INTRODUCTION
The Eastern Pacific is one of the most important feeding and nesting areas for four sea turtle species (Groombridge and Luxmoore, 1989; Seminoff et al., 2012). Its complex oceanography and biogeography, characterized by its narrow continental shelf, extensive deep-water areas, and the El Niño-Southern Oscillation (ENSO), constitutes highly dynamic habitats for sea turtles, which populations have adapted with plasticity in their biology, behavior, morphology, and demographics in comparison with their conspecifics in other regions of the world (Seminoff et al., 2012).
Sea turtles are highly migratory, with a complex life history that includes the migration of adults from feeding areas to distant reproduction zones and ontogenetic changes, which condition the distribution of juveniles in a variety of marine habitats (Jensen et al., 2013). In this complex life cycle, the nesting habitat takes up less than 1 % of the average lifespan (Bjorndal, 1999a), but it is there where most studies are conducted due to easier logistics and lower costs. Conducting studies in feeding areas is difficult and expensive (Rincón-Díaz and Rodríguez-Zárate, 2004), but in order to understand the life story of sea turtles and effectively manage their preservation, it is essential to make efforts in feeding, transit, and residence areas (Bjorndal, 1999b; Broderick et al., 2007; Gaos et al., 2012; Barrientos-Muñoz et al., 2015a. 2015b; Páez et al., 2015; Ramírez-Gallego et al., 2015). Therefore, continuous, systematic, and standardized monitoring is urgent to know the actual state of sea turtles, which also allow us to understand their contribution of sea turtles in this area for populations in the Eastern Pacific (Barrientos-Muñoz et al., 2015a, 2015b, 2020; Páez et al., 2015; Ramírez-Gallego et al., 2015).
Four out of the five marine turtle species identified in Colombia are present in the Pacific: the leatherback sea turtle (Dermochelys coriacea), the green Pacific turtle (Chelonia mydas, also known in the region as “black turtle”), the hawksbill sea turtle (Eretmochelys imbricata), and the olive ridley sea turtle (Lepidochelys olivacea). All of these are classified as species at some risk of extinction by the International Union for the Conservation of Nature (IUCN) (Seminoff, 2004; Abreu-Grobois and Plotkin, 2008; Mortimer and Donelly, 2008; Wallace et al., 2013) and, at the national level, by the Colombian Red Book of Reptiles (Libro rojo de reptiles de Colombia, 2015) (Barrientos-Muñoz et al., 2015a, 2015b; Páez et al., 2015; Ramírez-Gallego et al., 2015). Moreover, all of the species are in Appendix I of the CITES, and they are protected by different national and international regulations (see marine turtle sheets in Morales-Betancourt et al., 2015).
According to Barreto-Sánchez (2011), in the Colombian Pacific, 40 % of the research on sea turtles is conducted on nesting beaches and only 12 % in feeding areas. In the department of Chocó, studies have been carried out mainly on the nesting beaches of the olive ridley sea turtle in the Utría National Natural Park (PNN) and its buffer zone (Amorocho et al., 1992; Amorocho, 1993; Martínez, 1999; Martínez and Páez, 2000; Hinestroza and Páez, 2001; Barrientos-Muñoz et al., 2014, 2015b), and a sporadic nesting of the black turtle has been recorded (Barrientos-Muñoz et al., 2013), whereas hawksbill sea turtle juveniles have been observed in coral patches in Punta Diego and the Aguada reef of the Utría PNN (Ramírez-Gallego, unpublished data). In a similar way, in the department of Nariño, in the Sanquianga PNN, studies have focused on the nesting beaches of the olive ridley sea and black turtles (Ceballos-Fonseca et al., 2003; Caicedo et al., 2009; Muñoz et al., 2010). Studies involving nesting and feeding areas are only found in the department of Cauca, in the Gorgona PNN (McCormick, 1996; Sánchez and Quiroga, 2002; Pavia et al., 2007; Gaos et al., 2010; Payán et al., 2010; Zorrilla, 2011; Tobón-López and Amorocho, 2014), where the sporadic nesting of the olive ridley sea and black turtles has been recorded, in addition to the presence of the leatherback and hawksbill sea turtles in the water (McCormick, 1996; Amorocho et al., 2001; Sánchez and Quiroga, 2002; Payán et al., 2010; Zorilla, 2011; Rivera-Gómez et al., 2016).
In 2003, the Marine and Coastal Research Institute (Invemar) toured the Colombian Pacific to confirm the species and nesting and feeding sites of sea turtles, but the sector comprising the Buenaventura Bay and Cabo Corrientes was not included due to a lack of budget, and it has been argued that, since it is an area with mangroves and clips, it is not a typical habitat for sea turtles (Ceballos-Fonseca et al., 2003). Consequently, the department of Valle del Cauca is the place with the least information on sea turtles along the Colombian Pacific littoral. In the Malpelo Fauna and Flora Sanctuary, there have been sporadic sightings of hawksbill sea and green Pacific sea turtles during shark monitoring (Fundación Malpelo, pers. comm.). In the Málaga Bay, Puerto España beach has been recorded as a nesting site for the olive ridley sea turtle (Merizalde et al., 2005; Núñez, 2007; Barreto-Sánchez, 2011; Cubillos, 2016). The communities settled in the bay confirm their sightings in water and a continuous interaction with sea turtles in their artisanal fishing gears (Barrientos-Muñoz and Ramírez-Gallego, unpublished data). Barrientos et al. (2020) recorded the first hawksbill sea turtle female for the Colombian Pacific in the Málaga Bay Conservation Mosaic. This study is part of the first interinstitutional, intersectoral, and community effort made between 2016 and 2020 in the Colombian Pacific and aimed at identifying marine turtle species, as well as their sizes, population structure, potential feeding, residence, and/or transit sites, and threats, by means of aquatic monitoring and the local fishermen’s voluntary surrender of the sea turtles captured in the Málaga Bay Conservation Mosaic.
STUDY AREA
The Málaga Bay Mosaic (3º56’ - 4º05’ N, 77º19’ - 21’ O) is located in the Eastern Pacific, in the western slope of the Cordillera Occidental (Western Mountain Range), in the Colombian Pacific, department of Valle del Cauca, Buenaventura district (Cantera et al., 1999) (Figure 1). Its area is approximately 136 km², which corresponds to its coastal-marine component. The bay is recognized as an estuarine area with sandy, rocky, and muddy beaches, as well as with cliffs and approximately 3000 ha of mangrove forests (Cantera, 1991; Cantera et al., 1999). It has a relative humidity of 90 % and a precipitation of 9000 mm, with a very wet tropical forest (bmh-T).
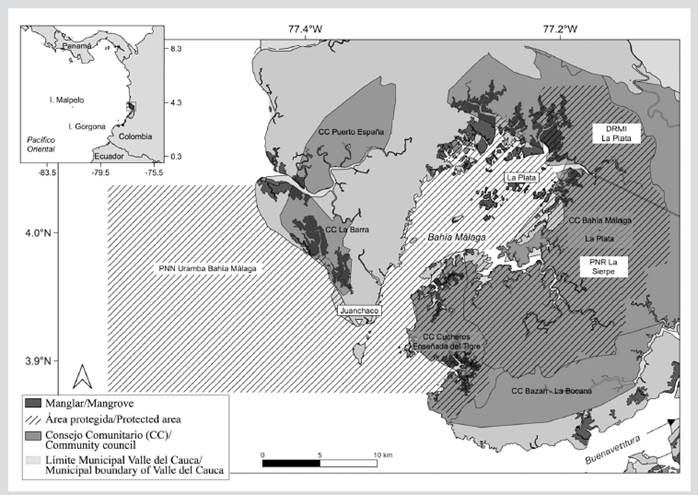
Figure 1 Study area. Málaga Bay Conservation Mosaic in the Colombian Pacific. The Mosaic comprises the following protected areas: the Uramba-Málaga Bay National Natural Park (PNN), La Plata Regional Integrated Management District (DRMI), La Sierpe National Regional Park (PNR), and the community councils (CC).
Málaga Bay is a strategic ecosystem of the tropical Eastern Pacific due to the environmental goods and services it provides and because it is a habitat with a wide diversity of species (Castellanos-Galindo et al., 2006; Invemar et al., 2006; Molina et al., 2006). The Conservation Mosaic comprises the Uramba-Málaga Bay PNN, La Sierpe Regional Natural Park, La Plata Integrated Management District, Málaga Bay, collective territories of Black communities, and indigenous reservations. The Mosaic is the result of the continuous and coordinated work between national and regional environmental authorities, such as National Natural Parks of Colombia and the Valle del Cauca Autonomous Regional Corporation (CVC), the La Plata-Bahía Málaga, Chucheros, La Barra, and Puerto España-Miramar community councils, and the Wounaan indigenous communities, aimed at managing, organizing, and preserving this global-scale natural biodiversity hotspot (Resolution 1501 of 2010).
According to non-structured interviews, there is traditional knowledge by the local population on sea turtles. These have been sighted within the bay and/or interacting with their artisanal fishing gears at or near the more than 101 islands and islets, as well as in a network of channels and estuaries identified as the La Plata Archipelago. The sites for the capture effort during aquatic monitoring were selected together with local fishermen and based on the exchange of traditional and scientific knowledge.
MATERIALS AND METHODS
Aquatic monitoring
The exchange of local and scientific knowledge, as well as the articulated work with Los Esteros Traditional Fishermen Association of Málaga Bay (ASOPES), allowed identifying and selecting the important sighting and potential capture sites of sea turtles in the bay. Aquatic monitoring was conducted, which included three working phases between 2016 and 2020, namely exploratory monitoring with trammel nets, continued/adapted monitoring with gillnets, and standardized monitoring.
Exploratory monitoring with trammel nets
During the first semester of 2016, two five-day aquatic monitoring sessions were conducted, using three to four chinchorro-type nets adapted for capturing sea turtles. These nets were manufactured with silk threads and were approximately 80 m long and 8 m high each. They had a 30-45 cm mesh size as well as less lead than a typical chinchorro in order to prevent the turtles from drowning when they were captured. Twice or thrice a day, the nets were extended and removed for periods of 1-2 h. Movements and sudden changes in speed and in the direction of the tide determined the effort time in which the net was extended. We recorded the name and GPS georeference of each site where the net was extended. If the safety and visibility conditions for entering the water allowed it, we proceeded to check the net every 40 minutes via snorkel diving in order to find entangled sea turtles and get them onto the boat (see similar methods in Carrión-Cortez et al., 2013; Chacón-Chaverri et al., 2015).
Continued/adapted sampling with gillnets
Starting in the second semester of 2016, a method for capturing sea turtles was designed and standardized together with the fishermen and leaders of the communities of Málaga Bay. By using a gillnet mate from twisted nylon multifilament (caliber 18), 100 m in length, 8 m deep, and with a mesh size of 50 cm, the capture effort was performed at different sites where the community had often sighted sea turtles and/or where they had interacted with the fishing gears. These sites are generally rocky promontories (locally named riscales) or trunks to which corals, sponges, and algae are associated. The nets were also deployed in islets and estuaries.
At each site where the nets were extended, a previous visual inspection was performed from a boat and/or from the riscales for a period of 15 minutes with the engine switched off, in order to detect turtles that came out to breathe and to visualize a precise point for extending the net, thus increasing the likelihood of captures. After 15 minutes, if no turtles were sighted, we proceeded to extend the net, which was deployed and retrieved for periods between 30 and 60 minutes with help from an outboard motorboat and a smaller rowboat. Two people in the small boat frequently checked the net manually, raising the superior cord from the bow in order to find entangled sea turtles and get them up. We recorded the name of the site and its coordinates, whether, tide (spring or neap), the state of the tide (rising or ebb tide), the time of turtle sighting (if the turtles were observed while coming out to breathe), and the net extension and retrieval times, as well as the people who performed them. However due to a lack of budget, between 2016 and 2018, no sampling sessions were conducted which were systematic in time.
Standardized sampling
Starting in the second semester of 2019, standardized sampling was carried out by means of the capture method described in the previous paragraph, adjusting the effort unit to the deployment of the gillnet (100 m x 8 m, mesh size = 50 cm) for 30 min. The catch per effort unit (CPUE) was calculated by dividing the total number of sea turtles captured in each sample, divided by the number of effort units. Four systematic aquatic monitoring sessions with a duration of 10 continuous days during the year (every three months) were defined, at different sites selected the night before the monitoring, where the community had often sighted sea turtles in recent times, employing four to six effort units (2-3 h) during the day, at 41 sites of the Mosaic.
The sites for the deployment of the net were almost always associated with rocky promontories, many of them submerged, which, with the reduced visibility in the water and the sudden changes in the tide of this area of the Colombian Pacific, constituted a risk for corals, sponges, algae, and the net itself. Therefore, in our effort unit, the deployment of the net was established at only 30 minutes, unlike the methods used in the Eastern Pacific, which employ continuous capture efforts of 24 hours (Seminoff et al., 2003), 4-8 hours (Carrión-Cortez et al., 2013), and 7 hours (Chacón-Chaverri et al., 2015).
Bycatch and surrender by the fishermen
In addition to the systematic and standardized aquatic monitoring, on the basis of the community strengthening carried out by the researchers in 2016, which was oriented towards the territory and its communities, and later under La Minga project starting in 2019, the Málaga Bay community council and ASOPES (Los Esteros) have led the process of voluntary surrender of the bycatch in their gears involving sea turtles. Moreover, in articulation with the researchers, we have reached agreements for the conservation of the territory, including sea turtles as objects of conservation value, as well as their associated ecosystems. ASOPES (Los Esteros) is located in Málaga Bay and comprises 56 organized active fishermen. The fishing gear is used and allowed in the territory are nets, namely multifilament (cloth) and monofilament (plastic) nets; fishhooks, such as espineles (fish trolling devices), hand lines, kites, and hawsers; and atajo nets (a kind of fishing net that uses tidal flow in narrow spaces to capture fish).
In articulation with the community council and ASOPES (Los Esteros), the turtles that were captured (alive or dead) during fishing were voluntarily surrendered for this study between 2016 and 2020. As the turtles were surrendered, the date, the species, the names of the fishermen, the fishing gear, and the capture site were recorded.
Turtle records
After being captured during aquatic monitoring and/or voluntarily surrendered as bycatch, the turtles were taken (according to their proximity) to the communities of La Plata or Miramar in order to continue with the protocol for recording information. This includes identifying the species, gathering biometric data, marking the individuals, making photographic records if possible, and releasing the individuals.
The recorded biometric data were the measurement of the minimum curved carapace length (LCCmín), the curved length of the nuchal-supracaudal carapace (LCCn-s), the curved width of the carapace (ACC) -all taken with a ± 0.1 cm metric tape- and the weight of each individual, which was taken by using a mechanical or digital balance with a precision of 0.01 kg. As for the marking process, a pair of Inconel 681C metallic tags (National Band & Tag, Newport, USA) with unique coding were applied in the second proximal scale of each front flipper, and, for some individuals, a DNAchip ISO-type integrated passive PIT tag (AVID Identification Systems, Inc, Norco, USA) was placed on the right front flipper with unique numbering. Subsequent capture events were registered as recaptures. Photographs of each side of the head were recorded per individual, and external physical conditions were assessed in order to detect injuries, mutilations, malformations, and/or the presence of fibropapilloma, as well as to continue by releasin the individuals at the same site or near the initial capture location within the next four hours. This exercise was carried out with the training and accompaniment of the researchers and public entities, and it was continued by the local experts trained in standardized protocols (Diez and Ottenwalder, 1999; Eckert et al., 1999; Ehrhart and Ogren, 1999).
Data analysis
In cases where one of the two LLC measurements was not taken in the field, the missing data were replaced by adding (to obtain the LCCn-s) or removing (to obtain the LCCmín) the average of the difference between the LCCmín and the LCCn-s for each species. For E. imbricata, the LCCn-s was converted into the straight length of the nuchal-supracaudal carapace (LRCn-s) by using the following formula: SCL = 0.9355 * LCCn-s + 0.4486 (Limpus, 1992). For C. mydas, the LCCmín was first converted into the minimum straight length of the carapace (LRCmín), and then into the LRCn-s by using the following formulas: LRCmin = 0.9240 * LCCmin +1.0205 and LRCn-s = (SCLmin + 0.0460)/0.9883, according to Meylan et al. (2011). The converted LRCn-s were used to calculate the body condition index (ICC) through the following formula: ICC = (Weight / LRC3) * 10 000 (Bjorndal et al., 2000). For all data analyses, the R v.v.3.4 software was used (R Core Team, 2020).
With the purpose of detecting the areas with the largest amount of marine turtle captures, heatmaps were elaborated which were based on the Kernel density estimation (KDE), implemented in the QGIS software (QGIS Development Team, 2020). The densities were calculated from the amount of GPS points for each capture and the action radius. The action radius was calculated based on the averages in the size of the area for 95 % of the use: 67.16 ha for E. imbricata juveniles (Carrión-Cortéz et al., 2013) and 1537 ha for C. mydas (Seminoff et al., 2002). This resulted in radii of 462.4 and 2211.9 m, respectively.
RESULTS
Aquatic monitoring
During the five years of aquatic monitoring, three phases were executed in the capture of sea turtles, for a total of 126 hours of effort that 41 sites and 10 captured turtles. During the exploratory monitoring with trammel nets, 22 hours of aquatic monitoring effort were employed (2016/1st semester) and a black turtle juvenile was captured. During the continued/adapted monitoring with gillnets between the second semester of 2016 and 2018, 40 hours of monitoring effort were spent, and three hawksbill sea turtle juveniles were captured. Finally, a black turtle juvenile and four hawksbill sea turtle juveniles (with an additional recapture) were caught during the standardized monitoring, where 128 effort units were spent, for a total of 64 hours of net effort regarding net extension (from October 2019 to October 2020). The greatest abundance of turtles captured with nets corresponded to hawksbill sea turtles (80 %, n = 8), followed by black turtles (20 %, n = 2).
Bycatch and surrender by the fishermen
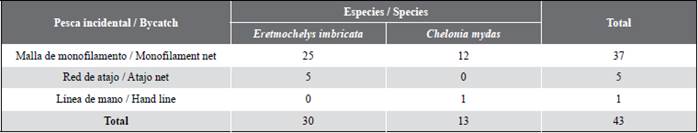
From May 2016 to October 2020, without a defined temporal tendency and along the Conservation Mosaic, a total of 43 sea turtles, comprising hawksbill sea (E. imbricata) and black turtles (C. mydas), were captured as bycatch and surrendered by the Asopes (Los Esteros) fishermen to the researchers and local experts. 86 % of these, namely 25 hawksbill sea turtles and 12 black turtles, were captured with monofilament nets. 11.6 %, which amounted to five hawksbill sea turtles, were captured with atajo nets, whereas 2.3 %, one black turtle, was captured using a hand line with a J-type fishhook (Table 1). Additionally, a hawksbill sea turtle juvenile was captured on the beach of El Cementerio Islet while a fisherman brought his boat ashore. The individual was close, got scared, and emerged from the sea, crawling towards the beach, where it was captured by hand.
Turtle records
During the five years of study, a total of 54 turtles have been recorded and identified as hawksbill sea and black turtles. Among these, 72 % (n = 39) were hawksbill sea turtles, and 28 % (n = 15) black turtles were recorded in the Málaga Bay Conservation Mosaic.
Hawksbill sea turtles were the species with the highest capture rate during the study. A total of 36 individuals were recorded and labeled within the bay. Eight hawksbill sea turtle captures occurred during aquatic monitoring, 30 were carried out by fishermen with monofilament and atajo nets, and one additional turtle was captured by hand by a fisherman. This was the only species with recapture records (n = 3): an individual was marked on July 3, 2017, and recaptured on March 1, 2020; another individual was marked on March 1, 2020, and recaptured on October 22, 2020; and one dead individual was recorded as bycatch by the fishermen months after its release. Based on the minimum size of the nesting females recorded in the geographically closest colony of Machalilla in Ecuador (LCC = 73.0 cm; Gaos et al., 2017), most individuals, namely 97 % (n = 35), were juvenile turtles, with an LCCmín average of 45.3 ± 11.6 and one adult female (3 %) with an LCCmín of 90 cm The latter was the first adult hawksbill sea turtle recorded for the Colombian Pacific (Barrientos-Muñoz et al., 2020) (Figure 2). With the recapture of two hawksbill sea turtle juveniles, the individual growth average (n = 2) oscillated between -0.29 and -0.76 cm month-1. The slowest growth rate was shown by a turtle whose LCC went from 52.1 to 61.1 cm in 31 months, whereas the fastest growing turtle’s LCC went from 40.2 to 45.5 cm in 7 months. The body condition index (ICC) varied from 0.8 to 2.2, with an average of 1.1 ± 0.2 (Table 2).
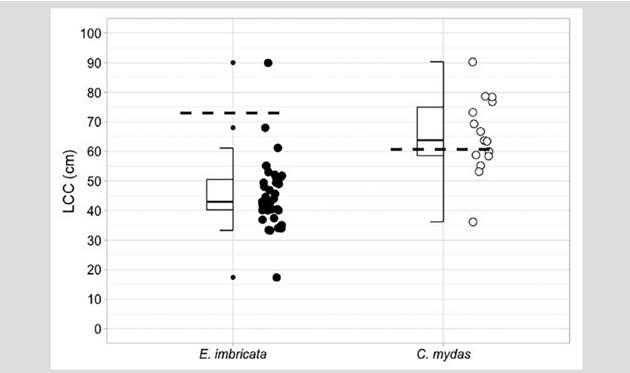
Figure 2 Median, quartiles, range, and extreme LCC (curved carapace length) values in cm for hawksbill sea turtle (E. imbricata) and black turtle (C. mydas) individuals captured in Málaga Bay. The minimum nesting sizes of each species are indicated (E. imbricata = 73.0 cm LCC, Gaos et al., 2017; C. mydas = 60.7 cm LCC, Zárate et al., 2003).
Table 2 Averages ± SD (range) of biometric measurements of hawksbill sea turtles (Eretmochelys imbricata) and black turtles (Chelonia mydas) recorded in Málaga Bay, Valle del Cauca, where LCCmín = minimum curved carapace length (cm); LCCn-s = curved length of the nuchal-supracaudal carapace (cm); LRC = straight carapace length (cm) converted according to Limpus (2002) for E. imbricata and according to Meylan et al. (2011) for C. mydas; P = weight (kg); and ICC = body condition index.
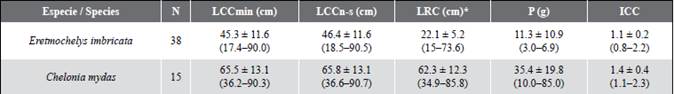
On the other hand, the 15 black turtles were also captured in La Plata Archipelago. Two of these were caught during the aquatic monitoring, 12 with monofilament nets, and one with a hooked hand line by fishermen. Based on the minimum nesting LCC size of 66.7 cm recorded in the geographically closest colony in the Galapagos Islands (Zárate et al., 2003), most of the C. mydas individuals were potential adults, with an average of 65.6 ± 13.1 cm (Table 2). The ICC varied from 1.1 to 2.3, with an average of 1.4 ± 0.4 (Table 2).
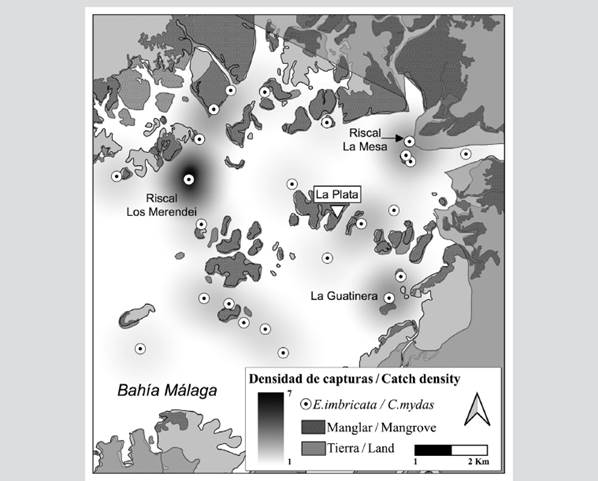
Among the 41 sites where individuals were captured using grillnets for turtles or as bycatch, the greatest density corresponded to Los Merendei riscal (n = 6; 5 E. imbricata, 1 C. mydas); Cementerio (n = 4; 3 E. imbricata, 1 C. mydas); La Guatinera (n = 3; 2 E. imbricata, 1 C. mydas); La Mesa riscal (n = 2; 1 E. imbricata, 1 C. mydas); and Parguera (n = 3 E. imbricata). On the other hand, La Jejenera, the riscal of Pital, Chontaduro, La Aguada recorded two E. imbricata captures per site (Figure 3).
DISCUSSION
Our study indicates that the Málaga Bay Conservation Mosaic harbors important sites for the development and feeding of different stages of E. imbricata and C. mydas individuals. Both species are mainly represented by juveniles, sub-adults, and potential adults to a lesser extent (Zárate et al., 2003; Chacón et al., 2015; Gaos et al., 2017; Barrientos-Muñoz et al., 2020). The knowledge of these stages and feeding areas is essential to generate effective strategies for the conservation of both species, which have been classified as being at some risk of extinction (Seminoff, 2004; Mortimer and Donelly, 2008; Hamann et al., 2010; Bjorndal et al., 2011; Rees et al., 2016; Llamas et al., 2017; Wildermann et al., 2018).
The hawksbill sea turtles found in Málaga Bay exhibit a wide range of sizes, which varies between 17.4 and 90.0 cm, with an LCCmín average of 45.3 cm (n = 38), whereas, in the Gorgona PNN, the range varies from 32.0 to 58.0 cm, with an average of 41.6 cm (n = 49) (Cañas-Uribe et al., 2020). However, the average size of hawksbill sea turtles suggests that most individuals inhabiting Málaga Bay have not reached the maturity age (Liles et al., 2011,2015; Heidemeyer et al., 2014; Tobón-López and Amorocho, 2014; Chacón-Chaverrí et al., 2015; Gaos et al., 2017; Llamas et al., 2017). The LCC size of the nesting females in the only known colony of the Southeastern Pacific, located in Machalilla, Ecuador, about 700 km away, is 91.1 cm, with a minimum size of 73.0 cm; the female found during our study is above this minimum value, thus confirming that there are adults in addition to juveniles (Gaos et al., 2017; Barrientos-Muñoz et al., 2020). In recruitment areas, hawksbill sea turtles are dominated by individuals with an LCCmín between 30 and 60 cm, which have been described along the Pacific coast of Latin America, as is the case of Gorgona Island (Tobón-López and Amorocho, 2014), Coiba Island in Panamá (Llamas et al., 2017), and Costa Rica (Carrión, 2010; Carrión-Cortez et al., 2013; Heidemeyer et al., 2014; Chacón-Chaverrí et al., 2015). Nevertheless, most of these sites represent coral reefs and rocky points, whereas Málaga Bay has unique habitats such as octocoral bottoms, mangroves on rocky substrate, dwarf mangroves, and soft rock cliffs (Invemar et al., 2006). This area is dominated by a great mangrove strip and turbid waters almost all year long, which is similar to the habitats described for adult hawksbill sea turtles in Central America (Gaos et al., 2012).
During sampling, a female with an LCCmín of 90 cm was captured, which validates the versatility of hawksbill sea turtles regarding their use of habitats throughout their development in the Eastern Pacific (Gaos et al., 2012; Barrientos-Muñoz et al., 2020), but this also suggests the possible of still undescribed nesting sites for this species in the Colombian Pacific. Genetic and telemetry studies on the hawksbill sea turtle of the Eastern Pacific evidence that it does not move much between nesting and foraging sites, which is why it is necessary to keep making efforts to identify and monitor potential nesting sites in the area (Meylan et al., 2011; Gaos et al., 2012a,2018; Barrientos-Muñoz et al., 2015, 2020). Even though the records for hawksbill sea turtles stem from mostly exploratory sampling that is sporadic and just recently standardized with respect to their methodology, the recapture of an individual after 2.5 years suggests that residence also takes place in Málaga Bay (Tobón-López and Amorocho 2014; Llamas et al., 2017; Cañas-Uribe et al., 2020).
On the other hand, black turtles show a similar pattern to that of hawksbill sea turtles: breeding females in the three main nesting colonies in the Eastern Pacific (Colola in Mexico, Galápagos Islands in Ecuador, and the northern coast of Costa Rica) (Cornelius, 1986; Márquez, 1990; Alvarado and Figueroa, 1991; Alvarado-Díaz et al., 2001; Zárate et al., 2003; Chaloupka et al., 2004; Delgado Trejo and Alvarado Díaz, 2012) recorded LCCmín averages of 85 cm, which is why most of the individuals captured in Málaga Bay correspond to large juveniles or sub-adults. Similar sizes have been found in other feeding areas for black turtles (López-Castro et al., 2010; Senko et al., 2010; Sampson et al., 2014). Variations in the LCCmín size were more consistent: 36.2-90.3, with an average of 65.5 ± 13.1 cm (n = 15). However, these values are higher than those occurring in the Gorgona PNN with black turtles, where the average LCCmín was 64.0 cm, with a range of 37.9-82.7 cm (n = 276) (Sampson et al., 2014).
The data from the marking-recapture of metallic tags applied to the turtles’ flippers (Green, 1979; Figueroa et al., 1993; Heidemeyer et al., 2018), as well as the satellite tracking of C. mydas in the Eastern Pacific (Blanco et al., 2012; Hart et al., 2015), have shown black turtles to have long migrations in order to recruit diverse feeding sites after the reproductive phase in their natal beaches. Apart from some reports evidencing the existence of nesting sites in the Pacific coast of Colombia (Barrientos-Muñoz et al., 2013), the presence of individuals close to the reproductive age in Málaga Bay suggests that this may be their final development site before they start their reproductive migration towards nesting beaches in Colombia and/or nearby countries such as Panama, Costa Rica, and/or Ecuador. Meylan et al. (2011) suggest that, in the Eastern Pacific, habitats primordially occupied by juveniles and adults are more mixed than segregated in comparison with other regions of the world. By comparing our still limited records with the data obtained from other feeding sites in the region (Isla Gorgona, Sampson et al., 2014), this seems to be true for Colombia.
It is necessary to keep conducting research and active and standardized monitoring of the sea turtles present in the Conservation Mosaic. An extension of this work to other communities is being considered, replicating the exercise with fishermen and evaluating the capture percentage of the fishing gears. This would even be a monitoring indicator for fishing organization and regulation regarding the use of gears and methods with less impact on hydro-biological resources. This work has been conducted by local communities and the Uramba-Málaga Bay PNN. The fishing areas used by the turtles in Málaga Bay must have a special fishery management in order to ensure their permanence in the riscales within the framework of conservation agreements articulated with the communities. With continuous monitoring and additional sampling techniques such as the use of satellite transmission and genetics, we expect to be able to obtain more information on the temporal and spatial use of marine turtles, as well as their origin, sizes, densities, abundance, growth rate, residence time, connectivity, and threats, which contributes to the knowledge and conservation of both species at local and regional levels.
CONCLUSIONS
This study highlights the importance of Málaga Bay as the site in the Colombian Pacific that exhibits the greatest diversity of sizes for the sea turtles E. imbricata and C. mydas. This is essential for the survival of the species in the Eastern Pacific. Despite the short time of this study (2016-2020), it is evidenced that Málaga Bay is a critical site as a feeding and residence area for the hawksbill sea turtle of the PO. The combination of unique habitats including mangroves, riscales, and bottoms with octocorals and sponges in Málaga Bay shows their great importance for the foraging of both species, given that, according to the body condition index, they allow for productive and healthy aggregations. The evidence of only one dead hawksbill sea turtle individual, as well as that of a black turtle (later commercialized) in the fishermen’s nets, does not reflect that the bycatch of turtles is low in the area. It is possible that, in the first years of this study, when they found dead individuals in their nets, the local fishermen were too scared or ashamed to report it, which is why this must be cautiously interpreted. In the next years of study with sea turtles in Málaga Bay, it is important to maintain a constant dialogue to strengthen local trust and to get more fishermen to record the bycatch of sea turtles. On the basis of interinstitutional, intersectoral, and community efforts, this study contributes with a baseline for knowledge and the generation of effective strategies for research and the conservation of these species and their ecosystems in the Málaga Bay Conservation Mosaic.











 text in
text in