INTRODUCTION
In the Caribbean Sea, bivalve mollusks have always been maintained under the expectation of being massively cultivated, particularly in the northeastern coast of Venezuela. Special attention was given to the cultivation of some species, such as the brown mussel Perna perna, the mangrove oyster Crassostrea rhizophorae and the American oyster C. virginica, which have been the subject of commercial cultivation for decades. Similarly, other species for which experimental crops have been developed include the scallops Euvola ziczac, Nodipecten nodosus and Argopecten nucleus, the green mussel Perna viridis, and the pearl oysters Pteria colymbus and Pinctada imbricata (Lodeiros and Freites, 2008).
One of the pearl oyster species with dual production targets (human protein source and pearl production) is the winged pearl oyster of the Atlantic, P. colymbus (Röding, 1798). This epibenthic species of the family Pteridae reaches a maximum shell height of 60-70 mm, inhabits the subtidal zone at depths between 3 and 10 m, attaches to hard substrates such as rocks and octocorals, and is distributed along the western Atlantic, from North Carolina to southern Brazil (Díaz and Puyana, 1994; Lodeiros et al., 1999a). In Colombia, Brazil and Venezuela, some studies aimed to determine the biological feasibility of P. colymbus cultivation have been performed, particularly to obtain seeds with artificial collectors and grow them to commercial size of 5-6 cm on-field (Lodeiros et al., 1999b; Márquez et al., 2000; Urban, 2000; Mengual et al., 2011; Freites et al., 2017). Further, Lara et al. (2009) and Romero et al. (2009) adapted and transferred this culturing technology to indigenous communities in the Guajira in the Colombian Caribbean area. So far, the results of integrating this technology as part of continuous livelihood training programs have been encouraging.
Developing optimal culture methods to maximize growth and survival in juvenile pearl oysters is important to reduce the time required to reach operable size for pearl production (Millione and Southgate, 2011). For P. sterna, a related winged pearl oyster species, some authors have determined a differential growth and survival of the animals placed in different types of enclosures in hanging culture (Millione and Southgate, 2011) or between suspended and bottom culture on the seabed (Gaytán-Mondragón et al., 1993). Gaytán-Mondragón et al. (1993), for example, reported 99 % survival of the oysters in pocket nets, 84 % in lanterns nets and 98 % in bottom cages, concluding that the enclosure device plays an important role on the cultivation of the species. Millione and Southgate (2011) showed that site selection and culture unit are important parameters for optimizing the growth and survival of P. penguin during its culture’s nursery phase. The enclosure system also exerts an influence on the quality of half pearls (mabés) produced by P. sterna (Ruiz-Rubio et al., 2006), as no significant differences occurred in the growth of the oysters held in plastic cages and pocket nets, but a greater yield of commercial quality pearls occurred in those grown in plastic cages.
The Southern Caribbean Sea, and particularly the northeastern coast of Venezuela, is under the influence of coastal upwelling (Okuda et al., 1978; Jury, 2018). The high concentrations of nutrients during particular periods of the year stimulates the productivity of phytoplankton, and it has been hypothesized that the cycles of upwelling and relaxation (non-upwelling) modulate the availability of planktonic food for some benthic species, influencing, in turn, their growth patterns (Puccinelli et al., 2016). The predominant trade winds blow parallel to the coast and the topography of the continental shelf favor the upwelling of sub-tropical waters rich in nutrients, with a seasonal intensification between December and April (Müller-Karger and Aparicio-Castro, 1994, Freites et al., 2017) Recently, Rueda-Roa et al. (2018) showed the presence of a mid-year upwelling cycle (June-August) in the northeastern coast of Venezuela, attributed to the intensification of the Caribbean Current during this period. This pattern helps to maintain a highly productive ecosystem and the variability in the water column, affecting, in turn, the temperature and chlorophyll traits (Okuda et al., 1978; Rueda-Roa, 2012; Müller-Karger et al., 2013).
The goal of this study was to evaluate the growth and survival of P. colymbus juveniles (until final grow-out) in five methods of hanging culture, during the two hydrographic seasons that occur within the Gulf of Cariaco (upwelling and non-upwelling). This as a strategy to advance in the optimization of a feasible culturing method of this bivalve species in shellfish farming in the Caribbean Sea.
MATERIALS AND METHODS
Source of juveniles and experimental design
Juveniles were obtained from the natural spatfall 2014 using bag collectors (green onion bags filled with five green onion bags) and then suspended from a 100-m long line placed in the Turpialito site (Figure 1), Sucre State, Venezuela (10° 26’56 “N; 64° 02’00” W). The long line was set parallel to the coastline, 50 m away from it, in a place with a depth of 7-8 m, where five culture methods were submerged at 4 m depth.
Each culture method studied included three enclosures (replicates) that confined the oysters: baskets top and bottom closed (BTBC), baskets bottom opened (BBO), and baskets top opened (BTO); as well as, two methods without enclosure: oysters fixed over baskets (OFOB) and oysters fixed over fishing net strings (FNS) using biodegradable mesh support designed for this purpose. Three replicates with fifteen juveniles each were used per each of the five culture methods (225 oysters), and for the initial sampling of the oyster’s biometrical variables, three additional replicates of five juveniles each were taken (15 extra oysters, for a total of 240 oysters per season). The dimensions of the different culture methods (approximately 1500 cm2 area) and main features are shown in Figure 2. All baskets were manufactured with 2×2 cm2 Ø polypropylene plastic net, with a design that allowed the evaluation of the method that’s less affected by the predatory action of ranellid gastropods. This, considering that unconfined bivalves usually show lower predation rates than confined bivalves (Semidey et al., 2010).
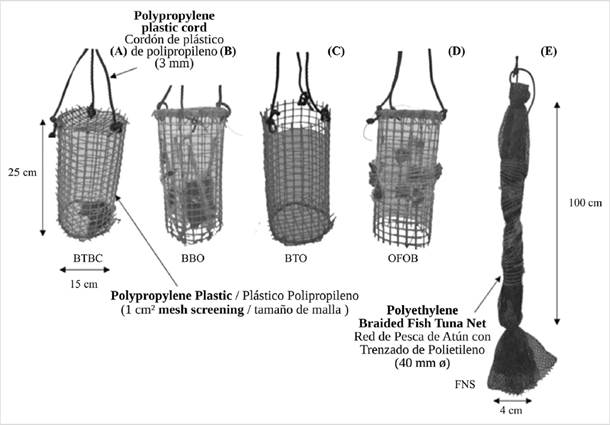
Figure 2 Different types of culture-hanging methods used for grow-out of juveniles of the winged pearl oyster P. colymbus with confined individuals: A) Baskets top and bottom closed (BTBC); B) Baskets bottom opened (BBO); C) Baskets top opened (BTO); and unconfined individuals: D) Oysters fixed over baskets (OFOB) and E) Fishing net strings (FNS).
Growth and survival
The shell height of the oysters was measured at 33.28 ± 2.2 mm at the start of the studies of both the upwelling and non-upwelling seasons. Growth and survival traits were evaluated for 90 days during each season, July-September 2014 (non-upwelling) and March-May 2015 (upwelling), established by the trade wind regimes and Caribbean Current, which allowed for the evaluation of influence of environmental variability. During both cultivation periods, neither predator nor competitor controls were carried out, only at the end of the periods.
To measure the growth of the oysters in each treatment, five oysters were randomly selected and taken from each of the treatments, to form three new replicates. After removing all the fouling fauna from the shell surface, shell height was measured with a digital caliper (Mitutoyo, ± 0.01 mm). The dry mass of the fouling, shell, and soft tissues dry mass (STDM) of the oysters were obtained by dehydration in an oven at 60 ºC for 72 h and weighing on a digital balance (OHAUS Adventurer, ± 0.001 g).
Survival was evaluated by counting the live specimens at the end of each season, in the different culture methods. The presence or absence of predators was considered in each case, to establish their influence on the oyster performance. The dimensionless condition index (CI) was calculated as the ratio of dry mass of soft tissues divided by the total dry mass of the oyster, by 100 (Narváez et al., 2008).
Environmental factors
Water temperature was continuously measured in intervals of 60 min, using an electronic thermograph (Minilog, Vemco, Canada, 0.01 °C). Weekly water samples were taken (at culture depths) with a Niskin bottle (5-L), including a subsample to determine the oxygen concentration with the Winkler method, according to recommendations of Strickland and Parsons (1972). The remaining water from the Niskin bottle was transferred to opaque plastic containers to be transported to the laboratory; after which two 1.5-L replicates were pre-filtered (153 μm) to remove large particulate matter and zooplankton and then used to determine chlorophyll a and organic particulate matter (POM). These samples were filtered on pre-combusted GF/F 0.7-μm filters (450 °C for 4 h), weighed, and rinsed with isotonic ammonium formate (0.5 M). Filters were dehydrated at 60 ºC/24 h to determine the POM by gravimetric methods, then after the incineration of the filters at 450 ºC/4 h in a muffle furnace, the inorganic seston was determined. Phytoplankton abundance was estimated as chlorophyll a (Chl-a) using the spectrophotometric method following Strickland and Parsons (1972). At the end of each season, the fouling on the oyster shells was carefully extracted and weighed after drying at 80 °C/72 h.
For the determination of the upwelling index, the methodology proposed by Freites et al. (2017) was considered, using the formula described by Bowden (1983) and modified by Lavin et al. (1991). For the determination of wind velocity and wave height (H) during the evaluated seasons, the daily database of the Windguru virtual platform (https://www.windguru.cz/67638) was used.
Statistical analysis
The homogeneity of the variance data was confirmed with the Levene test. Then, a one-way analysis of variance (ANOVA I) was used to check for significant differences in the increments in shell size, shell mass, soft tissue mass, Fouling, survival and oyster condition index between the five culture treatments, in each culture season (upwelling or non-upwelling), and the differences between the environmental variables seen in both seasons. The variables that showed significant differences (p< 0.05) were evaluated by a posteriori analysis of Tukey. The size and biomass data were previously transformed to Log10 (Zar, 1996). Additionally, a two-way analysis of variance (ANOVA II) was run to detect if the season, culture methods and the interaction of both of these variation sources influenced the results obtained. When significant differences were observed in some interactions, an orthogonal contrast analysis was developed following recommendations by Yossa and Verdegem (2015). In this case, significant differences among means were evaluated by the Duncan´s test using SPPS 23.0 software (IBM SPSS Statistics). All the remaining analyses were carried out with the statistical package SYSTAT 12.02.A.
The method of graphic management or Principal Components Analysis was used to identify the environmental variability modulation and its influence on the increase in soft tissues mass, CI, and mortality in both study seasons and culture methods (Chatfield and Collins, 1980). In this case, the CANOCO statistical package (v. 4) was used (Ter and Smilauer, 1998).
RESULTS
Shell growth
In general, the increments in shell height during the non-upwelling season were significantly higher (ANOVA I, p< 0.05) in oysters cultured in baskets BTBC (13.76 ± 0.35 mm), BTO (12.95 ± 0.29 mm) and FNS (11.50 ± 0.67 mm), compared to those cultivated in baskets OFOB (10.71 ± 1.00 mm) and BBO (10.20 ± 0.18 mm), where the growth was lower (Figure 3). During the upwelling season, the oysters with significantly higher increments in shell height (ANOVA I, p< 0.05), were those cultivated in FNS (30.98 ± 0.69 mm) and OFOB (30.24 ± 1.09 mm), in contrast to those cultivated in baskets BTBC (28.49 ± 0.24 mm), BTO (28.42 ± 0.24 mm), and BBO (27.63 ± 0.19 mm) where the growth was lower (Figure 3).
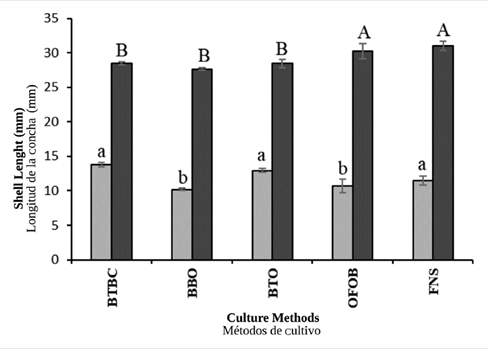
Figure 3 Shell height increments seen in the winged pearl oyster P. colymbus for each culture method and hydrographic season (light bars: non-upwelling; dark bars: upwelling). Identical superscript letters denote lack of significant differences at p < 0.05. The vertical lines indicate the standard deviation.
Soft tissue dry mass (STDM),
During the non-upwelling season, the increment of soft tissues dry mass was significantly greater (ANOVA I,p< 0.05), in oysters cultured in baskets BTBC (0.63 ± 0.04 g), FNS (0.61 ± 0.01 g) and OFOB (0.58 ± 0.01 g), compared to those cultivated in BTO (0.52 ± 0.03 g) and BBO (0.50 ± 0.04 g) where the growth was lower (Figure 4A). During the upwelling season, the oysters showing significantly higher increments in soft tissues dry mass (ANOVA I, p< 0.05) were those cultivated in FNS (5.28 ± 0.03 g) and OFOB (5.19 ± 0.11 g); the oysters with the lowest growth were cultivated in BTBC (4.77 ± 0.22 g), BBO (4.67 ± 0.29 g) and BTO (4.66 ± 0.30 g) (Figure 4A).
Shell dry mass
During the non-upwelling season, the increment of shell dry mass was significantly greater (ANOVA I, p< 0.05), in oysters cultured in baskets BTBC (4.45 ± 0.19 g), OFOB (4.44 ± 0.31 g), FNS (4.19 ± 0.35 g) and BTO (3.94 ± 0.45 g), compared to those cultivated in BBO (3.66 ± 0.41 g) where the growth was lower (Figure 4B). At the end of the upwelling season, the oysters showing significantly higher increments in shell dry mass (ANOVA I, p< 0.05) were those cultivated in FNS (10.20 ± 0.11 g), OFOB (9.91 ± 0.16 g) and BTBC (9.47 ± 0.59 g); and the oysters with the lowest growth were cultivated in BTO (8.93 ± 0.70 g) and BBO (8.73 ± 0.25 g) (Figure 4B).
Condition index (CI)
In the non-upwelling season, the CI did not show significant differences (ANOVA I, p> 0.05), between any of the methods, FNS (14.53 ± 0.31), BTBC (14.25 ± 0.37), BBO (13.93 ± 0.72), BTO (13.62 ± 0.48) and OFOB (13.57 ± 0.29) (Figs. 4C). During the upwelling season, the oysters showing significantly higher increments in CI (ANOVA I, p< 0.05) were those cultivated in OFOB (37.63 ± 0.59) and FNS (37.48 ± 0.09); while the oysters with lower growth were cultivated in BTO (36.23 ± 0.47) BTBC (35.58 ± 0.22) and BBO (35.33 ± 0.35) (Figure 4C).
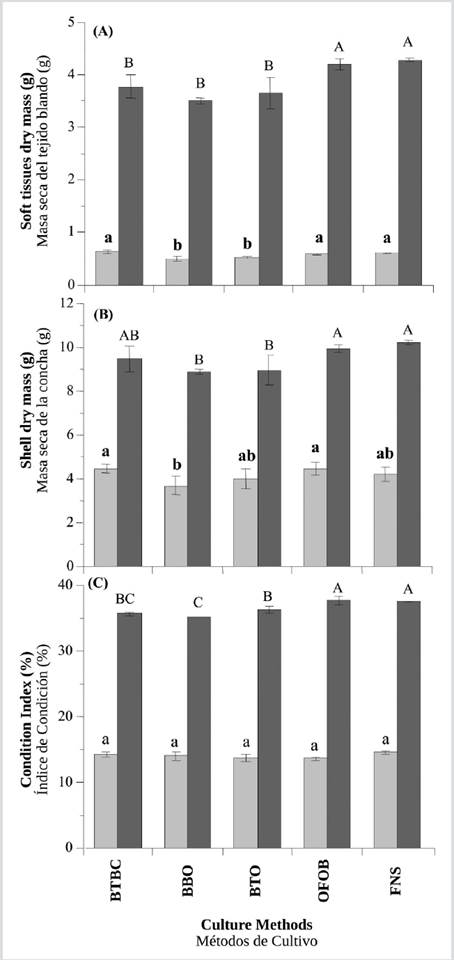
Figure 4 A) Dry mass of the soft tissues; B) shell and C) condition index of the juvenile pearl oyster P. colymbus, in each culture method and during the two seasons (light bars: no upwelling; dark bars: upwelling). Identical letters in superscript denote the lack of significant differences at p < 0.05. The vertical lines indicate the standard deviation.
Survival
No significant differences in survival rates were seen between culture methods (ANOVA I, p> 0.05), regardless of the variability in each treatment, especially during the non-upwelling season (Figure 5A). The baskets BTBC (52.78 ± 9.62 %) showed the highest survival during non-upwelling, while baskets BTO (88.90 ± 10.18 %) did it during the upwelling season.
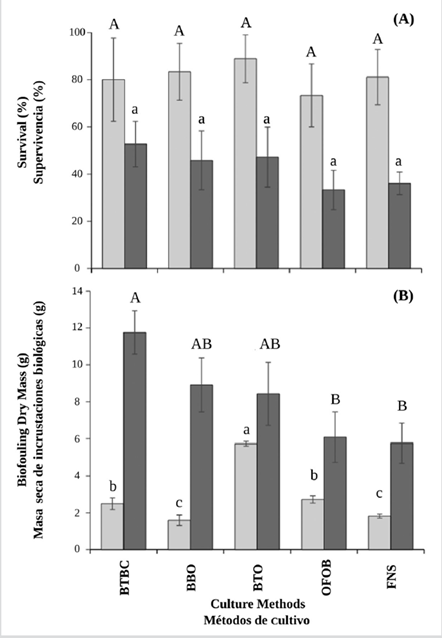
Figure 5 A) Survival and B) fouling dry mass fixed on the shell surface of the winged pearl oyster P. colymbus, in each culture method and during the two seasons (light bars: no upwelling; dark bars: upwelling). Identical superscript letters denote lack of significant differences at p < 0.05. The vertical lines indicate the standard deviation.
Fouling
During the non-upwelling season, significant differences in fouling biomass were seen between culture methods (ANOVA I, p< 0.05) with the higher fouling mass concentrations seen in the BTO treatment (5.73 ± 0.125 g) (Figure 5B). During the upwelling season, significantly higher dry fouling biomass (ANOVA I, p < 0.05) was observed in the oysters cultivated in the BTBC (11.76 ± 1.18 g), BBO (8.91 ± 1.47 g ) and BTO ( 8.43 ± 1.71 g) treatments.
Growth, fouling, condition index and survival inter seasons
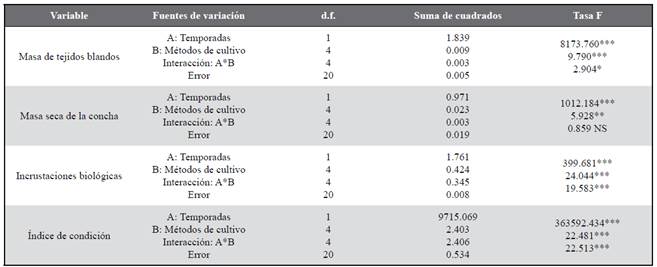
All biometric variables (shell height, survival, soft tissues dry mass, shell dry mass, fouling and condition index) showed significant differences between seasons and culture methods, as well as the interaction between both of these factors (ANOVA II, p< 0.05, Table 1, 2), except for survival, which didn’t show significant differences (p> 0.05) between culture methods and season and culture methods interaction. These results are due to the fact that all the biometric variables studied showed higher rates in the upwelling season than in the non-upwelling season, as well as in the interaction between both sources of variation (season and culture methods) which showed evident synergy.
Table 1 Results of the two-way ANOVA evaluating the effect of the season (upwelling and non-upwelling) and culture methods as factors on the parameters of growth and survival in the winged pearl oyster P. colymbus.
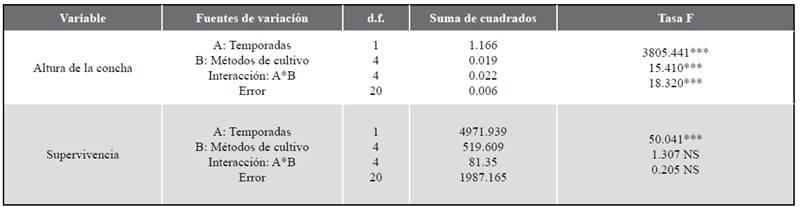
NS: no es significativo/ not significant; *P < 0,05; **P < 0,01; ***P < 0,001
Environmental variables
All physicochemical variables, except for temperature during the upwelling season (Figure 6E), showed marked fluctuations during the two culture seasons (Figure 6A, B, C, D). Except for the Chl-a data, the remaining physicochemical and biological parameters showed significant differences between each season (ANOVA I, p < 0.05).
Upwelling index: this index represented by the graphic record of the Ekman transport (Km3 s-1.1000) showed a higher value (2.4±0.24) during the upwelling season (March, April and May 2015) (Figure 6A) and dropped (0.3±0.31) during the non-upwelling season (July, August and September 2014), indicating strong water stratification. Dissolved oxygen: higher concentrations ranging from 6.1 mg L-1 to 9.6 mg L-1 occurred during the non-upwelling season, while minimum (3.5 mg L-1) values occurred during the upwelling season (Figure 6B). Chlorophyll a: the highest Chl-a values were seen during the upwelling season (Figure 6C), specifically in March and May (5.2 μg L-1 and 5.1 μg L-1, respectively). The lowest Chl-a concentrations were recorded during the non-upwelling season in August (0.3 g L-1). Seston: particulate organic matter (POM) showed variability in both seasons, with averages of 2.8±1.35 mg L-1 for the non-upwelling season, and 3.8 ± 1.01 mg L-1 for the upwelling season (Figure 6D). Temperature: this trait showed maximum values during the non-upwelling season in September (29.2 °C) and minimum values during the upwelling season in March (24.2 °C) (Figure 6E).
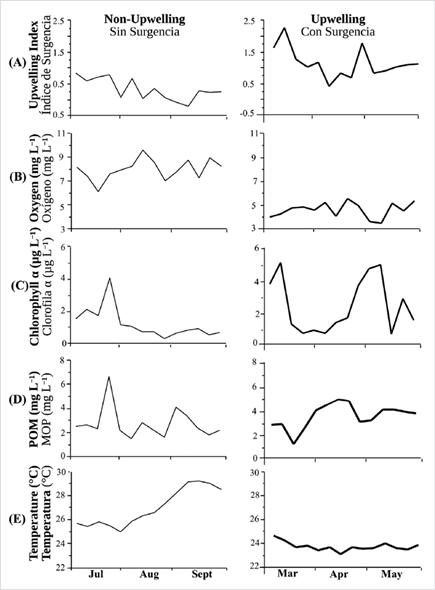
Figure 6 Weekly variation of physicochemical factors during the two hydrographic seasons in Turpialito site. A) upwelling index; B) dissolved oxygen; C) chlorophyll a; D) particle organic matter (POM) and E) temperature. Wide lines: Upwelling season (March through May); thin lines: Non-upwelling season (July through September).
Relationship between environmental variables, oyster growth, fouling, CI and mortality
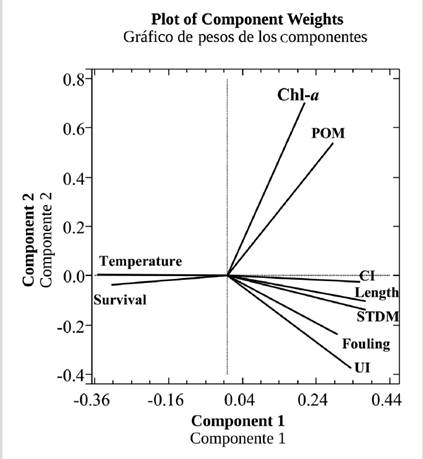
The Analysis of Principal Components showed that the first two components of the orthogonal representation (Figure 6) explained 89.7 % of the variation, indicating that the graphic representation is reliable. The variables that presented a greater positive correlation with the first component were, shell height, soft tissues dry biomass (STDM), condition index (CI), and upwelling index (UI), while survival and temperature were negatively correlated with these variables (Figure 7). The equation explaining the observed variance is as follows:
0.373101*shell height + 0.377168*STDM + 0.377216*CI + 0.302155*Fouling-0.352164*Survival + 0.207782*Chl- a-0.352679*Temperature + 0.285581*POM + 0,334394*UI
DISCUSSION
Several studies show that the cultivation method exerts an important effect on the growth and survival of different species of pearl oysters of the genus Pinctada (Southgate and Beer, 2000; Urban, 2000; Lodeiros et al., 2002) and Pteria (Saucedo and Monteforte, 1997; Fu et al., 2007; Millione and Southgate, 2011). These findings are consistent with our results, which show culture method had a significant effect on the growth of the shell, soft tissues mass and condition index of juvenile oysters. Thus, our results indicate that the selection of culture methods is an important parameter for optimizing the growth and survival of P. colymbus under suspended culture.
The performance (growth/survival) of the oysters grown in the outer section of the baskets (OFOB) is similar to that reported by Mengual et al. (2011) in Mochima bay (northeast Venezuela) with the same species. The authors attributed the success in the use of a tubular culture method (unconfined) to a lower predation rate by snails and crabs. However, the disadvantage of this tubular method is the loss of oysters by detachment of the byssus, probably due to the strong waves caused by the Trade winds present during the upwelling season. The effect of the wave action in the growth and survival in the culture of scallops Euvola ziczac and Nodipecten nodosus was demonstrated by Freites et al. (1999). A similar case was observed in the fishing mesh strings (FNS), where the presence of predators (snails, crabs) was not reported. Still, survival was < 60 %, and in this case, mortality could not be clearly attributed to the predation or detachment of individuals.
Future studies aimed to improve culture techniques through harnessing the settlement capacity of the oysters with the strength of their byssus are recommended. Compared to any other method, FMS was the only one not favoring the aggregation of oysters, likely because of a lower intraspecific competition, which in turn could be related to the higher growth values observed here. Consistently, the black-lip pearl oyster Pinctada margaritifera also showed higher growth rates in pocket nets where individuals are confined within a single compartment (Friedman and Southgate, 1999a, 1999b; Southgate and Beer, 2000).
Our results show a marked variation in the growth and survival of P. colymbus juveniles, which were influenced by environmental factors recorded in the Turpialito area during the two seasons. These results contrast with those reported by Lodeiros et al. (1999b), showing that P. colymbus was only lightly affected by marked changes in environmental conditions in the same locality. Freites et al. (2017) discussed how the study of Lodeiros et al. (1999b) which started in December 1993, and covered the complete upwelling and high productivity seasons (January to July), showed P. colymbus displaying rapid increases in shell and somatic tissue growth, reaching maximum shell heights of 60 mm during the non-upwelling season (August-October), which contrasts with our results, where clear differences were established in the growth of P. colymbus obtained in both seasons (upwelling and non-upwelling), indicating that environmental variability exerts an important influence on the growth pattern of the winged pearl oyster P. colymbus during both seasons.
During the months of major coastal upwelling, the growths in shell height and soft tissues dry mass surged to 246 % and 862 %, respectively, compared to what was registered during the months of coastal upwelling recession. Such increases are closely related to the high availability of phytoplankton biomass that responds to the greater magnitude of the trade winds causing the coastal upwelling, the mixing of the water column, and the decrease in temperature with its corresponding increase in nutrient concentrations (ammonium, nitrite, phosphate and silicate) and overall phytoplankton production (Calvo-Trujillo et al., 2015). In this study, overall contribution of environmental factors that explain a high percentage of the variability in the growth of corporal compartments during the two seasons was high (> 85 %) and maintained a negative relationship with water temperature and a positive relationship with Chl-a and POM traits.
In this experiment, the organic fraction of seston (POM) and Chl-a values (which were > 1 μg L-1) modulated most of the growth parameters of the oysters in both seasons (this considered as non-limiting for the growth of the organism by Saxby (2002). Lodeiros and Himmelman (2000) highlighted the effect that the energy contained in phytoplankton has on the growth of the scallop Euvola ziczac under suspended culture conditions in the Cariaco gulf. Venezuela. The authors analyzed the spatial and temporal variabilities in the communities of primary producers (dominated by nanoflagellates) during the non-upwelling season (October through December), and compared that to what was observed in the upwelling season, where diatoms dominate the phytoplankton community. Consequently, studying the role that the energy quality of POM plays in the diet of the bivalves is highly recommended to provide information that can be compared with the existing knowledge on the feeding mechanisms of the species.
It was also clear that the oysters experienced low mortality when primary productivity (non-upwelling season) fell, a circumstance that is confirmed by the result of the PCA analysis, where a direct relationship between survival and temperature is observed. In our study, low survival rates were observed, and were likely related to the presence of predators, such as the gastropod snails Linatella caudata and Cymatium pileare and the Decapod crab Pilumnus caribaeus. This pattern was more evident in the closed baskets where the oysters remained confined (qualitative data not shown) than in the devices where the oysters where fixed outside. Consistently, Malavé et al. (2012) reported an increment in the recruitment rates of predator snails of the family Cymatiidae during the upwelling seasons in the Gulf of Cariaco,Venezuela. This probably occurs because predators take advantage of the protection provided by the enclosure when they colonize it, in order to continue their predatory actions, which cannot happen with oysters fixed outside the enclosure.
Moreover, once the predator kills and unenclosed oyster, the oyster falls to the seabed, and it is likely that this causes the predator to fall down with it, taking it away from the rest of the bivalves, and stopping the negative effect on the rest of the oysters. Similarly, Freites et al. (2000) described how the predator crab Calappa cinerea takes advantage of the refuge provided by the bottom culture enclosures of the scallop Euvola ziczac, causing 27 % higher mortality than that occurred with other cultivation methods where the crab is exposed to certain predators. These results agree with numerous investigations highlighting the harmful effect of certain predators contained within bivalve culturing devices, mostly fish of the families Balistidae and Monacanthidae (Alagarswami, 1987, Freites et al., 2017). and crabs and snails (Monteforte and García-Gasca, 1994; Freites et al., 2000; Lodeiros et al., 2002; Villarroel et al., 2004; Semidey et al., 2010).
Given these results, two relatively effective strategies to counteract the presence of predators may be considered: 1) the location of the culture site for culturing the target bivalve species that withstand desiccation periods in the intertidal zone. such as oysters and mussels (Buitrago et al., 2009; Núñez et al., 2010), and 2) the introduction of certain invertebrate organisms such as sea urchins within culturing devices as biocontrol mechanisms for basket fouling (Lodeiros and García, 2004; Sonnenholzner et al., 2017) and also for reducing the incidence of species identified as predators (Malavé et al., 2012).
Considering that a continuous recruitment of spat has been observed throughout the year. with peaks in August and December (Márquez et al., 2000), we suggest deploying the spat collectors in November to harvest the juveniles in January. Similarly, it is recommended to hang-out the culture devices in the subsequent months to take advantage of the high primary productivity in the area, stimulated by coastal upwelling (January-August). This is a strategy to accelerate the time needed (within the first six-eight months) to harvest the oysters with size suitable for human consumption (Lodeiros et al., 1999b) or for half-pearl (mabé) and round pearl production.
The viability of the culture of P. colymbus is feasible using low-cost elements, particularly those that reduce the risks of incidence of predators (unconfined organisms), taking advantage of the natural and strong byssus production of this species. Given these results, we recommend the fishing net string (FNS) as the ideal device for the hanging culture of P. colymbus juveniles until final grow-out to commercial size (implantation, grafting). This in accordance with Southgate (2008) who concludes that cultivation methods without the enclosure of a cage or net increase food availability and greater circulation of water, favoring, in turn, the oyster’s performance.
CONCLUSIONS
Our results showed higher growth rates in the oysters cultivated during the upwelling periods, however, the survival rates were lower in this season, as a consequence of higher presence of predators. With this in mind, it is recommended to keep the culture under constant revisions during this period to control the incidence of predators, which would increase the performance (growth / survival) of the oysters in culture.
The culture method that showed the greatest growth of the winged pearl oysters P. colymbus was the unconfined method “fishing net strings” (FNS), followed by the “oysters fixed over baskets” (OFOB) method.
Interactions between temperature (inverse relationship), Chlorophyll a and Particle Organic matter (POM) (direct relationship). were able to explain a high percentage the growth variances in the individuals. showing the modulating effect of the environment has on the yield of the winged pearl oysters P. colymbus.











 text in
text in