingshausen Sea through passages that exist between the westernmost South Shetland Islands and through the Gerlache Strait; and it is also connected to the Drake passage through the Boyd Strait mainly (García et al., 2002). From a hydrographic point of view, the Bransfield Strait is best defined as a transition zone between the Bellingshausen Sea and the Weddell Sea. The basins of this strait are occupied mainly by water masses whose properties are controlled by the characteristics of the flows of the adjacent seas, a warm flow, and relatively low salinity of the Bellingshausen Sea (typically 0.5-3.0 °C and 33.1-33.9 in summer), and a relatively salty flow from the surface of the Weddell Sea and deep waters (generally with negative temperatures and salinity ranging from 34.1 to 34.6 in summer) (García et al., 2002).
The Gerlache Strait is located between the western coast of the Antarctic Peninsula and the Palmer Archipelago. Water flows from the Bellingshausen Sea into the Gerlache Strait through multiple pathways, passing over thresholds whose depths are less than 400 m, which imposes a significant restriction on circumpolar deep water. Most of this circumpolar deep water layer is lost and is replaced by transient zone waters influenced by the Weddell Sea. At the surface, a sharper transition is observed from Antarctic surface water to transient zonal waters influenced by the Bellingshausen Sea. This last type of water mentioned in the Gerlache Strait is colder and sweeter than in the Bransfield Strait. Returning to the deep layers, an isolated maximum temperature (θ> 0.2 °C) is observed near the eastern end of the Gerlache Strait at depths of the order of 200 m (García et al., 2002). Circulation patterns in the Bransfield and Gerlache Straits change seasonally (Zhou et al., 2002). The shallow waters and southwestern bays of the Bransfield and Gerlache Straits are highly productive areas for biota diversity at many trophic levels (Huntley et al., 1990; Brinton, 1991; Zhou et al., 1994).
MATERIALS AND METHODS
The collection of benthic molluscs (macro and meiofauna) in the study area was carried out during three southern summers (years 2014-2015, 2016-2017, and 2018-2019) in three scientific expeditions to Antarctica aboard the ship ARC 20 de Julio of the Republic of Colombia. Sampling was done at each station by launching a 3 L (0.04 m2) Shipek grab (Table 1). From the free fall of the grab, which closes mechanically in contact with the bottom, approximately 500 g of marine sediment was taken for which the epifauna was separated. The subsamples (500 g) were taken since the rest of the material from the grab was used for the development of other research projects within the framework of the Scientific Agenda of the Colombian Antarctic Program (CCO and CTN AA, 2014).
Table 1 Mollusc sampling stations, names of Colombian scientific expeditions to Antarctica, collection dates, and coordinates.
Once the sediment samples were obtained, they were introduced into thick plastic bags with a narcotic solution (70 g of magnesium chloride / 1 L water) that was left to act for 15 minutes. A 10 % formalin solution prepared with microfiltered seawater (40 µm sieve) and neutralized with borax (pH = 7.2) was added.
In addition to the benthic organisms collected by Shipek grab, others cataloged as epifauna were also obtained, which corresponded to specimens attached to the tide gauge (used to measure or record tides). All samples were duly labeled and transported to the Invemar optical laboratory (Santa Marta, Colombia), where 96 % alcohol was used to preserve them.
The identification of the specimens was carried out by visual examination of their morphology. A Leica S6D brand trinocular stereo-microscope and a Leica M205-A brand stereo-microscope with fusion and zoom optics with motorized focus (model MDG41), plus the complementary hardware for scientific digital photography (Leica Microsystems Application Suite LAS Version 4.5.0) were used. During this process, specialized scientific literature was reviewed and web portals derived from global bioinformatics projects were consulted, with systematic reviews, biological databases, and scientific photography, including Biodiversity Heritage Library -BHL (2019), World Register of Marine Species - WORMS (2019), Encyclopedia of Life -EOL (2019); Marine Biodiversity Information Network -SCAR -MarBin (Griffiths et al., 2011), which forms the regional Antarctic OBIS node (Ocean Biogeographic Information System -OBIS, 2019), and which in turn interacts with Census of Marine Life -COML (2019) and Global Biodiversity Information Facility -GBIF (2019). Additionally, photographic material was compared on websites from specimens registered in different natural history museums around the world, such as Natural Museum of Natural History -NMNH, Smithsonian Institution, United States of America (catalog numbers with the acronym USNM); National Museum Wales (NMW), UK; Natuurhistorish Museum Rotterdam (NMR), The Netherlands; Muséum National d’Histoire Naturelle (MNHN), France. The collected specimens are deposited in the Museum of Marine Natural History of Colombia (MHNMC) - Makuriwa, of Invemar.
RESULTS
The molluscs included in this list were assigned in the MHNMC -Makuriwa a catalog number with the acronym “INV MOL” (Invemar Molluscs). In total 15 specimens were collected, 10 of which were gastropods (including 5 microgastropods) and 5 bivalves. The most abundant species was the gastropod Nacella concinna, from which four specimens were collected, followed by the bivalves Philobrya sublaevis and Adacnarca nitens, with two specimens each. Most of the specimens were collected at station 541 in the Gerlache Strait, around Anvers Island (Palmer Archipelago), during the austral summer 2016-2017.
Systematics
For the 10 gastropod specimens, seven taxa were identified (two of them up to superfamily, two up to genus, and three up to species). The taxa identified up to genus and species were distributed in five families. For the five bivalve specimens, three species belonging to two families were identified.
Class Gastropoda Cuvier, 1795
Subclass Patellogastropoda Lindberg, 1986
Superfamily Patelloidea
Family Nacellidae Thiele, 1891
Genus Nacella Schumacher, 1817
Nacella concinna (Strebel, 1908)
Material examined. 4 specimens:
INV MOL10873. A specimen collected alive corresponding to epifauna on the tide gauge. Depth: 54 m; dimensions: 4.9 x 3.5 mm; E541: around Anvers Island (Palmer Archipelago). Figure 2a.
INV MOL10873. A specimen with an empty shell collected as a benthos sample. Depth: 54 m; dimensions: 0.8 x 0.5 mm; E541: around Anvers Island (Palmer Archipelago).
INV MOL10874. A specimen collected alive corresponding to epifauna on the tide gauge. Depth 5.31 m; dimensions: 3.9 x 2.5 mm; E641: Gerlache Strait, in front of the Antarctic Peninsula.
INV MOL10874. A specimen collected alive corresponding to epifauna on the tide gauge. Depth: 5.31 m; dimensions: 2.95 x 1.9 mm, E641: Gerlache Strait, in front of the Antarctic Peninsula.
Synonyms: see Aldea and Troncoso (2008); González-Wevar et al. (2018); WORMS (2019).
Observations: generally conical shell, ovoid, and laterally compressed on its anterior side. Apex is located beyond the longitudinal half of the shell towards the anterior edge. According to González-Wevar et al. (2018) the shape of the shell and the sculpture are highly variable in this species. This was taken into account during the identification process of the four N. concinna specimens reviewed, as well as the previously cited author’s concept regarding the identity of the identified specimens (Claudio González-Wevar, Com. Pers., 11 of October 2019).
Distribution: Southern Ocean, West Antarctica, on Antarctic islands and the Antarctic peninsula, as well as some subantarctic islands. Mainly in the Bellingshausen and Weddell seas. There are also records in the Scottish Sea (GBIF, 2019). Gónzalez-Wevar et al. (2018) examined specimens from Adelaide Island (Antarctic Peninsula), Anvers Island, Doumer Island (Palmer Archipelago, Antarctic Peninsula), James Ross Island (East Antarctic Peninsula), Livingstone Island, and King George Island (South Shetland Islands), Deception, Elephant Island, South Orkney Island, Signy Island (South Orkneys Islands) and South Georgia Island. It has also been reported in the South Sandwich Islands (Gónzalez-Wevar et al., 2010; González-Wevar et a1., 2017) and Bouvet Island (González-Wevar et a1., 2017).
Bathymetric range: between 0 and 150 m deep (González-Wevar et al., 2018).
Habitat: rocky, intertidal and subtidal (Suda et al., 2015; González-Wevar et a1., 2018). Sedimentary bottoms, subtidal (present study).
Trophic guild: herbivore (Suda et al., 2015; WORMS, 2019).
References and other material used for identification: Powell (1973), Valdovinos and Rüth (2005), Nakano and Ozawa (2007), Gónzalez-Wevar et al. (2010, 2017, 2018), WORMS (2019), GBIF (2019). Photos: USNM897627, USNM897851, USNM897627; NMR50329 and NMR6574.
Subclass Vetigastropoda Salvini-Plawen, 1980
Trochida Order
Superfamily Trochoidea Rafinesque, 1815
Family Calliostomatidae Thiele, 1924 (1847)
Subfamily Margarellinae S. T. Williams, 2013
Genus Margarella Thiele, 1893
Margarella antarctica (Lamy, 1906)
Material examined: INV MOL10875. A specimen collected alive as benthos. Depth: 54 m; dimensions: 9 x 7 mm; E641: Gerlache Strait, Antarctic Peninsula. Figure 2b.
Synonyms: see Aldea and Troncoso (2008), and WORMS (2019).
Observations: Orbicular shell provided with white-cream and blue-violet pearly shades. The lip is interrupted ending on the last lap. Deep navel and corneal operculum with spiral turns.
Distribution: West Antarctica, South Orkney Islands (Melvill and Standen, 1907; Powell, 1951; Linse, 2002), South Shetland Islands (Powell, 1951), Antarctic Peninsula (Lamy, 1906b; 1911; Thiele, 1912; Powell, 1951; Linse, 2002; Aldea and Troncoso, 2008) and the Bellingshausen Sea (Lamy, 1911).
Bathymetric range: between 0 (Powell, 1951) and 460 m (Lamy, 1911) deep.
Habitat: benthos (WORMS, 2019), sedimentary bottoms, as well as other specimens collected on a tide gauge (present study).
Trophic guild: according to WORMS (2019) it is a predator on sessile prey (deduced from Calliostomatidae Thiele, 1924 (1847)); however, other types of diet are not ruled out (see Zelaya, 2004).
References and other material used for identification: Aldea and Troncoso (2008); WORMS (2019). Photographs: NMR65742, MNHN-IM-2000-31126, USNM 886686.
Order Lepetellida Moskalev, 1971
Superfamily Scissurelloidea Gray, 1847
The family Anatomidae McLean, 1989
Genus Anatoma Woodward, 1859
Anatoma sp.
Material examined: INV MOL10876. A live specimen collected as benthos. Depth: 400 m; dimensions: 2 x 1 mm; E423: Strait of Gerlache, in front of Anvers Island. Figure 2c.
Synonyms: Scissurella (Anatoma) (Woodward, 1859) -original rank (WORMS, 2019), Anatomus (Adams, 1862); Schizotrochus (Monterosato, 1877), Hainella (Bandel, 1998); Thieleella (Bandel, 1998).
Observations: Microgastropod. Shell in bad condition, broken at the opening. Involute form, discoidal, translucent, moderately smooth (compared to the Scissurellidae family - Gray 1847, whose sculpture is more prominent), fragile, and with a concave apex. Selenizone present on the periphery of the turns.
Distribution: Northwest Pacific, Northeast Atlantic and Mediterranean, southern oceans of South America, southern oceans of Africa, the Pacific Ocean surrounding New Zealand, and waters surrounding Australia (Geiger, 2019).
Bathymetric range: from continental shelves (0 m) to bathyal and abyssal depths (Geiger, 2019).
Habitat: often on soft substrates (Geiger, 2019), sedimentary bottoms (present study).
References and other material used for identification: Geiger (2003, 2019), Geiger and McLean (2010), GBIF (2019).
Subclass Caenogastropoda Cox, 1960
Order Neogastropoda Wenz, 1938
Superfamily Buccinoidea Rafinesque, 1815
Family Buccinidae Rafinesque, 1815
Genus Prosipho Thiele, 1912
Prosipho turritus P. G.Oliver and Picken, 1984
Material examined: INV MOL10877. A live specimen collected as benthos. Depth: 54 m; dimensions: 2.45 x 1.20 mm; E541: around Anvers Island (Palmer Archipelago). Figure 2d.
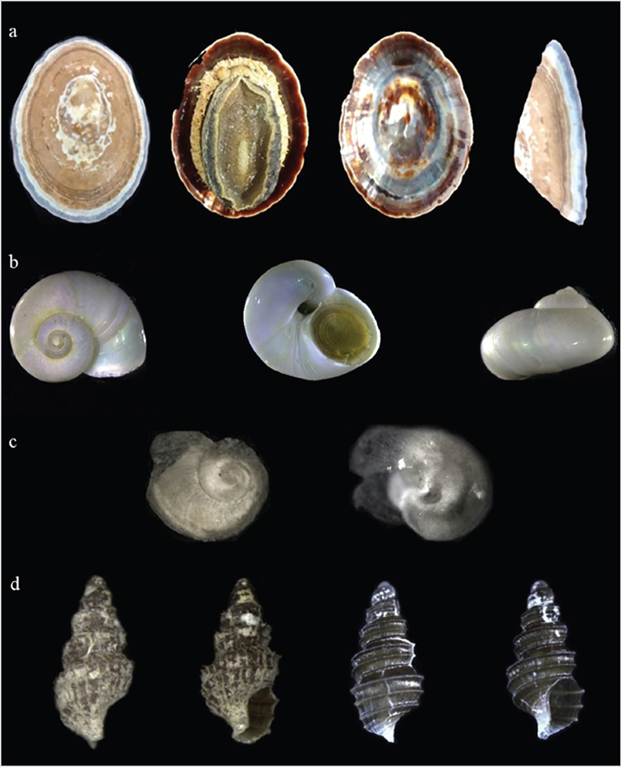
Figure 2 Gastropods collected in the present study: a. Nacella concinna INV MOL10873, 4.9 x 3.5 mm; b. Margarella antarctica INV MOL10875, 9 x 7 mm; c. Anatoma sp. INV MOL10876, 2 x 1 mm; d. Prosipho turritus INV MOL10877, 2.45 x 1.20 mm. Left: specimen with hairy structures, right: the same specimen without hairy structures.
Synonyms: see Aldea and Troncoso (2008), WORMS (2019).
Observations: Microgastropod. Elongated, fusiform shell, without umbilicus, brown periostraque. Smooth inner lip, devoid of folds. External lip provided with spiral ribs and cords similar to those that make up the rest of the shell, which are arranged almost parallel to each other. These protrude outside the outer lip, giving it a crenulated appearance, and are also visible on the inside. Two spiral ribs on each turn, separated by a thin suture. Fine radial lines on the spiral cords. Smooth, whitish, semi-translucent proto-shell and rolled up. Short curved siphon canal. Initially, the shell of the collected specimen was covered with periostracum hairy structures, which fell off during the identification process, making the ornamentation more evident. Thereafter the specimen was compared with P. astrolabiensis (Strebel, 1908), which was later discarded based on the original description and drawings of the holotype (synonymy Sipho (Mohnia) astrolabiensis) in Strebel (1908) and in USNM 886664.
Distribution: Southern Ocean. West Antarctica. Cam Rocks, Borge Bay, Signy Island (South Orkney Islands) (Oliver and Picken, 1984). Weddell Sea (Hain, 1990), Antarctic Peninsula, Bellingshausen Sea (Aldea and Troncoso, 2008).
Bathymetric range: from 2 (Oliver and Picken, 1984) to 300 m (Hain, 1990) deep.
Habitat: benthos (WORMS, 2019), sedimentary bottoms (present study).
Trophic guild: predator (WORMS, 2019).
References and other material used for identification: Oliver and Picken (1984), Hain (1990), Aldea and Troncoso (2008), WORMS (2019), holotype NMW.Z.1979.002.00078, NMW M013549.
Order Littorinimorpha Golikov and Starobogatov, 1975
Cingulopsoidea sp.
Material examined: INV MOL10878. A specimen collected alive as benthos. Depth: 54 m; dimensions: 1.15 x 0.75 mm; E541: around Anvers Island (Palmer Archipelago). Figure 3a.
Observations: Microgastropod. Shell ovoid, turbinate, globose, gray, almost smooth, with slight dark gray radial lines. Edge of the outer lip folded inward to the opening and in the middle of the opening. This fold has a convex-type projection in the middle, and above this there are 5 small teeth on the upper edge of the outer lip. The upper part of the outer lip ends on the last lap. The collected specimen probably belongs to the Cingulopsidae family (Fretter and Patil, 1958); however, further evaluation is required to delve into the taxonomic category, which is difficult due to the small size and fragility of the shell.
Habitat: benthos (WORMS, 2019), sedimentary bottoms (present study).
References and other material used for identification: Golikov and Starobogatov (1975), Ponder (1983), Criscione and Ponder (2013). Photographs: family Cingulopsidae (NMR).
Truncatelloidea sp.
Material examined: A specimen collected as benthos. Depth: 54 m; dimensions: 1 x 0.75 mm; E541: around Anvers Island (Palmer Archipelago). Figure 3b.
Observations: Microgastropod. Turbinate shell, elongated, conical, white, smooth, and with a blunt apex. The edge of the lip slightly retracted outwards. The upper part of the outer lip ends on the last lap. The collected specimen probably belongs to the Hydrobiidae family (Stimpson, 1865); however, further evaluation is required to verify this.
Habitat: benthos (WORMS, 2019), sedimentary bottoms (present study).
References and other material used for identification: Stimpson (1865), Golikov and Starobogatov (1975), Ponder (1983), Criscione and Ponder (2013). Photographs: Family Hydrobiidae (NMR).
Superfamily Cingulopsoidea Fretter and Patil, 1958
Family Eatoniellidae Ponder, 1965
Genus Eatoniella Dall, 1876
Eatoniella sp.
Material examined: INV MOL10879. A specimen collected alive as benthos. Depth: 54 m; dimensions: 0.75 x 0.58 mm; E541: around Anvers Island (Palmer Archipelago). Figure 3c.

Figure 3 a. Cingulopsoidea sp. INV MOL10878, 1.15 x 0.75 mm; b. Truncatelloidea sp., 1 x 0.75 mm; c. Eatoniella sp. INV MOL10879, 0.75 x 0.58 mm.
Observations: Microgastropod. Shell ovoid, turbinate and globose, white and with a blunt apex. Round lip, continuous, without termination in the last turn and with the edge, retracted out of the opening. The shell is almost translucent after the last turn (white) to the apex.
Synonyms: see WORMS (2019).
Distribution: In GBIF (2019) the genus Eatoniella is registered almost exclusively in the coasts and seas of the Southern Hemisphere.
Habitat: sedimentary bottoms (present study).
Trophic guild: According to WORMS (2019) the genus Eatoniella is a detritivore and/or herbivore.
References and other material used for identification: Ponder (1983), Troncoso et al. (2007), Aldea and Troncoso (2008), Criscione and Ponder (2013). Photographs: E. kerguelensis regularis: NMR 65743, family Eatoniellidae (NMR), E. kerguelensis: USNM 881555, E. cana: holotype NMW.Z.1979.002.00031, E. varicifera: holotype NMW.Z.1979.002.00038.
Class Bivalvia Linnaeus, 1758
Subclass Autobranchia Grobben, 1894
Infraclass Pteriomorphia Beurlen, 1944
Order Arcida Stoliczka, 1871
Superfamily Limopsoidea Dall, 1895
Family Philobryidae F. Bernard, 1897
Genus Philobrya J. G. Cooper, 1867
Philobrya sublaevisPelseneer, 1903
Material examined. 2 specimens:
a) A live specimen collected as benthos. Depth: 98 m; dimensions: 7 x 6.1 mm; E543: Trinity Island, between the Gerlache Strait and the Bransfield Strait.
b) INV MOL10880. A live specimen collected as benthos. Depth: 54 m; dimensions: 8 x 5 mm; E541: around Anvers Island (Palmer Archipelago). Figure 4a.
Synonyms: Philobrya antarctica (Thiele, 1931). See WORMS (2019).
Observations: Both are similar specimens. Fragile shell, whitish and uneven cream color. Semi-glossy periostrack with a tendency to dryness and flaking; a part of it protrudes beyond the calcareous valves as a semi-translucent and easily brittle sheet. It has radial ribs that become more prominent from the umbo towards the margin of the shell, intercepted by growth lines. Prodisoconch with a granular appearance at the stereoscope.
Distribution: Southern Ocean. It is one of the most common circumantarctic bivalves, its distribution extends around the Antarctic continent including the Antarctic peninsula. South Shetland Islands, South Orkneys Islands, South Sandwich Islands, South Georgia Island, and Bouvet Island. Ross Sea (GBIF, 2019). It does not appear to extend to the Magallanes region (Dell, 1990). South of the Polar Front, the Falkland Islands, the Weddell Sea, the Scottish Sea, and the Antarctic Peninsula. Common in New Zealand and Australia (Jackson et al., 2015).
Bathymetric range: from the intertidal zone to deep waters> 1000 m (Jackson et al., 2015) deep.
Habitat: benthos (WORMS, 2019). They have byssus filaments to attach themselves to substrates such as rocks, algae, hydrozoans, or hedgehogs (Jackson et al., 2015). Sedimentary bottoms (present and study).
Trophic guild: suspended feeder (EOL, 2019).
References and other material used for identification: Aldea and Troncoso (2008), Jackson et al. (2015), WORMS (2019). Photographs: USNM 882415 (EOL, 2019), USNM 904336.
Genus Adacnarca Pelseneer, 1903
Adacnarca nitens Pelseneer, 1903
Material examined: INV MOL10881. Two specimens were collected alive as benthos. Depth: 98 m; dimensions: 5.1 x 5 mm and 5 x 5 mm, each one attached to the same piece of wood utilizing byssus filaments; E543: Trinity Island, between the Gerlache Strait and the Bransfield Strait. Figures 4b, 4c, and 4d.
Synonyms: see WORMS, 2019.
Observations: bright cream-white shell with shades between yellow and olive green. Ornamented with slightly prominent radial ribs intersected by even lighter concentric lines, exhibiting a very delicate reticulated appearance. It looks almost smooth without a stereoscope. The ornamentation of the prodisoconch is characteristic (see Jackson et al., 2015). The inner edge of the valves was toothed, straight hinge, and provided with small teeth. Slightly translucent valves. Through these, as internally in one of the specimens, the presence of oocytes in an advanced stage of development was observed, some in the veliger phase with a developing shell, a characteristic feature of this species (Higgs et al., 2009).
Distribution: Southern Ocean, Antarctica, Falkland Islands, Weddell Sea, Scottish Sea, and Antarctic Peninsula. Throughout the southern hemisphere (distributed in patches), common in New Zealand and Australia (Jackson et al., 2015).
Bathymetric range: intertidal (Jackson et al., 2015) at 2350 m (Dell, 1990) depth.
Habitat: benthos (WORMS, 2019). They have byssus filaments with which they adhere to substrates such as rocks, algae, hydrozoans, or urchins (Jackson et al., 2015). Specimens attached to a piece of wood found on a sedimentary bottom (present study).
References and other material used for identification: Dell (1990), Aldea and Troncoso (2008), Higgs et al. (2009), Jackson et al. (2015), WORMS (2019). Photographs: USNM 898190.
Infraclass Heteroconchia J. E. Gray, 1854
Subterclass Euheterodonta Giribet & Distel, 2003
Superorder Anomalodesmata Dall, 1889
Superfamily Thracioidea Stoliczka, 1870 (1839)
Family Thraciidae Stoliczka, 1870 (1839)
Genus Thracia Blainville, 1824
Thracia meridionalis E. A.Smith, 1885
Material examined: INV MOL10882. A specimen with an empty shell collected as benthos. Depth: 29 m; 3.7 x 3 mm; E546: Greenwich Island (South Shetland Islands), in front of the Bransfield Strait. Figure 4e.

Figure 4 a. Philobrya sublaevis INV MOL10880, 8 x 5 mm; b. Adacnarca nitens, one attached to a piece of wood by byssus filaments (INV MOL10881); c. A. nitens INV MOL10881, 5.1 x 5 mm; d. A. nitens INV MOL10881, 5 x 5 mm; e. Thracia meridionalis INV MOL10882, 3.7 x 3 mm.
Synonyms: Mysella truncata, Mysella frigida (Thiele, 1912).
Observations: specimen in poor condition, broken. Uneven shell, eroded. Both valves present. Creamy white color, opaque. Dorsally ornate valves with comarginal growth lines from the umbo to the edge. Secondary ligament with thickened periostracum and heterodontic hinge.
Distribution: Southern Ocean. Weddell Sea (Hain, 1990). Around the Antarctic continent, along the Antarctic Peninsula, South Shetland Islands, South Orkneys Islands, South Sandwich Islands, South Georgia Island to the Magellanic region. Falkland Islands, Kerguelen, Marion, and Prince Edward. Ross Sea (Dell, 1990). Bouvet Island (Thiele and Jaeckel, 1931; Linse, 2006). Peter I Island (Soot-Ryen, 1951; Aldea and Troncoso, 2008) and the Bellingshausen Sea to the surroundings of Thurston Island (Aldea and Troncoso, 2008), Beagle Channel (Linse, 1997), Shackleton Ice Platform (Hedley, 1916), Davis Sea (Thiele, 1912; Egorova, 1982).
Bathymetric range: 4 m to 836 m (Dell, 1990) deep. 4 m to 836 m (Dell, 1990) deep.
Habitat: variety of soft bottoms, mud, muddy sand, sand, and gravel (Nicol, 1966; Mühlenhardt-Siegel, 1989). Sedimentary bottoms (present study).
Trophic guild: suspension feeder (Sartori and Domaneschi, 2005).
References and other material used for identification: Sartori and Domaneschi (2005), Aldea and Troncoso (2008). Photographs: USNM 886980.
DISCUSSION
Three expeditions of the Colombian Antarctic Program carried out in southern summers, collected molluscs at the stations sampled in the geographical area of the Strait of Gerlache. Although they are specific samples from expeditions that had different objectives and not specifically benthic samples, these are the first Antarctic molluscs to be recorded in a collection of marine fauna in Colombia. The closest records correspond to the subantarctic zone of the Indian Ocean, collected in 1982 and which are deposited in the mollusc collection of the University of Valle. The identified molluscs are previously reported in some locations in western Antarctica, but the records of some species in the stations carried out by the three Colombian expeditions constitute new sites in their distribution, as is the case of Philobrya sublaevis and Adacnarca nitens in the station E543 of the expedition III “Admiral Padilla” 2016/2017 carried out near Trinity Island.
Most of the mollusc species found south of the subantarctic convergence have distribution throughout the strip that surrounds the Antarctic continent (Cantera and Arnaud, 1984; Hain, 1990), and although biogeographic provinces are recognized, the circumpolar current Antarctica (Jackson et al., 2015), together with the possibility that some species adhere to floating material “rafting” (Gutt, 2007; Higgs et al., 2009) and the existence of species with relatively long larval stages, allow a wide geographical distribution of the species that inhabit this region of the world (Linse et al., 2006; Heilmayer et al., 2008).
Gastropods of the genus Nacella, which together with the bivalves of the Philobryidae family were the most abundant taxa found in Colombian expeditions, are also one of the most dominant molluscs in the Southern Ocean, where they are widely distributed (Linse et al., 2006; Higgs et al., 2009; Jackson et al., 2015; González-Wevar et al., 2017). However, the species of the genus Nacella presents a marked regionalization in this area, as established by González-Wevar et al. (2010) who, based on molecular analysis, determined that geographically distant Nacella species belong to different genetic lineages. The Patagonian species are separated from those of the Antarctic Peninsula, central Chile, and the subantarctic Heard Island in the Indian Ocean of East Antarctica. Nacella concinna, a species found in the present study with four specimens, also exhibited considerable phenotypic plasticity, making it difficult to identify and confusing its identity, which only became evident when relating it to the study area (cf. González-Wevar et al., 2017). This particularity makes its taxonomic determination at a specific level present difficulties.
The other species were found in low numbers, sometimes a single individual. In this geographical area, the difficulties in collecting benthic material are great, due to the existence of very different substrates with an abundance of large rocks, and it is necessary to use various equipment, in addition to intense benthic sampling to achieve a systematic collection of benthic organisms. It should be noted that the Colombian expeditions had different objectives and the mollusc collections were carried out with a Shipek grab. For molluscs from this region, it is necessary to combine (Arnaud 1974, Cantera and Arnaud, 1984) the use of the medium to large size grabs (for example, Charcot Picard, Okean SIPAN grabs) and trawling nets (Blake, “de percha”). These two factors (difficult substrates and use of only one type of grab) may explain the low abundance of specimens and species of molluscs. Likewise, some of the specimens, especially the smaller ones, have been preserved in the museum in a poor state of preservation, showing the shells quite eroded, most likely due to the fixation system used during the expedition (formalin, for example) that, for calcareous organisms in general, but to a greater extent for micromolluscs it is not suitable. Furthermore, tiny and small caenogastropods make up a large proportion of the world’s gastropod diversity, but even more so in Antarctica (Bouchet et al., 2002). The classification of tiny prosobranchs has proven to be usually difficult and probably the least understood, these being loosely identified as “Rissoacea” (Ponder, 1983). Its small and simple morphology can make an identification, even at the family level, difficult (Hershler and Ponder 1998; Geiger et al., 2007; Criscione and Ponder, 2013). However, the specimens found have allowed the identification conservatively, at the superfamily level of the most deteriorated (Cingulopsoidea sp. and Truncatelloidea sp.), at the genus level of two of the microgastropods (Anatoma sp. and Eatoniella sp.), and at species level to Prosipho turritus.
The continuity of this type of basic research, both locally, regionally, and globally is very important. The Antarctic region has been key to establishing global patterns of marine diversity, and one of the pioneering taxa that have allowed the establishment of such patterns has been the phylum Mollusca (Linse et al., 2006), more specifically bivalves and gastropods. These authors, when analyzing the database of molluscs of the Southern Ocean (ie. Southern Ocean Mollusc Database -SOMBASE, Griffiths et al., 2003) indicated that in the Southern Ocean, the biodiversity of gastropods and bivalves is distributed by patches (a scale of 1000 km) and that, for almost all the areas of their study and at all taxonomic levels, gastropods present greater richness than bivalves. This trend can be observed in the present work and also in the tropics and globally (Bouchet et al., 2002; Rosenberg, 2014). In the same way, they determined that at the date of the study, approximately 895 gastropods with shells and 379 species of bivalves were known in the Southern Ocean. In the same work, the authors divided the area into 12 subregions using multivariate analyzes of recent records of these molluscs. Two of the 12 subregions were the Antarctic Peninsula and the South Shetland Islands, within which the mollusc collection stations of the present study were located. The Antarctic Peninsula subregion includes the Palmer Archipelago, the Gerlache Strait, and the region closest to the Bransfield Strait peninsula. The other part of the Bransfield Strait falls within the South Shetland Islands subregion.
Based on SOMEBASE, 131 species, 81 genera, and 47 families of molluscs (including only shelled gastropods and bivalves) are recorded for the Antarctic peninsula. For the South Shetland Islands subregion, 160 species, 90 genera, and 56 families of molluscs are recorded. In other subregions of the seas that are related to the studied area, the Bellingshausen Sea is known to be “very poorly sampled”, with 11 species, 9 genera and 7 families of bivalves (no gastropods). The Weddell Sea, by contrast, represents one of the three “hotspots” of West Antarctica, in terms of mollusc richness (along with South Georgia and the Ross Sea), with 301 species, 126 genera, and 69 families of molluscs (Linse et al., 2006).
This research constitutes a small fraction of the mollusc species registered for Antarctica, but it represents for Colombia, its first foray into malacological studies of the Southern Ocean, adding to inventories of species cataloged in natural history museums that have material from this region and global bio-informatics project platforms (eg. Ocean Biodiversity Information System - OBIS and SCAR-MarBIN). In the future, it is intended that the analysis of data collected together in these platforms contributes, both to explain biogeographic patterns over time, and to provide bases to define possible conservation areas, following the objective of the “BioGerlache-Antarctica” Project Invemar and the Colombian expeditions to Antarctica.











 texto en
texto en 



