INTRODUCTION
Marine diversity is threatened by different disturbances of anthropic origin such as overexploitation, introduction of invasive species, alteration of land use, contamination and climate change (Beatley, 1991; National Research Council, 1995; Irish and Norse, 1996; Ormond et al., 1997; Tickel, 1997; Snelgrove, 1999; Mancera et al., 2013). Among these threats, plastic debris contamination constitutes a great hazard to marine life (Liebezeit and Dubaish, 2012).
Solid wastes in the marine environment have only recently been treated as a complex scientific problem. Today the fragmentation and accumulation of non-degradable debris in the marine environment is considered the ‘‘most ubiquitous and long-lasting recent change to the surface of our planet’’ (Barnes et al., 2009).
Although the types of debris found in the ocean are diverse, plastics account for a considerable quantity because they tend not to decompose (Galgani et al., 1996). Metal or glass objects together with discarded or derelict fishing gear are also among the commonly reported debris types (Hess et al., 1999; Backhurst and Cole, 2000; Chiappone et al., 2002; Keller et al., 2010).
Plastics, synthesized for just over a century (Gorman, 1993), are lightweight, strong, durable and inexpensive organic polymers (Laist, 1987). Due to these same characteristics, plastic waste represents a serious hazard to the marine environment (Laist, 1987; Pruter, 1987).
Due to the high buoyancy, plastic debris is dispersed over large areas, generating significant and increasing loads that can settle in sediments and persist for centuries (Ryan, 1987b; Hansen, 1990; Goldberg, 1995, 1997). Floating marine debris may serve as introduction vector for a variety of organisms to new habitats, facilitating the spread of invasive species (Bravo et al., 2009, Rech et al., 2016).
Moreover, floating debris pose a threat to marine wildlife. Animals like seabirds, marine mammals, turtles and fish often confuse plastic bags, plastic pellets, styrofoam and other floating plastic with their food; the ingestion of these materials reduce effective stomach volume, introduce toxic chemicals and, in extreme cases, cause death by suffocation or blocking the digestive tract (Ryan, 2008; Mrosovsky et al., 2009; Tourinho et al., 2010; Lazar and Gracan, 2011; Possatto et al., 2011). Furthermore, wildlife may get entangled in these residues, which cause injuries or may threaten the life of the animals trapped in them (Boren et al., 2006; Moore et al., 2009; Raum-Suryan et al., 2009; Udyawer et al., 2013). Plastic partition in the ocean sediments induces anoxia and hypoxia, interfering with ecosystem functions and affecting the organisms living in those sediments (Islam and Tanaka, 2004; Aloy et al., 2011).
In addition to the environmental problems, the incidence of marine litter has been related to economic losses due to the loss of the aesthetic value of the beach, and risks for beach users (Santos et al., 2005). This relationship needs to be better documented and understood in order to make coastal zone management more efficient.
The Seaflower Biosphere Reserve , one of the biggest marine protected areas in the Caribbean, receives more than 600 thousand tourists ever year, thus tourism and commerce represent the main income for this archipelago. San Andres, the biggest island, fits well as a typical Caribbean insular country: it is highly dependent on tourism, its population increased with little or no land planification, leading to a chaotic coastal development, and the main tourist attraction is the “sun, sand and sea” formula (Gavio et al., 2010). Considering that beach litter may seriously compromise the tourism business in the island, as well as the wildlife and natural settings the island has been recognized for by Unesco, the objective of this article was to determine the amount and the type of beach debris on the island’s coast, as a first step for a specific management plan.
MATERIALS AND METHODS
Study site
San Andrés is a small island (Figure 1) that lies between 12°28´58´´ and 12°35´55´´ N and 81°40’49´´ and 81°43´23´´ W (IGAC, 1986). The beach area is around 0,1 km2. It forms part of the Archipelago of San Andrès, Providencia and Santa Catalina, and it is the administrative center of the Department, and the main touristic target. The population is concentrated in the northern part of the island (around 7000 habitants/km2), as well as the tourism. Towards the center and the south, the population density is much lower, and the island still maintains rural characteristics.
The island is volcanic in origin (Diaz et al., 1996). The marine platform on the eastern side is shallow and gets to the coral reefs in the open ocean, which mitigate the waves. On this submarine terrace there are deposits of corals, coralline algae, sea urchins, and other animals, which produce calcareous sand (Geister, 1975). On the west side of the island, the transition is much more abrupt, with cliffs indicating strong marine erosion and absence of beaches (IGAC, 1986).
The island lies in the transition zone between tropical dry and tropical wet climate. The annual mean precipitation is 1797.8 mm, unevenly distributed in a dry season (January-April), with stronger winds, and a wet season (October-December) when 80 % of the annual rain falls. During the period May-July the rain is moderate in intensity (IDEAM, 1995). The predominant currents around San Andres Island are caused by the north-east trade winds (Díaz et al., 1995). Currents intercept the island from the northeast, running through the barrier reef and diverging to the south of the island. Current velocity is reduced by the barrier reef and becomes weaker on the western coast of the island (Díaz et al., 1995).
Monitoring protocol
According to the Secretary of Tourism, a total of 678 850 tourists visited the island in 2013, with 183 094 visitors in the first three months of the year (Newball, 2013) . There is no peak touristic season in the island, and the tourist affluence is constant throughout the year.
Three touristic beaches were selected as sample sites: Spratt Bight, Paradise Beach and Tom Hooker (Figure 1). Spratt Bight is the main beach located in the northern part of the island, where most local population lives and where the center of the town, the commerce and the majority of hotels are located. Therefore, it is the most accessible beach to both locals and tourists. Paradise beach and Tom Hooker are located on the eastern side of the island, towards the south. Paradise beach is a touristic beach, where small restaurants are present directly on the beach, and few hotels are present nearby. The small population of San Luis is nearby. It is a touristic beach, but with much less influx than Spratt Bight. Tom Hooker, further south, is a small beach where few tourists normally turn up. The beach is mainly visited by residents living close by. Therefore, the three beaches were chosen on the basis of their differential affluence of people as well as the type of people mostly visiting them, tourists versus residents.
Beaches are cleaned daily by the Department around 6:30 a.m. Furthermore, in Spratt Bight the people renting chairs and umbrella clean the area where they install their equipment. Each site was visited once a week for a total of 10 weeks, from February to April 2013, at 6 a.m., before the beach cleanup. While most studies on beach litter sample just once, the sampling in the present work was designed in order to report weekly accumulation of litter at each site and avoid long-term accumulation biases. The beach was sampled always in the same spot during the ten weeks.
Two 50 m transects parallel to the coastline were disposed at each site, one on the shoreline, the other approximately at a 5 m distance from the first, in the recreational area of the beach, where tourists spend most of the time. For each survey, recorders walked back and forth along the 50 m transects and collected all visible anthropogenic litter present within 1 m span from the transect (50 cm by each side). Only large wood material (branches, tree fragments) was counted but not weighed if the dimension of the litter was too big to be transported by hand. The total area surveyed per each transect was 50 m2. The litter was deposited in labeled plastic bags. In the laboratory, individual debris were washed to remove any sand, dried, counted and weighted on a digital balance Onhaus Adventurer Pro AV264 with a precision of 0.0001 g. The items were grouped into 10 major categories reflecting their nature.
Analysis
Particles were classified in ten major categories: plastic, glass, metal, construction material, cigarette filter, paper /cardboard, wood, organic, fabric, and others. Beach cleanliness was assessed with the Clean Coast Index (CCI) (Alkalay et al., 2007). The CCI index was calculated for each site every week, and then we calculated the mean CCI per site.
We applied the following equation (Laglbauer et al., 2014):
CCI = (Total plastic parts of the transect/Total area of the transect) *k
Where CCI is the number of plastic items m-2, the plastic parts taken into account for the formula where > 2 cm (Alkalay et al., 2007), the area of each transect is 50 m2 and k (constant) = 20. The sites were classified according to the scale provided by Alkalay et al. (2007) (Table 1).
Table 1 Clean Coast Index. Values, grades and visual assessment of the Clean Coast Index (Alkalay et al., 2007).

Statistical analysis
Statistical analysis of the litter data (count and weight) was performed using the program STATISTICA 6 StatSoft, Inc. Variance analysis were performed to compare sites and transects (shoreline vs. recreational area).
Differences in litter as well as Clean Coast Index were tested among beaches using a one-way analysis of variance (ANOVA). Data were transformed (ln (x + 1)) to meet ANOVA assumptions (Zar, 2010). Post hoc pairwise comparisons were performed with Tukey test when significant differences (P < 0.05) were observed within a main effect.
RESULTS
Units
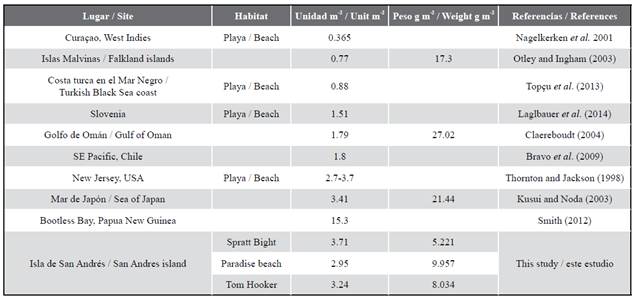
We collected a total of 9894 units of litter during the ten weeks of the study. The litter units consisted mainly of plastic (between 84 and 89 %); in Tom Hooker the second litter component was glass (10 %); while in Paradise Beach was fabric (5 %) and in Spratt Bight was paper and cigarette filters (4 % each) (Figure 2). Paradise beach had a mean density of 2.95 unit m-2, Tom Hooker a mean of 3.24 unit m-2 and Spratt Bight had a mean density of 3.71 unit m-2 (Table 2).
The litter was found mainly along the shoreline, with a less percentage in the recreational area. This was especially true for Spratt Bight, where 72 % of the litter units were collected from the shoreline and only 28 % along the recreational area. For Tom Hooker, 63 % was in the shoreline and 37 % in the recreational area and in Paradise beach 53 % was on the shoreline and 47 % on the recreational area. No statistical difference was observed among the three beaches.
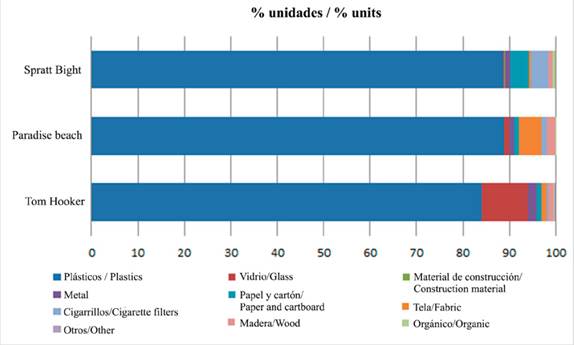
Figure 2 Proportion (% of number of items per m-2) of litter of the ten major groups observed at each study site.
CCI Index
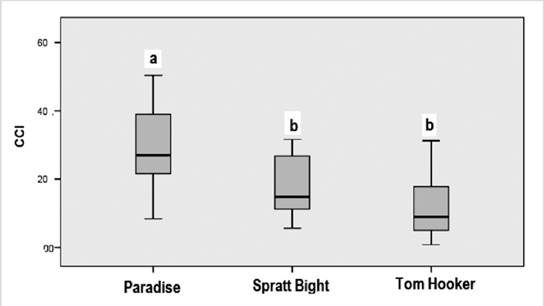
The index was calculated week by week, and the mean index was extrapolated. Two of the three study sites classified as “extremely dirty” (Figure 3), and only Tom Hooker classified as “dirty”. The most polluted site were Paradise beach ( range 16-44.4), Spratt Bight (range 7.2-48) and finally Tom Hooker ( range 5.6-22.6). CCI was significantly (F(2,57) = 12.57, P = 0.000029) higher in Paradise beach (29.6 ± 12) (mean ± ED) compared to Tom Hooker (12.5 ± 9.6) and Spratt Bight (21.8 ± 18.6) (Figure 4).
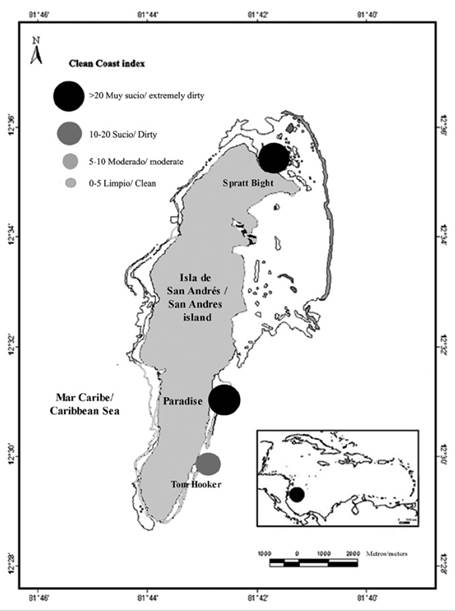
Figure 3 Pollution status of the study sites according to the Clean Coast Index. 1. Spratt Bight; 2. Paradise Beach; 3. Tom Hooker.
Weight
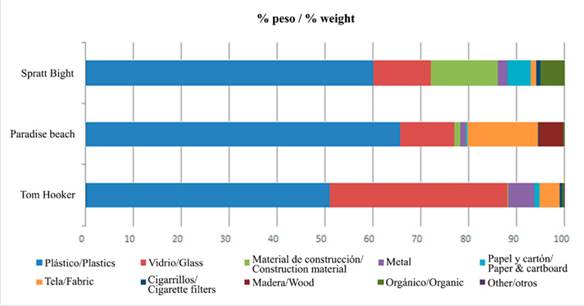
The total litter we collected weighted 23 212.47 g; plastic constituted 51 % (Tom Hooker), 60 % (Spratt Bight) and 66 % (Paradise Beach) of the total litter weight, followed by glass in Tom Hooker (37 %), construction material in Spratt Bight (14 %) or fabric in Paradise beach (15 %) (Figure 5). The mean litter weight per unit area was 9957 g/m2 in Paradise Beach, 5221 g/m2 in Spratt Bight and 8034 g/m2 in Tom Hooker (Table 2).
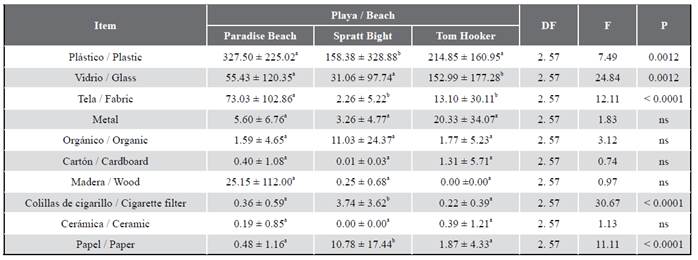
Statistical differences were observed among the three beaches in term of litter weight for plastics, glass, fabric, cigarette filter, and paper (Table 3). Paradise beach presented in average higher amount of plastic (327.5 g m-2) than Spratt Bight (158.38 g m-2) and Tom Hooker (214.85 g m-2). In Tom Hooker there was a much higher amount of glass (152.99 g m-2) than in Paradise beach (55.43 g m-2) and Spratt Bight (31.06 g m-2). Regarding fabric, Paradise beach showed higher average (73.03 g m-2) than Spratt Bight (2.26 g m-2) and Tom Hooker (13.10 g m-2). Spratt Bight with 3.74 g m-2 of cigarette filter and 10.78 g m-2 of paper, presented significant higher averages than Paradise Beach (0.36 g m-2; 0.48 g m-2) and Tom Hooker (0.22 g m-2 ; 1.87 g m-2).
Table 3 Statistical results of litter at the beaches in San Andres island, Colombia.Means (± ED) followed by different letters across each row are significantly different for each variable (Tukey post hoc test, p < 0.05). ns, not significant p > 0.05
Temporal analysis
No visible temporal pattern was observed for the three sites. The variability between weeks was not statistically different. Only in Spratt Bight, during week four, we collected a considerable amount of litter on the shoreline. This was possibly due to a cold front hitting the island during that week, which transported ashore a great quantity of litter. However, this was not observed at the other two sites.
DISCUSSION
During the present study, 9894 units of litter were collected, which weighted a total of 23 212.47 g for 3000 m2 surveyed. We collected a mean of 7.73 g m-2 and 3298 units m-2 on the beach shore. The litter was mainly composed of plastic (88 %), followed by glass (4 %). Other studies around the world show that plastic contributes the most significant part of marine litter, reaching about 80 % of debris (e.g. Islam and Tanaka, 2004; Morishige et al., 2007; Hinojosa and Thiel, 2009). Our data corroborate this worldwide trend.
The local government has a daily cleaning program for the most touristic beaches. People comb the beach early in the morning, although they normally bury the anthropic litter instead of collecting it, especially if the item is small. Furthermore, the persons who rent chairs and tents to tourists also clean the beach when they install their equipment. Therefore, there is little or no accumulation of litter on the beaches between days. Despite this cleaning program, the shore litter is abundant, and two of the three sites were classified as “extremely dirty”, while the third was classified as “dirty”. It is important to notice that most studies on marine litter collect just once, and therefore it is not possible to determine the daily deposit of litter. In this study we wanted to determine daily litter import, which shows to be great.
Spratt Bight, the most touristic beach of the island, classified as “extremely dirty”, as well as Paradise Beach. This despite the daily cleaning program of the local government, and the locals that rent chairs and tents, which also contribute to the cleaning of the beach where they are located. Tom Hooker, a much less visited beach, classified as dirty. From these results, we can postulate that litter is dumped on the beach not only by tourists; local residents contribute in great part to the inadequate disposal of solid residues.
Recent research in Panama has highlighted that microplastic pollution is more severe along the Caribbean coast of the country, compared to the Pacific side (Delvalle de Borrero et al., 2020). The authors argue that on the Pacific coast, anthropogenic activities are much more intense, and the higher concentration of microplastic along the Caribbean beaches is due to current dynamics. However, this does not seem to be the case for San Andrés, where both tourists and residents are the main responsible for the litter on the beach.
Plastics, glass, and other solid wastes have been included among the potential anthropogenic threats for beach ecosystems (Chapman, 2007), and are defined as factors of human origin that may cause damage to the quality of ecosystem services (Beaumont et al., 2007; Defeo et al., 2009). There is a plethora of studies on marine debris accumulations, highlighting the importance of the problem and its extension to all the coasts of the world. Only very recently, this problem has received attention also in Colombia. Rangel-Buitrago et al. (2018) reported an average of 7 items m-2 for 13 beaches in the Atlántico Department, on the continental Caribbean coast. Along the central Caribbean coast of the country, Rangel-Buitrago et al. (2020) reported an average of 6.05 items m-2, with plastic accounting for 88.9 % of the residues. In the Ciénaga Grande de Santa Marta, a mangrove ecosystem, Garcés-Ordóñez et al. (2019) found 540 and 31 items/ha, near and far from populated centers, respectively.
Even at remote or inhabited sites, solid residues are present, and plastic is always the dominant material. For example, at Albuquerque cay, a small atoll with two emerged cays 37 km southwest of San Andrés island, Portz et al. (2020) determined an average of 0.5 items m-2. The cays support a permanent military population of only 12 marines, therefore the direct anthropic impact is minimal.
The present study addresses the problem of marine litter in San Andres Island, which is one of the main touristic places in Colombia, and is part of the International Biosphere Reserve Seaflower. Unfortunately, marine debris are ubiquitous and abundant in the island, despite the daily cleaning program of the main beaches. Our results suggest that mass tourism of San Andres Island may be increasing the intensity of pressures associated with pollution and without effective cleaning controls, the economic system of the island could be strongly affected.
CONCLUSIONS
San Andres Island has a great amount of litter along its coast. The main components of the litter are plastics and glass, consistent with most studies published on the topic. Despite the cleaning program of the local Department, two of the three sites we monitored are classified as “extremely dirty”, and only one as “dirty”. These results underline the necessity of a major control by the authorities on garbage disposal, and of an educational program for both residents and tourists on litter disposal practices. Moreover, further studies are required to determine the effects of coastal litter on the nearshore ecosystems of the island, such as seagrass beds and coral reefs. Most litter we found was plastics, which is both lightweight and durable, and its negative effects on marine fauna has been extensively proved. Such quantities of marine debris may prove deleterious for the marine ecosystems the Reserve is supposed to protect and should be taken into account for better management practices in the Biosphere Reserve.











 text in
text in