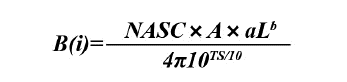INTRODUCTION
Anchovy (Engraulis ringens) is an endemic and dominant species of the Northern Humboldt Current System (SNCH), with an estimated biomass between 1.7 and 12.7 million t in the last 20 years, which sustains the largest sustainable fishery in the world (Vildoso et al., 1999; Castillo et al., 2020a). On the other hand, the red squat lobster (Pleuroncodes monodon) is a decapod crustacean that inhabits the neritic pelagic ecosystem and is considered to be a potential fishing resource due to its abundance levels, which vary between 1.5 and 3.5 million t during the summer and spring (Chirichigno, 1970; Gutiérrez et al., 2008; Castillo et al., 2020a, 2020b).
The Paracas National Reserve (RNP), located in the southern Peruvian provinces of Pisco and Ica, was created on September 25, 1975, with an extension of 335 000 ha, out of which 35 % corresponds to the mainland and 65 % to marine waters (Decreto Supremo N° 1281- 75-AG; Jacinto, 2014; Guezel and Wickel, 2015). The RNP marine ecosystem has a high productivity, given the occurrence of upwelling processes and an intense oxygen minimum zone (OMZ), which is typical of the SNCH (Strub et al., 1998; Messié and Chavez, 2015). This sustains the great marine biodiversity and biomass of the Peruvian-Chilean province (temperate-cold) consisting of resident and migrant birds, as well as cetaceans, dolphins, sea lions, sea turtles, fish, crustaceans, and invertebrates, where the following are highlighted due to their higher occurrence frequency: the anchovy (Engraulis ringens), the Peruvian morwong (Cheilodaytilus variegatus), the purple scallop (Argopecten purpuratus), the ribbed mussel (Aulacomya atra), the swollen frog shell (Crossata ventricosa), the Thaisella chocolata sea snail, the fine clam (Transennella pannosa), the jaiba (Cancer porteri), the jaiba peluda (Romaleon setosus), the Lessonia trabeculate kelp, the lapa negra (Fissurella latimarginata), and the red sea urchin (Loxechinus albus) (Vélez, 1975; Vildoso et al., 1999; Chirichigno and Cornejo, 2001; Paz et al., 2002; Reyes et al., 2007; Guezel and Wickel. 2015; Flores et al., 2019; Goya et al., 2020). However, there are few studies on the population density of marine species in marine protected areas (MPA). Said studies are important management tools for the preservation and recovery of marine ecosystems and their services, as well as for mitigation mechanisms regarding the effects of climate change (FAO, 2012; Spalding et al., 2013; Cutipa-Luque et al., 2020).
The objective of this study is to determine the distribution and biomass of anchovy and red squat lobster, as well as their relationship with the oceanographic characteristics of the coastal-marine area of the RNP.
MATERIALS AND METHODS
Study area
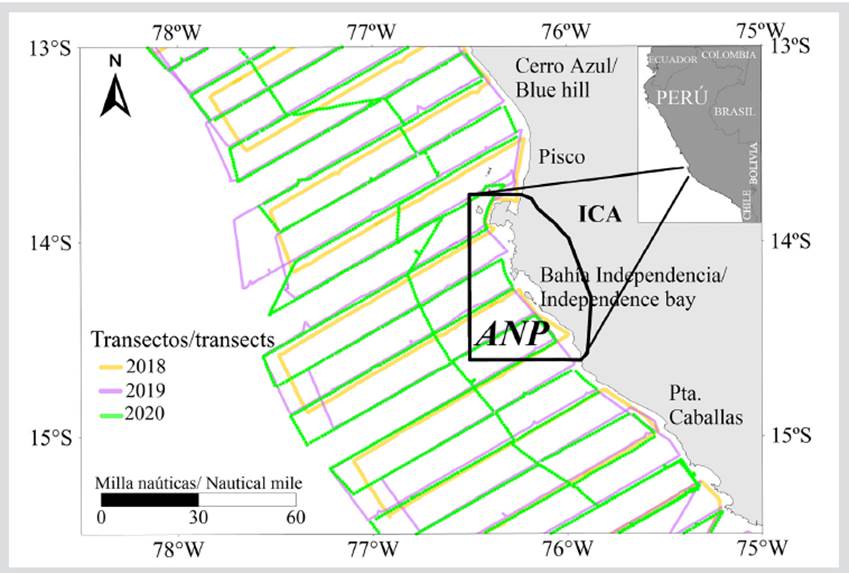
The geographic scope of the study encompassed the marine ecosystem corresponding to the direct and indirect influence areas of the RNP’s MPA, in the southern region of the Peruvian Southwestern Pacific Humboldt Current, between Cerro Azul (13°01´S, 76°20´W) and San Juan (15°20´S, 75°19´W) (Figure 1). The RNP itself was considered as a direct influence area and the adjacent areas between Cerro Azul and Punta Caballas as indirect influence areas. This implies a horizontal scope from 0,56 km (0.3 nautical miles (nmi)) up to 185.20 km (100 nmi) from the coast.
Acoustic, biological, and oceanographic data collection and analysis
Acoustic, biological, and oceanographic data were collected during the Cruises for Hydroacousitc Assessment of Pelagic Resources carried out by Sea Institute of Peru (Imarpe), with the participation of the José Olaya Balandra, Luis Flores Portugal, and Humboldt Scientific Research Vessels (SRV), during the months of summer (February-March) and spring (September-October) of 2018, 2019, and 2020. These cruises had the purpose of determining the distribution and biomass of the pelagic resources along the entirety of the Peruvian coast (Tumbes 03°30’ -Tacna 18°20’ S). For this work, the RNP, between 13°00’ (Cerro Azul) and 15°30’S (south of Punta Caballas), was used as a study area.
Acoustic data
The acoustic data (Nautical Area Scattering Coefficient, NASC (m2 mn2)) (MacLennan et al., 2002) for anchovy and red squat lobster were collected during the 1802-04, 1809-11, 1902-03, 1909-11, and 2002-03 cruises (the first two digits of each code correspond to the two last digits of the year, followed by the digits corresponding to the months in which the cruise was carried out).
The acoustic sampling design was performed using the systematic method (Simmonds and Maclennan, 2005), which consisted of transects or profiles perpendicular to the coastline and parallel to each other, with a separation of 18.25 km (10 nmi). The length or distance of the transects was from 0,56 to 185.25 km (0.3-100 nmi) from the coast. The cruise navigation speed was 18.5 km/h (10 knots), and the basic acoustic sampling unit was 1.85 (1 nmi). The total nautical miles evaluated were 4453 (8246.96 km) in 2018, 4411 (8169.17 km) in 2019, and 2320 (4296.64 km) in 2020. Acoustic measurements of the target species were conducted with Simrad Kongsberg EK80 (18, 38, 70, 120, and 200 kHz) and EK60 (38 and 120 kHz) scientific multifrequency echo sounders. Calibrations of the echo sounders were performed before the cruises started according to the standard methodology (Simmonds and MacLennan, 2005; Demer et al., 2015), using copper (Cu) and tungsten carbide (WC) calibration spheres. The calibration results were acceptable (a mean squared error lower than 0.4 dB), according to the manufacturer’s indicators and recommendations from the manufacturer and the International Council for the Exploration of the Seas’ (ICES) expert group regarding fisheries acoustics.
Biological data
Data were collected on the size structure (cm), and the a and b parameters of the length-to-weight ratio (cm-g) of anchovy and red squat lobster were estimated. As a sampler, Granton-type pelagic midwater trawl nets were used, with vertical openings of 12 m for SRV José Olaya Balandra (with NOTUS wireless fishing sensors), 8 m for SRV Luis Flores Portugal, and 14 m for SRV Humboldt.
Oceanographic data
To establish the environmental parameters of preference for anchovy and red squat lobster, superficial and vertical CTD-O Seabird data were used (Current-Temperature-Depth-Dissolved Oxygen). Oceanographic stations were set up on the transects per navigation hour, which allowed collecting physical parameters from the surface of the sea (temperature, salinity, and oxygen). The measurements of the temperature (°C) in the water column were taken at the six stations (5, 10, 20, 50, 70, and 100 nmi from the coast) regarding the oceanographic profile in front of Pisco (13° 42’S) with a CTD-O and a surface thermometer. The surface salinity samples from the sea were analyzed on board with a Portasal Guideline Model 8410A by means of the induction method. Vertical sampling of physical parameters (temperature, salinity, and dissolved oxygen) was performed by deploying the CTD-O in the oceanographic profile of Pisco, from the sea surface down to a depth of 500 m.
Acoustic data processing and biomass estimation
Acoustic discrimination was performed with the EchoView software, version 9 (Myriax, Hobart; www.echoview.com). Identification of the echo-traces from the detected species (anchovy and red squat lobster) was carried out according to the following aspects: the composition of what was captured by the fishing set, the type of echo-trace typical of each species, and multifrequency analysis (acoustic response graphs with the frequency of anchovy shoals and the red squat lobster aggregations) (Castillo et al., 2009; La Cruz et al., 2017).
The postprocessing of information and the handling of acoustic data were performed in accordance with the technical protocol for the acoustic evaluation of distribution and pelagic resource abundance areas in the Peruvian sea (Castillo et al., 2009).
The determination of the density distribution areas and the concentrations of anchovy and red squat lobster was carried out by interpolation of the NASC data, which is a measurement of the backscattered acoustic energy from a target (evaluated species) in the water column (echo integration). These are obtained by exporting the shoals and aggregations detected on the EchoView software.
Moreover, for the vertical distribution of the species, the acoustic backscattering intensity was used (Sv, dB re 1 m-1), which indicates the density of an aggregation or shoal and is directly proportional to the biomass as well as expressed in decibels (Simmonds and MacLennan, 2005). The abundance or biomass estimates were obtained using the stratification method in Iso-para-littoral areas (AIP) of 18.52 × 55.56 km (10 × 30 nmi), whose formulation is described in Castillo et al. (2009):
Where B(i) refers to the biomass in AIP(n), A is the area of each AIP expressed in nmi2, parameters a and b are constants of the length-to-weight ratio (cm-g), and L refers to the mean size. TS refers to the target strength equation for each species and specific size range (Castillo et al., 2009).
Analysis of the resource-environment relationship
The relationship of the presence of anchovy and red squat lobster with the oceanographic variables was determined by means of the Generalized Additive Models (GAM) of the Gaussian family and an identity link function. An exploratory analysis was previously conducted with the purpose of identifying the spatial autocorrelations and the co-linearity of the explicated variables using the Spearman method (parcels) (Zuur, 2012). The GAMs are applied to a set of acoustic and physical-oceanographic data to examine the effects of the environmental variables on the distributions and/or densities of anchovy and red squat lobster.
The dependent variable corresponded to the acoustic samples of 1 nmi with the NASC values of anchovy and red squat lobster, and the independent variables were temperature, salinity, and dissolved oxygen on the sea surface. The analyses were conducted for each research cruise. The NASC values of the acoustic samples were logarithmically transformed given the wide range of values.
The GAM analyses were performed with the mgcv (version 1,8-34) package of the R software (R Development Core Team, 2020). The explained deviation (analogous to the variance in a linear regression) was adjusted according to r2 scores and generalized cross-validation (GCV). The functional forms for the selected co-variables were outlined. When the slopes of the functional forms are positive, the co-variables are positively related to the dependent variables or vice versa (Wood, 2006, 2017).
RESULTS
Surface oceanographic conditions
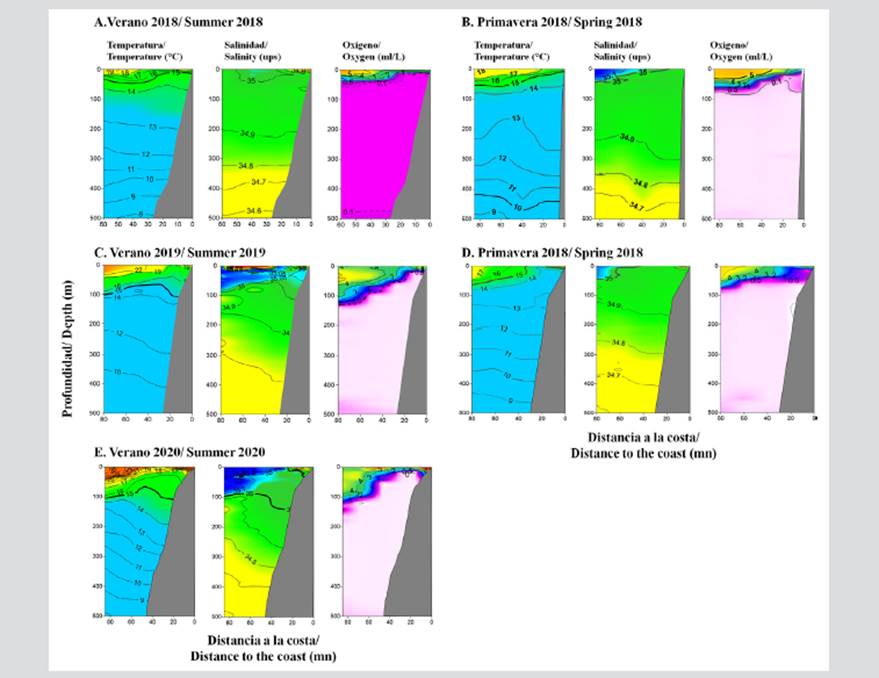
The surface oceanographic conditions of the profile in front of Pisco in the summer of 2018 showed a predominance of cold-water conditions, with temperatures around 19 °C, with values higher than 21 °C found outside the 92.6 km (50 mni) area. The surface salinity was found to be between 34.8 and 35.1 PSU, and the surface dissolved oxygen values were higher than 2 ml/l. In the summer of 2019, the conditions were warmer, with temperatures between entre 21 and 23 °C, salinities 34.8 and 35.3 PSU, and oxygen values higher than 0.5 ml/l. In 2020, the temperature fluctuated between 19 and 24 °C, with salinities of 34.8 PSU very close to the coast and up to 35.4 PSU away from it, as well as with oxygen values between 5 and 7 ml/l.
Oceanographic conditions in the water column
The oceanographic conditions of the profile in front of Pisco corresponded to a coastal upwelling zone of Cold Coastal Waters (CCW) in spring; and of mixture water formed by CCW and Subtropical Surface Waters (SSW) in summer. The oceanographic profile of Pisco was characterized by a 15 °C increase in the isotherm mainly at a depth of 50 m during 2018 and 2018, as well as below 80 m in the summers of 2019 and 2020.
The salinity distribution reported the presence of CCW and SSW mixture waters with an isohaline of 34.9 PSU located between 100 and 250 m deep. The upper edge of the OMZ (values of 0.5 ml/l) was recorded, which was very superficial over the continental platform, mainly during the summer and spring of 2018, and deep, between 50 and 120 m, during 2019 and 2020 (Figure 2).
Horizontal distribution of anchovy and red squat lobster
The distribution of anchovy was mainly coastal, with two characteristics: (i) a discontinuous distribution that was also very restricted to the coast within 37.04 km (20 nmi) for 2019; and (ii) continuous distribution for the summers and springs of 2018 and 2020 (Figure 3a). It is worth noting that, in 2018, the distribution of anchovy reached the 133.34 km mark (72 nmi). Along the coast, significant high-concentration nuclei were observed, which were mainly located north of the RNP. However, the RNP area concentrated significant densities of anchovy, associated with a deepening of the OMZ in 2020.
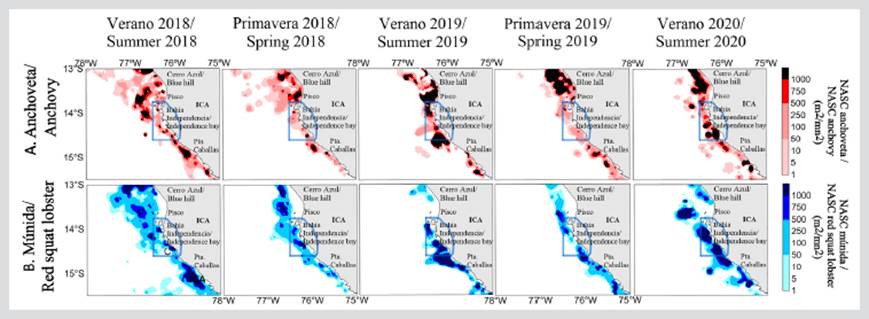
Figure 3 Distribution and spatial concentration of A) anchovy and B) red squat lobster during 2018 - 2020 in front of the Marine Protected Area of the Paracas National Reserve (RNP), Pisco (Peru) (RNP, continuous blue line polygon).
The red squat lobster was recorded along the coast; it generally had a continuous distribution during 2018 and a discontinuous one during 2019 and 2020. The nuclei with greater abundance were located in the central and southern areas of the RNP. It is worth indicating that, during the summer of 2018, the red squat lobster showed its highest amplitude, being recorded as far as 138.9 km (75 nmi) from the coast (Figure 3b), due to a superficial OMZ.
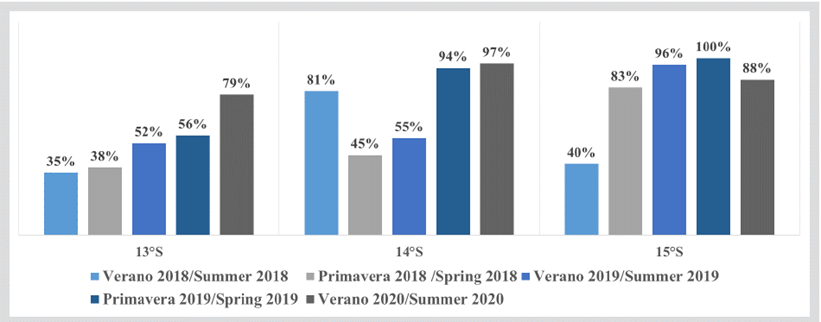
In some areas north and south of the RNP such as Cañete, Punta Azua, and San Nicolás, acoustic recordings of high-abundance anchovy shoals were observed, forming plume- and spot-type shoals with a size range between 7 and 16 cm of total length (LT), sharing the habitat with aggregations or swarms of red squat lobster (Figure 4). The structure by size of anchovy captured in the evaluated area per latitude degree showed the dominant presence of juvenile individuals (LT ratios lower than 12 cm). The highest juvenile percentages were recorded at 15°S, followed by 14°S and 13°S (Figure 5).
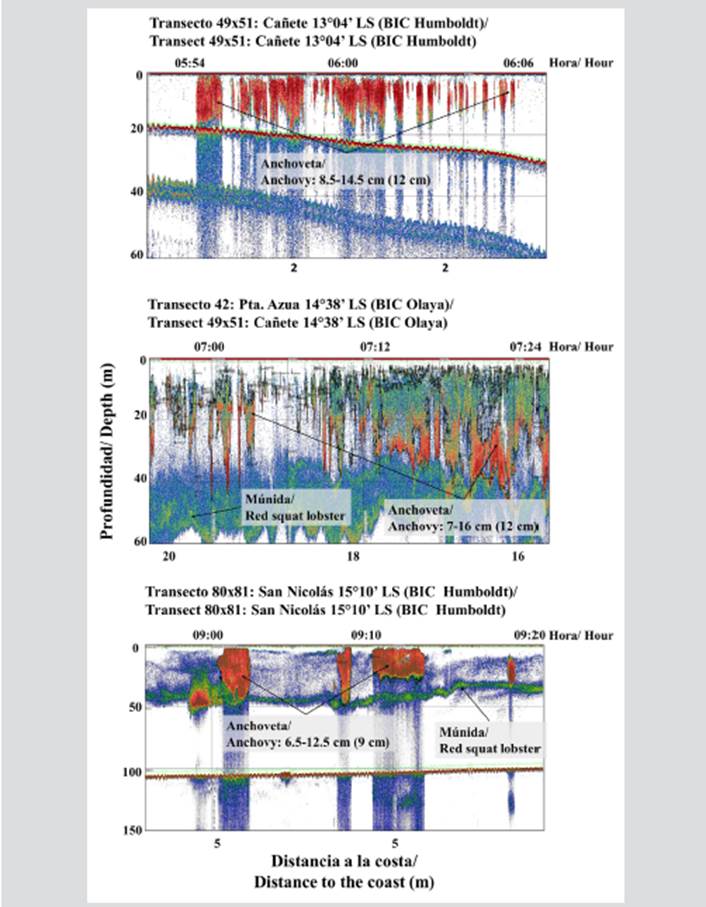
Figure 4 Typical records of anchovy and red squat lobster in front of the Paracas National Reserve (RNP). Each image shows the range of anchovy sizes and the mode in parentheses.
Vertical distribution of anchovy and red squat lobster
Vertically, anchovy shoals were found at a mean depth of 9.84 m and a depth range from the surface of up to 86.30 m. The most superficial shoals were recorded in front of Cerro Azul, and the deepest ones south of Bahía Independencia, thus evidencing, in front of the RNP, a transition area between the most superficial and the deepest shoals. The deepening of anchovy shoals in the southern zone was due to the oxygenation of the deepest layers of the water column (> 30 m). The greatest abundances of anchovy (> -45 dB) were found mainly in the first 20 m of the water column (Figure 6a). With regard to the time of day, the densest shoals were detected during daytime hours.
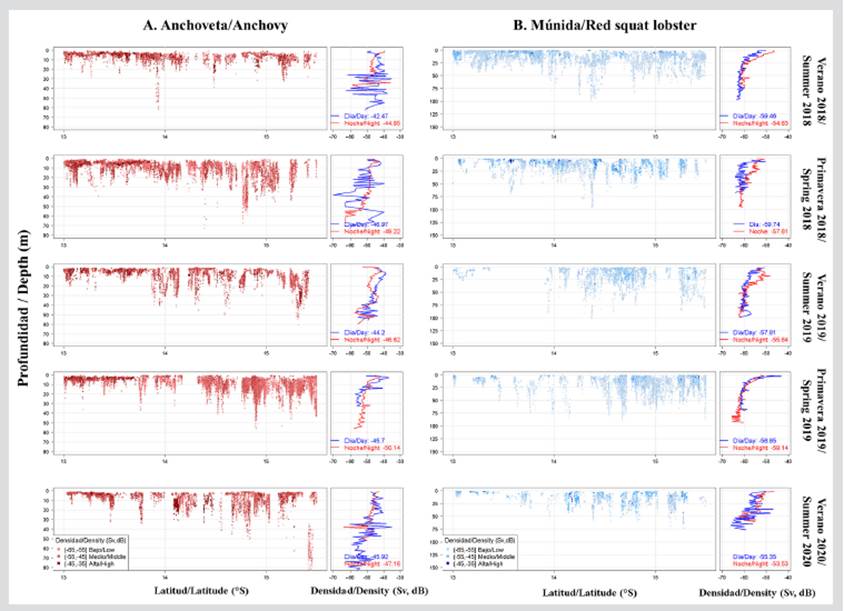
Figure 6 Vertical distribution of a) anchovy and b) red squat lobster in front of the Paracas National Reserve, Pisco (Perú) during 2018-2020.
Vertically, the red squat lobster was recorded between 1.20 and 100.89 m deep, with a mean value of 18.23 m. The deepest records were located mainly in front of Bahía Independencia, whereas the most superficial aggregations were recorded in front of Cerro Azul. The greatest abundances (> -55 dB) were found mainly in the first 25 m of the water column and during nighttime hours (Figure 6b).
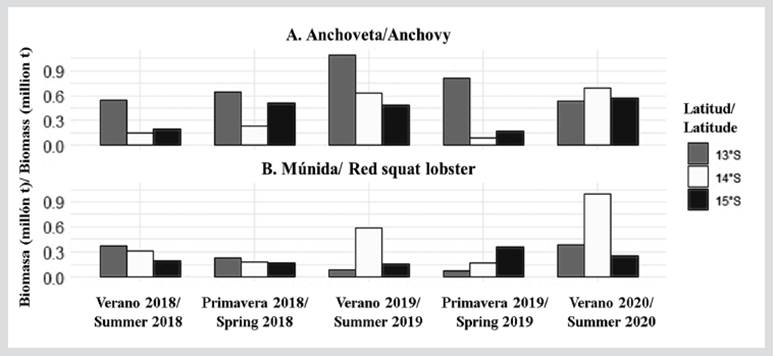
Acoustic biomass of anchovy and red squat lobster
In the evaluated study area, the greatest acoustic biomasses of anchovy were estimated at 13°S with 1 092 775.53 t (summer of 2019) and 810 398.57 t (spring of 2019). In front of 14°S and 15°S, the anchovy biomasses showed similar values, with the maximum values being reported during the summers of 2019 and 2020 (Figure 7). The acoustic biomass of red squat lobster has been variable in front of the RNP, showing a tendency to increase between 2018 and 2020. In the summer of 2020, the greatest biomass was recorded at 14°S with 995 972 t (Figure 7).
Relationship of anchovy and red squat lobster with superficial oceanographic variables
Anchovy was recorded mainly when the sea surface temperature (SST) recorded values between 16 and 18 °C during 2018, as well as with values higher than 18 °C during 2019 and 2020; when the sea surface salinity (SSS) was between 34.9 and 35.05 PSU; and when the sea surface oxygen (SSO) was mainly 4.0-5.4 and 6.3-7.7 ml/l (Figure 8).
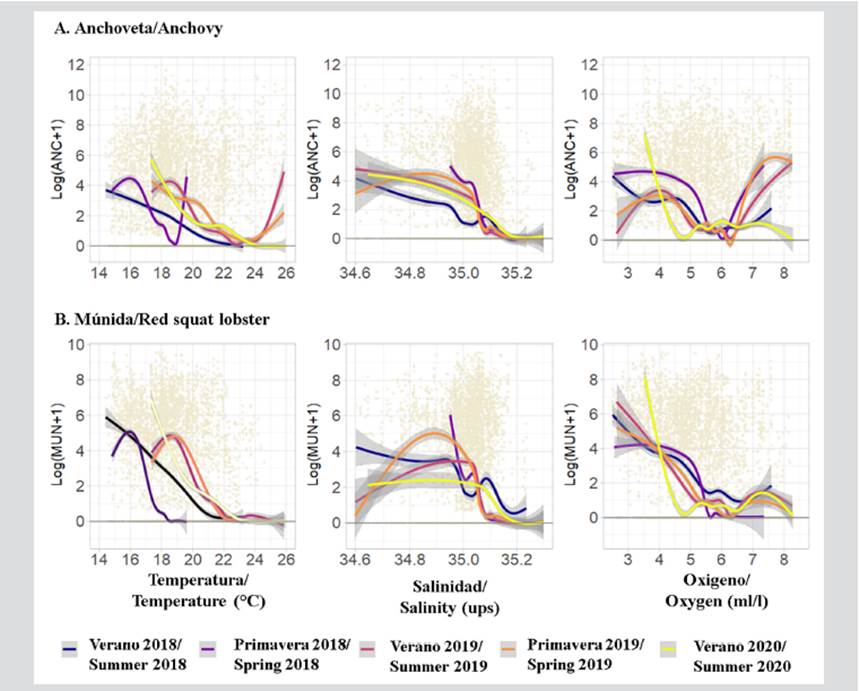
Figure 8 Results of the Generalized Additive Models (GAM) of a) anchovy and b) red squat lobster with respect to temperature, salinity, and surface oxygen of the sea in front of the Paracas National Reserve (RNP), Pisco (Perú), during 2018-2020
Regarding the red squat lobster, it was mainly detected when the SST showed values between 14 and 17 °C during 2018 and between 18 and 19 °C during 2019 in 2020, as well as an SSS between 35.05-35.1 ups and an SSO mainly between 3.0 and 4.0 ml/l (Figure 8).
DISCUSSION
The oceanographic conditions in the RNP were variable during spring and summer due to the influence of intense coastal upwelling processes and the presence of an OMZ, which determined the spatial occupation of anchovy and red squat lobster in the pelagic habitat. In the Peruvian marine ecosystem, a high oceanographic variability is normal (Espino, 2013; Flores et al., 2013). In the RNP’s area of influence, in front of Pisco (13° 42.5´S) and San Juan (15° 20.0´S), the Peruvian sea’s most intense upwelling zones have been reported, mainly between June and August and from December to February (Zuta and Guillen, 1970; Walsh, 1975; Calienes, 2014; Palash, 2019). In these upwelling areas, cold water comes very close to the coast and advects offshore in a plume between 50 and 75 m deep. Palash (2019) recorded, between 11° and 16°S, an oxicline gradient that is shallow near the coast and deep in intermediate waters offshore, with records of high zooplankton abundance mainly in the coastal zone. This oceanographic process occurs in all upwelling areas, and this environmental heterogeneity may be key in the development of the short food chain in the area of Pisco and San Juan: diatoms-copepods-engraulidae (Walsh and Dugdale, 1972; Ayon et al., 2008; Calienes, 2014) - as well as of the production of fishing biomass (Bakun and Weeks, 2008).
Hydroacoustic tests allowed mapping the habitats of anchovy and red squat lobster populations in the pelagic ecosystem of the RNP. Between 2018 and 2020, high biomasses were estimated for anchovy (1.7 - 3.3 × 106 t) and red squat lobster (1.43 - 1.60 × 106 t). The anchovy shoals were dominated by juvenile specimens distributed in the coastal strip from 9 to 86 m deep (20 m on average) with high concentrations during the day, whereas red squat lobster swarms recorded a deeper distribution, reaching 100 m (18 m on average) and with records of high concentrations during nighttime.
The results are consistent with the distribution and abundance of the North-Center stock (02°-16°S) of anchovy within the Temperate Southeastern Pacific Province, influenced by the Humboldt Current (Bouchon et al., 2010; Castillo et al., 2019; Oliveros-Ramos et al., 2020), with biomasses fluctuating between 6 and 11 × 106 t and captures by the industrial seine fleet (operating beyond 9.26 km (5 nmi) from the coast) between 2 and 4 × 106 t in recent years (Castillo et al., 2019; Oliveros-Ramos et al., 2020). However, the study area corresponds to the southern limit of the North-Center Stock of anchovy, characterized by a higher incidence of shoals with juvenile individuals (Imarpe, 2020); whereas the red squat lobster presents a continuous geographical distribution with higher abundance nuclei in the Peruvian central and southern coasts (Castillo et al., 2020b).
The spatial distribution of neritic pelagic resources such as anchovy and red squat lobster is associated with CCW and SSW masses and coastal fronts, which follow the cold water plume in upwelling areas for zooplankton foraging (Swartzman et al., 2008; Calienes, 2014). Red squat lobster is considered to be a biological indicator of the presence of cold environmental conditions and distributed in the main upwelling foci (Aliaga et al., 2004; Gutiérrez, 2016; Santibáñez, 2017; Castillo et al., 2020b).
Acoustic records indicated that there is a spatial overlap between anchovy and red squat lobster during the nychthemeral cycle, regulated by the presence of the OMZ. During the day and night, anchovy is found forming dense shoals (plumes and capes) within the first meters of the water column, limited to oxygenated conditions; whereas the red squat lobster, during the day, performs vertical migrations, forming dense swarms in the anoxic zone of the OMZ, and, during nighttime, this pelagic crustacean ascends to the surface forming little substructures (Gutiérrez et al., 2008; Kiko et al., 2015; Gutiérrez and Gerlotto, 2016; Sanfuentes, 2017; Castillo et al., 2020b).
In the Peruvian marine ecosystem, the presence of a superficial and intense OMZ (< 0,5 ml/l, ~50-500 m) regulates the vertical distribution of neritic and oceanic pelagic species for the development of bioecological processes (Cornejo, 2011; Lachkar and Gruber, 2012; Flores et al., 2013; Calienes, 2014; Espinoza-Morriberon et al., 2017; Palash, 2019; Castillo et al., 2020b).
In this way, the MPA of the RNP has a beneficial effect in the protection of the structure, function, and integrity of marine ecosystems and fishing productivity (Kellner et al., 2007;Boerder et al., 2017; Elliot et al., 2017; Laffoley et al., 2019, Cutipa-Luque et al., 2020), thus allowing to improve the reproductive, nurturing, and nourishment capacity of local and regional stocks of anchovy and red squat lobster, strengthening their recruitment through the effect of the overflow of adults and juveniles towards areas adjacent to the MPA. In the same way, the RNP and the MPAs of the Peruvian coast may offer an alternative to ensure that the extraction levels do not exceed the sustainability limits or that the population size does not decrease beyond the minimum necessary level, thus ensuring the viability and sustainability of the anchovy stock, as well as of other pelagic resources in the SNCH, in the medium and long term.
It is necessary to develop an integrative research program in the RNP’s MPA and its neighboring areas, considering the monitoring and scientific evaluation of population parameters for hydrobiological resources, marine biodiversity census, among others, with the purpose of promoting the sustainable utilization and preservation of the biological diversity of marine-coastal ecosystems by means of ecological and economic zoning. This includes the value proposal involving the promotion of other MPAs in the Peruvian marine ecosystem, given that it has a low coverage of protected marine territory at the Latin American and Caribbean levels.
CONCLUSIONS
The spatial occupation of the pelagic habitat of anchovy and red squat lobster in the RNP was influenced by the dynamics of upwellings, water masses, and a superficial and intense OMZ. A spatial overlap of high anchovy and red squat lobster biomasses was recorded in the superficial layer during nighttime, albeit with the presence of dense red squat lobster swarms in anoxic intermediate waters of the OMZ during the day. The RNP’s MPA serves as a protection zone for the structure and functioning of the neritic pelagic ecosystem, and it strengthens the fishing production of the anchovy stock in the SNCH.











 text in
text in