INTRODUCTION
The sardine Sardinella aurita Valenciennes, 1847 is the most abundant fish species in the Caribbean (Stromme and Saetersdal, 1989). It is the main Venezuelan fishery resource, and it is captured in the states of Sucre and Nueva Esparta, where its fishing began in 1927 (Guzmán et al., 2003) and its canning in 1934 (Gómez et al., 2008). Its abundance is related to the marine fertility caused by water upwelling, the Orinoco River, and other factors (Gómez and Acero, 2020). Evaluations indicate the availability of 850 000 ton (Gerlotto and Ginés, 1988; Stromme and Saetersdal, 1989; Cárdenas and Achury, 2002) and a biomass of 1 300 000 ton (Cárdenas, 2003). As of 2017, fishery had been practiced for nine decades, which is surprising because it was unregulated for almost 50 years and depletion was estimated in 1993 (FAO, 1963).
Since 1996, fisheries around the world have decreased their yields to 1.2 million ton/year, and their collapse has been estimated for 2018 (Pauly and Zeller, 2017). ‘Small pelagics’ such as sardine have been almost depleted by industrial fishing (Lluch-Belda et al., 1989) given the excess demand of fish flour. Furthermore, they undergo a strong trophic pressure since they are the forage that feeds predators (Checkley et al., 2017). In 2018, the worldwide captures by fishing were 96 433 763 ton, and small pelagics reached 19 820 354 ton (FAO, 2020), which constitutes 20.6 % of the total, albeit lower than the 33 % reported in 2000 (Pauly, 2009). In this century, seven Sardinella species (Froese and Pauly, 2020) have reported average captures of 1 017 400 ton/year (FAO, 2020). Until recent years, there was a consensus on the underexploitation of Venezuelan sardine (Guzmán and Gómez, 2000; Mendoza and Guzmán, 2000; Freón and Mendoza, 2003), even though most stocks had been completely exploited (FAO, 1994). Sardine is directly consumed (Christensen et al., 2014), which is locally valid, as it is an important national food. It is interesting to compare the sardine activity for 90 years and its collapse in California, where it disappeared (1968) 80 years after fishing began (Ueber and MacCall, 1992). Fishing was then resumed in 1990, and sardine recollapsed in 2014 (Hill et al., 2015).
The objective of this review is to estimate the captures of this resource throughout nine decades. Four periods are considered: an initial, unregulated one (1927-1973) and other three in which handling regulations were applied. For each of them, the captures are calculated along with their average, their operating gears, and the captures by effort unit. Relevant studies are cited. The causes for the permanence of fishery and measures for its continuity are discussed.
STUDY AREA
The northwestern region of Venezuela comprises the States of Sucre and Nueva Esparta (Figure 1), which are the main fisheries, as they capture > 70 % of the national total. They are characterized by the upwelling of subsurface waters, which increases marine fertility, promoting fishing richness and abundance of filtering species and the abundance of filtering species such as S. aurita, which has a rapid growth (Gómez, 2001). Numerous studies have been conducted on this upwelling (Gómez and Barceló, 2014). It spans from the Paria Peninsula to La Tortuga Island (61° 50’ to 65° 25’ W) and the edge of the continental shelf (11° 30’ N), with an area of 52 000-55 000 km2 (Gómez, 1996; Castellanos et al., 2002), which is where the sardine lives. In peripheral areas, sardine-predating fish are captured, such as scombrids, carangids, trichiurids, coryphenids, lutjanids, istiophorids, elasmobranchs, among others.
METHODS
Sardine captures were estimated using official data from 1952 to 2012 as cited by FAO, with information submitted by fishing authorities and mentioned in FishBase (Froese and Pauly, 2020). Captures from 2012 to 2017 were provided by Insopesca (Instituto Socialista de Pesca y Acuicultura de Venezuela, Venezuelan Socialist Institute for Fishing and Acuiculture). To estimate fishing between 1927 and 1949 (no records), we consulted the owner of the cannery that has been in operation since 1944 in Margarita, thus assigning equal figures for those years. The approximation to the yearly catch per unit effort (cpue) for each period was estimated by means of the quotient of the average captures and the number of gears at the end of the interval. The information published in journals and books, theses and university works (promotion), and scientific and canning industry reports is mentioned.
RESULTS AND DISCUSSION
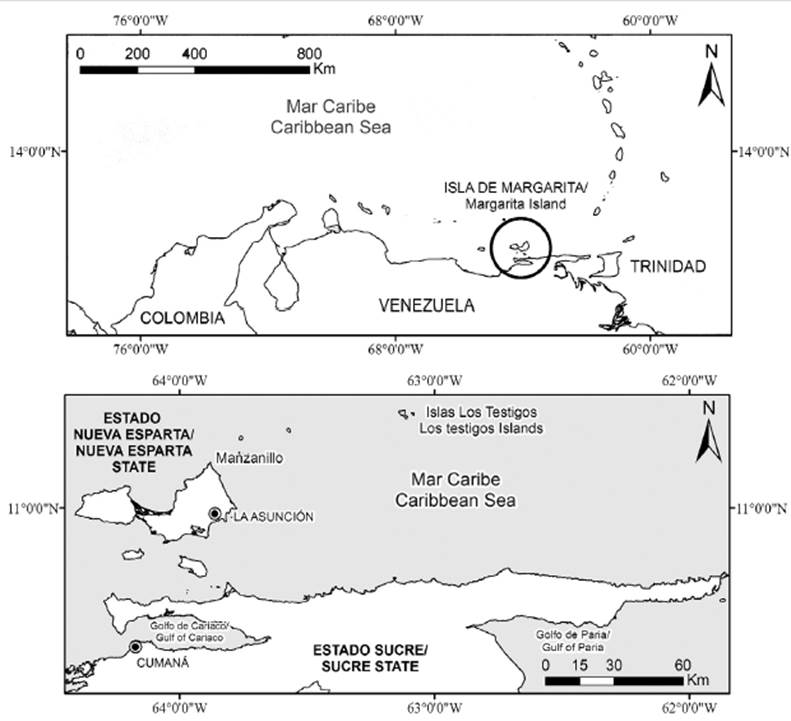
1927-1973 PERIOD: For 47 years, there was no regulation for sardine fishing. There are no data for the period between 1927 in 1949. By consulting the owner of the cannery, captures amounting to 10 000 tons per year (ton/year) were estimated, a moderate value, since five canneries were operating in Sucre (Gaviota, Margarita, Productos Mar, and CAIP) during that decade, as well as El Faro in Margarita (Teobaldo Castañeda, Marine Museum, Margarita Island, Venezuela, 2017, Com. Pers.). In the 1950s, the average was 19 170 ton/year. Between 1950 and 1973, the catch ranged from 8 400 (1954) to 47 500 ton (1973). A total of 739 500 ton were fished (34 662 ton/year on average), a figure higher than the fish landings until 1966 (Simpson and Griffiths, 1967). Between 1950 and 1957, as well as between 1964 and 1973, the averages were 30 000 and 40 000 ton/year (Freón et al., 2003). In the decade of 1960, canned sardine fluctuated between 22 300 and 25 300 ton/year, ranking fourth worldwide (Griffiths and Simpson, 1967). In the period between 1927 and 1973, the total captures were 969 500 ton, namely 230 000 ton for years with no record and 739 500 between 1950 and 1973. The average for the latter was 30 812 ton/year; with this figure, and with 194 chincorros operating in 1970 (Etchevers, 1974), a cpue of 159 ton/gear/y was estimated (Figure 2).
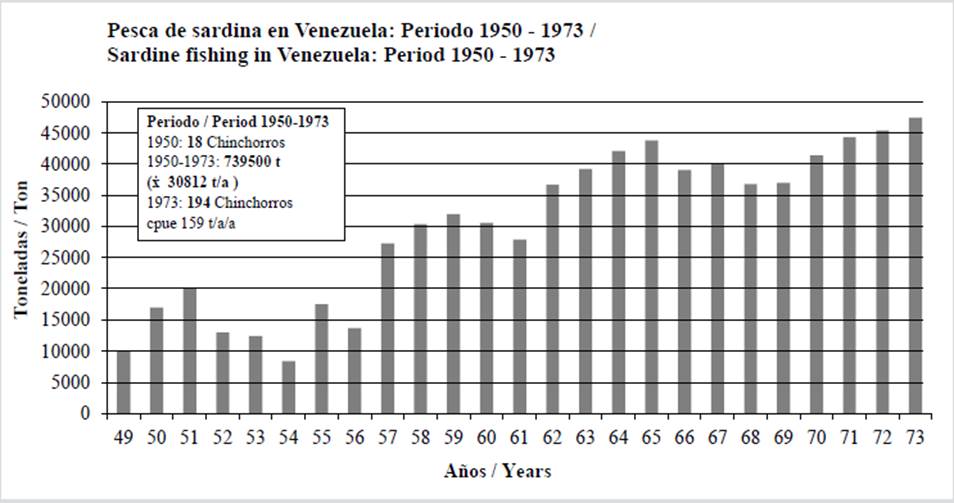
Figure 2 Annual variation of sardine fishing in Venezuela. 1950-1973 Period: the total catch, annual average (ton/year), number of gears, and estimated cpue (ton/gear/year) are shown. An estimated catch of 1949 is observed, as an example for previous years.
The shoals could be located from the coast. The chinchorros was 200-300 m long (4-6 m high), and it would be extended using boats with oars, thus surrounding the sardines. Up to 20 fishermen operated in the area, who manually brought over with the live sardines, which was anchored for up to 15 days. It was then transported to the factories in vessels (with a capacity of up to 8 ton). Up until 1966, the catch per haul varied between 78 and 133 ton. Chinchorros were regarded as primitive but efficient (Griffiths and Simpson, 1967).
In the 1950s, international experts started to study this matter. They identified the species as S. anchovia, and it was captured along with S. brasiliensis (Simpson and Griffiths, 1967). It was also classified as Clupanodon pseudohispanicus (Martin and González, 1960), but it was later made clear that it was S. aurita (Whitehead, 1973). Its body height shows a great variability, and some of its features are determined by the temperature, given that larvae born during the upwelling are greater in size (Etchevers, 1974). In the Eastern Atlantic, temperature and vertebrae are negatively correlated (Gheno and Poinsard, 1968). Since 1950, the tuna fleet uses sardine as bait (Griffiths and Simpson, 1967), as well as the artisanal medium-height fleet (> 400 ships). Some factories had fishing gear, but this became a complex issue, so they made it easy for fishermen to buy boats and nets in order to intensify the activity. They made changes to the chinchorros (length of up to 900 m; 16-20 m height), to the distance from the coast for fishing (2-3 km), and to the motorized traction for setting and hauling gear, which increased from 18 to 194 (Etchevers, 1974).
Around 1950, canning factories came into operation, which bought machinery used in California, given the sardine collapse. They acquired equipment from Germany, Sweden, and Portugal, as well as automated systems from Spain. The personnel, with experience in California, searched for raw materials in other latitudes (Popovici, 1964). They set up plants in Perú, Chile, and South Africa, and they also moved fishing boats (Ueber and MacCall, 1992).
In the 1960s, the results of the first studies emerged: the sardine captured since 1957 did not show a tendency towards a lower size, thus indicating that it was not affected by fishing (Simpson and Griffiths, 1967). Spawning occurred between December and April (Simpson, 1963), and mature, 13.4 cm sardines were found (Simpson and González, 1967). The mean total length (LT) maturity size (Lm 50 %) was estimated to be 19.5 cm (FAO, 1963; Simpson, 1963). Despite the fact that this size was known, which could have aided in managing this resource, there was no regulation. A sardine collapse was also proposed in 1993 (FAO, 1963) because the capture size was lower than the maturity size. Factory owners were worried about depletion, and, in order to expand exploitation, knowing about possible captures was prioritized. In 1966, the government decided to evaluate the resource (MAC-FAO-PNUD program) by estimating the abundance of shoals and determining how to capture them (Trujillo, 1975a). By means of acoustic techniques, it was concluded that the shoals are near-shore in January-April. Reproductive areas were cited (López, 1972), and changing the fishing technique was suggested (Oodegard, 1971a, 1971b). The shoals were quantified through aerial prospection. A gear change and a landing expansion of up to 150 000 ton were proposed (Trujillo, 1975a, 1975b). The evaluations reconfirmed the areas with greater concentrations (Martin and González, 1960), and fishing 200 000 ton was advised (Ginés, 1972).
The factories bought sardine; instead of fishing, the sardine was extracted with brailers and deposited on motorboats (20-60 ton capacity) to be taken to the factories. Since 1950, companies submitted their fishing data to the authorities based on purchases from fishermen (Trujillo, 1977; Freón and Mendoza, 2003). Until the beginning of the 21stcentury, the Venezuelan Chamber of the Canning Industry for Fishing (Cavenpesca) provided the annual purchased amount (Freón and Mendoza, 2003a).
1974-2005 PERIOD: at the end of 1973, the first ordinance appears (Gaceta Oficial 30283,18 December 1973), which set the capture size at 15 cm (LT) and allowed extracting 30 % of the individuals with inferior length, as well as 10 % of sardines of less than 12 cm (Novoa et al., 1998; Freón et al., 2003b; Huq, 2003). It is surprising that the first regulation after 47 years of fishing would allow juveniles to be fished and have a validity of 32 years.
Captures show great variation due to incomplete statistics, as in all countries (Pauly and Zeller, 2017). Between 1973-1979, they varied from 22 835 ton (1974) to 47 608 ton (1975). In the 1980s, they fluctuated between 27 974 ton (1981) and 82 759 ton (1987); and, in the 1990s, between 56 949 ton (1990) and 186 060 ton (1998). Between 2000 and 2005, they varied from 73 534 ton (2000) to 200 232 ton in 2004 (the year with the highest captures). The sardine crisis began in 2005. Fishing decreased by 50 % in comparison with 2003-2004, and it was lower than 50 000 ton/year for > 10 years. The captures for this period were 2 738 010 ton (85 563 ton/year on average), with 246 gears in operation (Figure 3). The estimated cpue was 348 ton/gear/y. It is beyond belief that the capture of juveniles (15 cm) and even of individuals shorter in length was allowed, given that, in 1973, the FAO report of 1963 was already known, which recognized a mean maturity size (19.5 cm) larger than that of fishing (< 15 cm). It would seem as though the ordinance intended to deplete the sardine. Perhaps the industry influenced the imposition regarding size due to the machines that were used for canning. The prolonged validity of the handling may be related to the production of fish flour, which has always had a high international price. During this period, 12 processing factories were in opperation (Simpson and Griffiths, 1967).
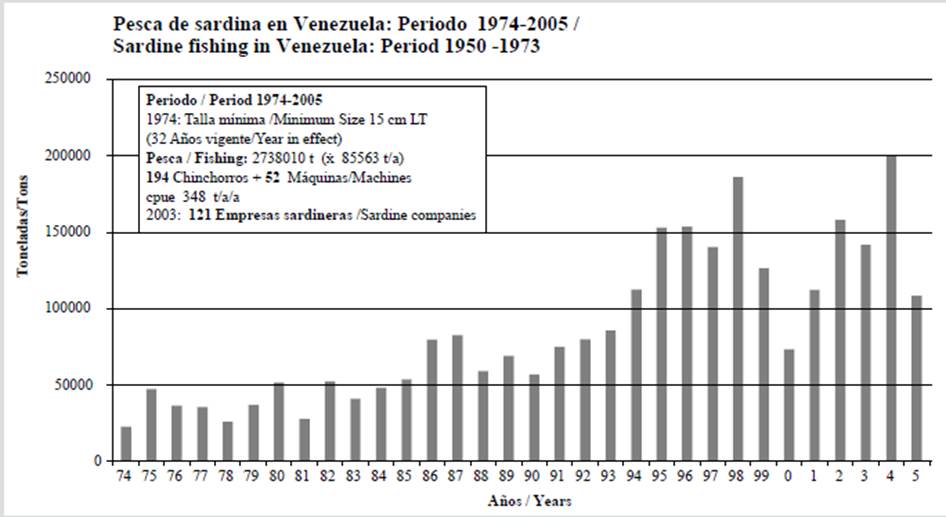
Figure 3 Annual variation of sardine fishing in Venezuela, 1974-2005 period. Total catch, annual average (t/a), number of gears, companies, and calculated cpue ton/gear/year (t/a/a) are shown.
To increase the captures, it was necessary to change the fishing methods (Etchevers, 1974). Purse seines were used with scarce results (Griffiths and Simpson, 1967). In Venezuela, this gear is called ‘ring machine, ring fence, sardine fence, or bag’ because the inferior headrope has rings through which a rope is inserted in order to close the net. Once the sardines are located, and end of the gear is thrown into the water, and the lines remain in the auxiliary boat surrounding the shoal and closing the inferior net (Marval and Cervigón, 2009). It is similar to the tuna purse seine, albeit with a length of up to 400 m and a height of 40 m (Gaceta Oficial 40308/2013). 52 of these were fishing in Sucre in 1996 (Barrios et al., 2010).
Between 1976 and 1989, the recruitment figures went from 40-1000 million juveniles/y (Mendoza, 1996; Freón and Mendoza, 2003) to over 3300 million (González et al., 2016). With a record number of sardines per ton that were fished in a catch (Gómez and González, 2008), it was calculated that, in 2004 (200 232 ton fished), between 2023 and 2344 million sardines were captured, to which natural mortality and predator consumption had to be added. In 1978, quantifications were resumed (Cárdenas, 2003). A biomass of 1.4 million ton was estimated, and intensive exploitation with other gears was suggested (Gerlotto and Elguezábal, 1986; Gerlotto and Ginés, 1988). In 1988 the Fridjotf Nansen (NORAD/UNDP/FAO) ship conducted biomass prospection activities between Colombia and Surinam. 800 000 ton in Venezuela and 100 000 ton in Colombia were estimated (Stromme and Saetersdal, 1989). Between 1995 and 1998, the Hermano Ginés ship executed the VECEP Project (European Economic Community), estimating a biomass of 1.3 million ton, with 855 000 ton available (Cárdenas and Achury, 2002). Until 1998, ~ 30 evaluations were carried out, which agreed on their estimates (Gerlotto and Ginés, 1988; Stromme and Saetersdal, 1989; Cárdenas and Achury, 2002).
At the beginning of the 21st century, an underexploited stock that was also under moderate conditions was cited (Guzmán, 2000; Freón and Mendoza, 2003). Sustainable captures were between 160 000 and 470 000 ton/year (Mendoza and Guzmán, 2000). Sardine-related activities surged, given that seven major canneries, along with 25 medium- and small-sized ones, 12 freezing companies, 32 cutting enterprises (cutting the head, tail, and guts off to obtain sardine torsos), and 45 salting-smoking ventures were in operation. A total of 121 companies utilized 140 000 ton/year, which includes 5000 ton for bait and 18 000 ton for direct consumption (Gastón, 2003). Frozen sardine was exported to Brazil, Costa Rica, Argentina, and Asia. The industry considered sardine to be of worldwide importance, as 800 000 ton/year were available, but only 17 % were exploited (Gastón, 2000). In 2004, 200 032 ton were fished, but only 140 000 ton/year were used (Gastón, 2003), which might have enabled the fact that 60 232 ton were not registered (Rueda-Roa et al., 2017) -perhaps in Sucre, because thorough records were kept in Nueva Esparta (Gómez, 2006; Gómez et al., 2008). In 2006, the sardine crisis intensified (Figs. 4 and 5). For a decade, the annual captures did not exceed 50 000 ton.
2006-2013 PERIOD: the second ordinance (Gaceta Oficial 38377, 10 January 2006) increased the capture size to 17 cm, with a limit of 200 000 tons. Perhaps the authorities acknowledged that the captures could reach 25 % of the stock (Cárdenas, 2000). In spite of the crisis (Figures 4 and 5), the captures varied between 36 157 ton (2008) and 63 426 ton (2006), for a total of 373 645 ton (46 706 ton/year) with 294 gears in operation. The cpue was estimated to be 159 ton/gear/y.
Figure 4 Annual variation of sardine fishing in Venezuela. Period 2006-2013, during the validity of second ordinance (catch size 17 cm LT) and the third one since 2014 (catch size 19 cm and ban). Total catch, annual average (ton/year), operating gears, and calculated cpue (ton/gear/y) are shown.
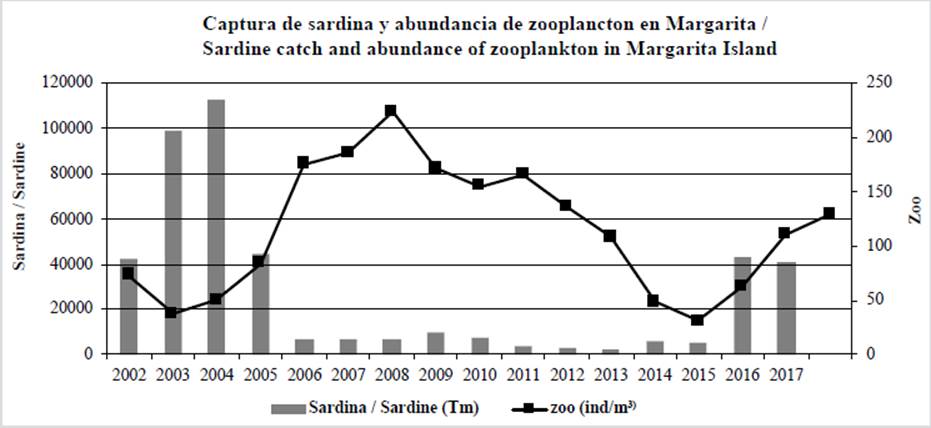
Figure 5 Sardine catch (ton) and abundance of zooplankton (ind/m3) in Margarita Island, Venezuela (Gómez, 2019). Regime change observed between 2005 and 2015.
The capture size was increased to 17 cm with some restraints, as it was considered as a fact that ~20 cm is the mean maturity size (FAO, 1963; Freón and Mendoza, 2003). However, several studies have concluded that it is < 17 cm because, within the 162-166,9 mm interval, the cumulative frequency of mature individuals was 62.15 % in females and 65.29 % in males (Kortnik, 2005; Kortnik and Posada, 2006). The same maturity size has been determined in the Eastern Atlantic (Quaatey and Maravelius, 1999; Gaamour et al., 2004; Tsikliras and Antonopoulou, 2006; Mustac and Sinovcic, 2012). If the mean reproduction size were not < 17 cm, Venezuelan sardines would have already been depleted, as argued by FAO (1963, 1979). In 2009, the last assessment indicated a decrease in the abundance of sardine (Mendoza, 2015), varying between 81 040 and 289 940 ton. Therefore, reducing the efforts and captures was recommended (Gassman et al., 2012b).
2014-2017 PERIOD: the third ordinance (Gaceta Oficial 40308, 4 December 2013) established a ban (three months) and increased the capture size to 19 cm. The catch varied between 48 777 ton (2014) and 91 565 ton (2017), with 305 603 ton being captured (76 401 ton/year on average) (Figure 4). The cpue was 214 ton/gear/y. In Sucre, 100 fences (purse seines) were in operation in 2015 (Gaceta Oficial 40573/2015), which increased to 115 in 2017, along with 242 chinchorros being authorized (Gaceta Oficial 41295/2017), for a total of 357 gears.
Sardinella aurita breeds all year; in a few hours, the gonadosomatic index increases from 5 to 20 % (Somarakis et al., 2004), which is why the monthly or biweekly gonad indices are questionable and the proposed handling is wrong. Moreover, this species has parceled spawning; it releases mature oocytes every day (Alheit, 1993), around 10 % of those present in the gonads (Bernal et al., 2011). The fishing authorities acknowledge that reproduction takes place between December and April, as concluded 60 years ago (Simpson, 1963).
The established capture size of the third ordinance (19 cm) is lower than that accepted for maturity (20 cm). This ordinance was incoherent and wrong, and it strayed from biological reality. Its implementation affected fishermen, factories, and consumers who acquired large amounts of sardine at an affordable price. The ban issued at the beginning of the year is questioned because an 11-year study conducted on the abundance of sardine eggs in plankton indicates a higher reproduction between October and December (Gómez, 2015). Moreover, according to another study, it is concluded that sardines > 17 cm in size have more lipids in the months after the upwelling (Tornes et al., 1971) which constitutes a particular breeding strategy (Freón et al., 1997, 2003a).
The current handling is not ecosystemic. Perhaps sardine is considered to be an independent variable in failed monospecific models, but it is another species that is affected by the environment. The sardine fluctuations in California are a lesson. Since 2000 years before fishing began (Baumgartner et al., 1992), small pelagics showed biomass variations due to environmental changes in regions with upwelling (Rykaczewsky and Checkley, 2008). The Venezuelan crisis began in 2005, but, between 2006 and 2013 (Figure 4), the captures were 373 645 ton (46 706 ton/year on average). It became unneccesay to increase the capture size because a natural fluctuation was perhaps taking place (Gómez, 2016, 2018a), as well as to handle fishing without approaching the ecosystem (García, 2000; Essington et al., 2015). A sardine collapse due to overfishing was cited at the coast (Rueda-Roa et al., 2017). The captures noticeably decreased in comparison with 2003-2004, albeit without a collapse (sensu stricto). The decrease in captures laid the foundations for the current handling. It is beyond understanding that an increase in gears was authorized in 2017 (with 357 in operation). The ordinance must have had good results after two or three years, given that recruitment takes place at six months of age and sexual maturity upon the year (Freón et al., 2003a; Mendoza et al., 2003). The exploited clupeids recovered quickly (Hutchings, 2000).
Between 2015 and 2017, fishing moderately recovered (85 609 ton), and the preliminary cpue calculations seem to prove it, given the increase from 159 to 214 ton/gear/y (Figure 4). Since 2014, the increased fertility has favored an increase in the resource (Gómez et al., 2016), unlike previous years (Gómez et al., 2012; Gómez and Barceló, 2014). Not changing the measures is suggested (González et al., 2016), a delicate matter given the widespread consumption of sardine. The handling has been ineffective, as the captures obtained between 2000 and 2004 have not been recovered (137 237 ton on average) (Figure 3), which may have been achieved by reducing the ring seines and the effort.
The considered fishing periods indicate an increase in the cpue: it was 159 ton/year/y between 1950 and 1973; it increased to 348 in 1974-2005; it decreased to 159 in 2006-2013 (crisis); and it increased to 214 between 2014 and 2017 (Figures 2, 3, 4). Assuming the effort to be the catch per set, between 1973 and 1989, it varied between 27.46 and 54.03 ton/set (Freón et al., 2003b), whereas, in Margarita, between 2003 and 2005, 168 204 ton were captured in 1552 sets (Gómez, 2006), that is, a cpue between 88.94 and 130.10 ton/ set (108.38 ton on average). The effort in Sucre (where the use of purse seines prevails) is unknown. The catch per set may be an indicator of abundance despite the uncertainties (Guzmán et al., 2003) because sardine regroups once the shoal is upset (Freón et al., 2003a).
Sardine captures have been associated with upwelling. By means of regression, it has been related to the winds, and it has been established that captures, effort, and upwelling have a high determination coefficient, even though this is not considered to be a valid proof because the effort employed is not observable without considering the captures, unlike the conventional one. The regression confirms the influence of upwelling with the captures (Freón et al., 2003b). A higher availability of sardine during upwelling has been mentioned, considering the positive correlation between wind and captures (González et al., 2007), although it was previously found to be negative (Trujillo, 1980). Other studies do not verify the correlations between sardine biomass and nutrients and temperature (Cárdenas and Achury, 2000; Rueda-Roa et al., 2017). Fishing researchers find it difficult to relate environmental factors to captures because the upwelling and the influence of the Orinoco alternate their maximums, with a high productivity throughout the year (Freón et al., 1997).
In nine decades of sardine exploitation, a capture of 4 386 758 ton is estimated, which may increase to 5 746 653 ton by adding the underestimation (31 %). This, by comparing the official captures with the production of canned sardine and flour (Trujillo, 1977). Between 1950 and 2017, 4 156 758 ton were fished, a figure lower than the captures of S. brasiliensis in Brazil (5 019 089 ton) and very low in comparison with the fishing (33 million ton) of Brevoortia patronus (Gulf of Mexico) (Table 1). Both of these are exploited in Area 31 FAO. This does not mean that the Venezuelan sardine is underexploited; it evidences deficiencies in fishing figures, as well as a higher underestimation. The variation is due to non-reliable statistics (Nascimiento and Rojas, 1971; Guzmán et al., 2003). The handling of the resource will be faulted as long as the real captures remain unknown.
Table 1 Capture (ton) of sardine Sardinella aurita in Venezuela between 1950-2017 and 2000-2017, compared to the global catch of the species, with S. brasiliensis from Brazil and the Gulf menhaden Brevortia patronus (Gulf of Mexico) in Area 31 (Source FAO, 2020; Froese and Pauly, 2020). Average ton/year in parentheses.
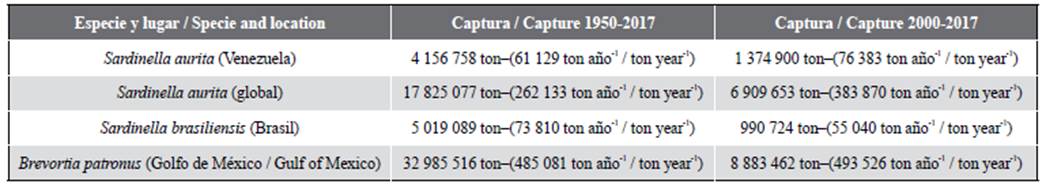
Between 1950 and 2017, the global captures of S. aurita were 17 825 077 ton. In Venezuela, 4 156 758 ton were captured (Table 1), which amounts to 23.3 %. The captures for this century (2000-2017) were 6 909 653 ton and 19.9 % (1 374 900 ton) in Venezuela, despite the crisis. The Venezuelan captures are low considering that sardine is an important food. For example, between 2000 and 2017 Sardina pilchardus and Clupea harengus captures were 19 126 441 and 34 039 392 ton, respectively (FAO, 2020), and those of S. aurita were equivalent to 7.2 and 4.0 percent of the species. Therefore, utilizing a globally relevant sardine resource must be reconsidered (Gastón, 2000), even though it has directly provided the country with food and supplied canneries for almost 90 years. There is a unique fishing wealth in the Caribbean, which is a sea poor in extractable resources (Gómez, 1996, 2001). It is cited that, in Venezuela (2017), 126 400 F of sardine were captured (FAO, 2020). The F represents an estimation, given that no official record was received; in reality, it was 91 565 ton (Figure 4).
The Venezuelan sardine crisis was caused by a change in the ecological regime which affected pelagic production, as verified through plankton. At a local level, a decrease in the phytoplankton biomass took place (Gómez et al., 2014), as well as a variation in the abundance and composition of zooplankton (Gómez 2018a, 2019) (Figure 5). Sardine larvae and juveniles selectively feed on small zooplankton. They filter large phytoplankton cells, but, during the crisis, smaller sizes prevailed (Pinckney et al., 2015), which were inadequate for the recruits. Strong upwellings favor larger phytoplankton, which is adequate for sardine (Gómez, 2015).
It is mentioned that sardine collapsed because, in the warmer months (September-October), the shoals come closer to the coast (< 10 km) and are easily captured, which results in overfishing (Mendoza, 2015; Rueda-Roa et al., 2017). This may have occurred in Sucre, where ring fences (purse seine) were used. In Nueva Esparta, chinchorros are used for fishing up to 3 km off the coast. In 2003-2004, the highest captures were in June-July (Gómez, 2006, 2008), when there was an abundance of phytoplankton (density > 100 000 cells/ml), and the lowest in September-October (Cellamare and Gómez, 2007). Sardine has not collapsed, as indicated by fishing between 2006 and 2014 (Figure 4), given that a population fluctuation occurred due to the regime affecting filtering fish, which was verified by real collapses (Alheit et al., 2009; Jiao, 2009; Deyle et al., 2013; Kelly et al., 2016).
FACTORS THAT ALLOWED THE PERMANENCE OF SARDINE FISHING FOR 90 YEARS AND THE DANGERS TO ITS CONTINUITY
Since 1927, sardines have been caught by means of artisanal fishing with chinchorros and at their mean reproduction size. There have been warnings about the excessive use of ring fences to capture it, a gear that is considered to be artisanal but is instead a purse seine, as is the case of industrial fisheries.
1. Sardine fishing with chinchorros near the coast
In Margarita, fishing has been carried out since pre-Columbian times. Chinchorros, a Mediterranean gear called seine or trawl (jábega or barredera), were shipped to Santo Domingo in 1495. These were common since the 16th century (Marval and Cervigón, 2009), and, as of 1873, 12 of them were in operation (Suárez and Bethencourt, 1994). The islands of Nueva Esparta are located at the center of the upwelling, which explains the abundance of sardine, a reason for the start of fishing and canning activities. There are works about fisheries and vessels (Méndez-Arocha, 1963; Cervigón, 1983; Iriarte, 1997). Given the importance of artisanal fishing, these communities, settlements, and fishing grounds are protected by the Constitution of Venezuela, which, in light of the global fishing crisis, gains value as they are appropriate for sustainability (Marval and Cervigón, 2009); encouraging artisanal fisheries is therefore advised for fishing to be sustainable (Pauly, 2019).
In Nueva Esparta, sardine is captured only with chinchorros (Méndez-Arocha, 1963; Iriarte, 1997; Gómez, 2006; Gómez et al., 2008). Traction used to be manual, but motors have been used since 1950. The gear has a rectangular shape and consists of a central part (sack or codend) and two lateral ones (extension pieces). Its length is over 1000 m, and its height is 20-22 m. Since 1990, fishermen-divers are part of this operation, with scuba equipment to observe the movement of shoals and indicate operations for the setting and transport of the gear towards the beach in order to avoid obstacles or repair the net. The record capture in a set is 1655 ton at 300 m from the coast (Gómez and González, 2008).
The fishermen practice a non-aggressive type of fishing; they wait for the shoals to approach the coast in order to surround them with the chinchorro. If a shoal moves through an area that is over 20 m deep, they do not fish. The gears with the live sardines are transported to a place near the coast, where they are anchored (Figure 6). For around 60 years, chinchorros were the only gear in operation. They are considered to be appropriate for exploitation because, once the capture has taken place, sardines are kept alive in order for their size to be verified by the authorities, who determine if they can be extracted. Otherwise, the sardines are released by raising the chinchorro.
2. Mean reproduction size of sardine (< 17 cm LT)
Fishing authorities accepted that the mean maturity size is 20 cm (FAO, 1963; Guzmán et al., 1998, 1999; Guzmán and Gómez, 2000; Freón and Mendoza, 2003; Mendialdúa, 2004; Gassman, 2005; Tagliafico, 2005). In light of the crisis, they suggested a ban and increasing the fishing size to 20 cm (Tagliafico et al., 2008; Gassman et al., 2012a), which would mean banning sardine fishing altogether, given that the capture size is lower than 20 cm (Gómez, 2018a). Several research works have cited a lower maturity size (Haugen, 1969; Etchevers, 1974; Ramírez and Huq, 1986; Huq and Rodríguez, 1988), including some studies in Margarita, which estimate it to be < 17 cm (Kortnik, 2005; Kortnik and Posada, 2006), as well as some in the Eastern Atlantic (Quaatey and Maravelius, 1999; Gaamour et al., 2004; Tsikliras and Antonopoulou, 2006; Mustac and Sinovcic, 2012). This is explained by the genetic relationship (De Donato et al., 2005), as well as by appropriate sampling (Gómez, 2018a). Consequently, the minimum capture size should be 18 cm.
If the mean reproduction size of sardine were not < 17 cm, an overfishing of the recruitment would have occurred years ago because the capture size (19 cm) is lower than the accepted mean maturity size (~20 cm). After 90 years of exploitation, sardine is still being fished because it breeds at a lower in length than the capture size (Gómez, 2018a). The unbelievable first handling (1973) allowed fishing juvenile sardines (< 15 cm) for 32 years. This resource was not depleted thanks to captures with chinchorros. If the fishing gear had been ring fences, sardine would have been depleted as predicted by the FAO (1963)
3. Intensive fishing with ring machines (purse seine) will cause a sardine collapse
Industrial overfishing caused the depletion of sardine in many places through the use of purse seine (Armstrong and Thomas, 1989; Armstrong et al., 1989; Lluch-Belda et al., 1989; Schwartzlosel et al., 1999). By the end of the last world war, the captures increased until 1968, when the collapses began (Pauly, 2009), and populations were depleted in 1980 (Schwartzlosel et al., 1999). In Venezuela, fishing continued, as industrial fishing was not encouraged (Etchevers, 1974).
Around 1960, the canning industry (12 factories) requested suggestions for increasing the captures. The experts recommended using purse seine with lights (Griffiths and Simpson, 1967) and fishing away from the coast (Odegaard, 1971a, 1971b; Ginés, 1972; Etchevers, 1974; Trujillo, 1975a, 1975b; Gerlotto and Elguezábal, 1986; Gerlotto and Ginés, 1988). Thus, the existence of an underexploited biomass was ensured, which was recognized by scientists, the government, and businesspeople. Moreover, innovations (purse seiners) were recommended, thus expanding the exploitation (Mendoza, 1996) to 250 000 ton/year (Mendoza and Guzmán, 2000).
In 1980 in Sucre nets (machines) of up to 1500 m in length began to be utilized (Novoa et al., 1998), a highly aggressive type of gear considered to be artisanal, albeit a purse seine of up to 400 and 40 m in length and height, respectively (Gaceta Oficial 40308/2013). Their intensive activity both away from and close to the coast (> 60 km) negatively affects the resource because they reach the bottom in the northwestern shelf, as is the case of Los Testigos bank, which is 37 m deep (Maloney, 1971). Captured sardines die in a matter of minutes, thus making it possible for juveniles to prevail, which have recently been incorporated to the pelagic area, which they actively exploit, feeding by filtration (Gómez, 2015). If pre-adults were predominant in these captures, their implementation would be of great harm, given that avoiding juvenile fishing is fundamental for stock sustainability (Gómez and Pérez, 2021). Ring fences must be strictly regulated; if there is any overfishing, it will be caused by intensive fishing with this gear.
Towards the end of the last century, the media informed of the existence of a million tons of sardine: a surplus. In turn, in Brazil, captures were lower than 30 000 ton (Schwartzlosel et al., 1999). The industry requested that measures were taken in Sucre, where non-traditional gear (machines or sacks) were authorized and intensively utilized, with a reduction in the captures and an increase in the effort (Gastón, 2003). As of 1996, 52 gears were in operation (Barrios et al., 2010). In 2014, they increased to 100 (Gaceta Oficial 40573/2015), as well as to 115 in 2017 (Gaceta Oficial 41295/2017), not accounting for 130 illegal gears (González et al., 2016). According to the fishermen, around 400 gears are currently in operation. If this increase is sustained along with the intensive operation of machines, the sustainability of the resource will be compromised and the real collapse will come to be, which may be soon, as the first sardine industrial ship arrived from Spain in 2021 (Prensa MinPesca 19/05/2021).
In 2005, sardine captures started to decrease. There was a fluctuation due to changes in the ecological regime (Gómez, 2018b, 2019) as well as in other filtering species. These are many times associated with overfishing, which did not occur in Margarita (Gómez, 2018a). Moreover, between 2006 and 2013, 373 645 ton (46 706 ton/year) were captured. If the captures are not recovered, the use of ring machines (purse seines) must be limited. So far, fishing with chinchorros has proven to be appropriate for the sustainability of Venezuelan sardine.
CONCLUSIONS
In 90 years of sardine exploitation, the total captures are estimated to be 4 386 758 ton (48 742 ton/year), a modest volume due to deficient statistics.
Sardine fishing was unregulated for 46 years. The first ordinance (1973) set the capture size at 15 cm (juveniles), which was valid for 32 years. The last ordinance (2013) increased the capture size to 19 cm. However, after seven years, the previous captures (2000-2004) were not recovered, thus rendering the authorization of an increase in gears unjustified (357 were in operation in 2017).
The nine-decade continuity of S. aurita fishing is explained by artisanal fishing with chinchorros, as well as by the mean maturity size (< 17 cm). The capture size should be 18 cm. Fresh sardine is the most affordable animal protein for the low-income population. If the intensive use of machines (purse seines) is not limited, the sustainability of the resource is in danger.











 texto en
texto en