NOTE
The Cytochrome Oxidase I gene confirms the presence of the nemertine Carcinonemertes conanobrieni, a parasite of the Caribbean spiny lobster (Panulirus argus) in Colombia
1Grupo de Investigación MIKU, Universidad del Magdalena, Carrera 32 # 22-08, Santa Marta DTHC, Colombia. amyberbenhenriquez23@gmail.com
2Grupo de Investigación MIKU, Universidad del Magdalena, Carrera 32 # 22-08, Santa Marta DTHC, Colombia.
3Grupo de Investigación MIKU, Universidad del Magdalena, Carrera 32 # 22-08, Santa Marta DTHC, Colombia.
4Facultad de Ciencias Básicas, Programa de Biología, Universidad del Magdalena, carrera 32 # 22-08, Santa Marta DTHC, Colombia. sigmerquiroga@unimagdalena.edu.co
ABSTRACT
Morphological identifications complemented with molecular analysis are important for the delimitation of cryptic species. This document contains annotations on the molecular identification of the parasitic nemertine Carcinonemertes conanobrieni in the Caribbean spiny lobsters Panulirus argus. The collected nemerteans were found parasitizing ovigerous lobsters from the Gulf of Salamanca (Puebloviejo), Colombia. DNA was extracted from a specimen and a fragment of the mitochondrial gene Cytochrome Oxidase I (COI) was amplified using Folmer’s universal primers, the sequence was edited with ProSeq 3.5 and aligned with all the C. conanobrieni sequences available in GenBank. A sequence of 488 base pairs corresponding to the section of the genetic barcode of the COI gene was obtained, the matrix of genetic distances showed little variation when comparing the obtained sequence with those available in the GenBank database. These results confirm the presence of this nemertean in the Panulirus argus lobsters in Colombia providing valuable information for the correct identification and detection of this parasitic species in the country, thus facilitating its monitoring.
Keywords: Carcinonemertidae; COI; Puebloviejo.
RESUMEN
Las identificaciones morfológicas, complementadas con análisis moleculares son importantes para la delimitación de especies crípticas. Este documento contiene anotaciones sobre la identificación molecular del nemertino parásito Carcinonemertes conanobrieni en langostas espinosas del Caribe Panulirus argus. Los nemertinos recolectados se encontraron parasitando langostas ovadas del golfo de Salamanca (Puebloviejo), Colombia. Se extrajo ADN de un individuo y se amplificó un fragmento del gen mitocondrial Citocromo Oxidasa I (COI) utilizando los primers universales de Folmer, la secuencia obtenida se editó con ProSeq 3,5 y alineó con todas las secuencias de C. conanobrieni disponibles en GenBank. Se obtuvo una secuencia de 488 pares de bases correspondientes a la sección del código de barras genético del gen COI, la matriz de distancias genéticas mostró poca variación al comparar la secuencia obtenida con las disponibles en la base de datos GenBank. Estos resultados confirman la presencia de este nemertino en las langostas Panulirus argus en Colombia, y aportan valiosa información para la correcta identificación y detección de esta especie parasita en el país, facilitando así su monitoreo.
Palabras claves: Carcinonemertidae; COI; Puebloviejo.
The Caribbean spiny lobster Panulirus argus (Latreille, 1804) is a decapod crustacean distributed in the Caribbean coasts of Colombia, including the insular regions of San Andrés, Providencia and Santa Catalina (Gracia and Díaz, 2002). This lobster is considered one of the most important fishing resources for the populations of artisanal marine fishermen since it has the highest economic value within the crustaceans that are fished in the greater Caribbean and the Gulf of Mexico (Seijo, 2007; FAO, 2015, 2019).
In the departments of Magdalena, La Guajira and the Archipelago of San Andrés, Providencia and Santa Catalina, this lobster is socially and economically important, since its extraction is essential for the livelihood of artisanal fishing communities. However, it is considered as a vulnerable species in Colombia (Gracia and Díaz, 2002; Minambiente, 2017), due to the overexploitation of its fishing stock and the systematic destruction of its habitat. Additionally, there are natural factors that have contributed to the decline of P. argus populations, such as diseases (e.g., Panulirus argus virus 1-PaV1) (Shields y Behringer, 2004) and parasites (e.g., nemerteans) (Atherley et al., 2020a). Specifically, the parasite Carcinonemertes conanobrieniSimpson, Ambrosio and Baeza, 2017 is a nemertean that feeds on the embryos that ovigerous females carry in their pleopods, causing negative effects on the reproductive performance of the lobster (Baeza et al., 2016; Simpson et al., 2017).
In 2018, C. conanobrieni was recorded for the first time on the Caribbean coast of Colombia (Gonzalez-Cueto and Quiroga, 2018), based on the morphological analysis of four individuals found in an ovigerous lobster from the Gulf of Salamanca (Magdalena). However, it was necessary to complement this information with genetic sequence data, essential to delimit cryptic species within the phylum (Sundberg et al., 2016). Furthermore, in the case of nemertean species associated with P. argus, molecular identifications are necessary since in this lobster there are at least two species of the genus Carcinonemertes (Atherley et al., 2020b).
Ovigerous lobsters of the P. argus species were captured by artisanal fishermen from the Gulf of Salamanca (10.97982; -74.32758), Puebloviejo municipality (Magdalena) (Figure 1). Upon examination, nemerteans were isolated at different stages of development; some of which were fixed in 100 % ETOH.
DNA from one of the individuals identified as C. conanobrieni was extracted using the QuickExtract ™ kit following the manufacturer’s protocol (Lucigen). The “Folmer” region was amplified with universal primers (Folmer et al., 1994). DNA extractions were amplified using 2 μL of DNA in a PCR mix with a final volume of 23 μL containing 1.25 μL MgCl2, 5 μL 5X Buffer (BIOLINE), 1 μL of each primer, 0.625 μL of dNTPs, 13.625 μL of H2O and 0.5 μL of Taq polymerase (BIOLASETM, BIOLINE). The thermo-cycler program was: 1 min at 95 °C, followed by 35 cycles of 15 s at 95 °C, 1 min at 40 °C, 1.5 min at 72 °C and a final extension period of 5 min at 72 °C. The sequence obtained was edited with ProSeq 3.5 and aligned with all the C. conanobrieni sequences available in GenBank using the ClustalW algorithm in MEGA (Tamura et al., 2011). Other sequences of Carcinonemertes sp. reported in P. argus by Atherley et al. (2020b) were included in the analysis. A matrix of intraspecific and interspecific evolutionary genetic distances was made (Hebert et al., 2003), using the K2P model (Kimura, 1980).
A sequence of 488 base pairs corresponding to the section of the genetic barcode of the COI gene was obtained, this was deposited in GenBank with the access code MW205898. The sequence obtained, possess a genetic distance that varies between 0 % and 0.2 % when compared with other individuals of C. conanobrieni and 22.5 % with other species of this genus found in P. argus (Table 1).
Table 1 Matrix of interspecific and intraspecific genetic distances based on 488 bp sequences of the Cytochrome Oxidase I (COI) gene using the K2P nucleotide substitution evolution model (Kimura, 1980). The sequence generated in this study is highlighted in bold; the rest of the sequences were extracted from the GenBank genetic sequence database of the “National Center for Biotechnology Information” (https://www.ncbi.nlm.nih.gov/).
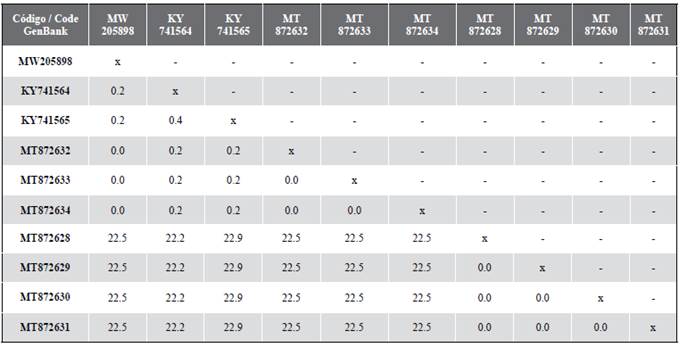
These results lead to the confirmation of the presence of C. conanobrieni in Colombia, since the genetic distance found was less than 0.5 % when compared with other individuals of C. conanobrieni sequenced by Simpson et al. (2017) in their original description of the species. Within the Hoplonemertea group, which contains the genus Carcinonemertes, it is estimated that the interspecific distances are greater than 5 % (Sundberg et al., 2016).
The information herein provides tools that allow for correct identification of the species of nemerteans that can be found parasitizing the lobster. The results of this study indicate monitoring programs should be established to evaluate the effect of C. conanobrieni on the lobster populations of Colombia. In order to maintain the present and future fishing and market values of P. argus, emerging pathogens and parasites must be considered, particularly in light of a changing environment.
ACKNOWLEDGEMENTS
This work was derived from the project “Efecto del parásito de crustáceos Carcinonemertes conanobrieni sobre el desempeño reproductivo de la langosta espinosa del caribe, en un contexto de manejo pesquero participativo en el departamento del Magdalena, Colombia” funded by Fonciencias 2017 of the Universidad del Magdalena, with Permit resolution number 0080 of January 17, 2019 by the Corporación Autónoma regional del Magdalena CORPAMAG. Special thanks to the artisanal fishermen of the Gulf of Salamanca, who helped in obtaining the lobsters specimens.
LITERATURE CITED
Atherley, N.A.M., M.A. Freeman and M.M. Dennis. 2020a. Post-mortem examination of the Caribbean spiny lobster (Panulirus argus, Latreille 1804) and pathology in a fishery of the Lesser Antilles. J. Invertebr. Pathol., 175:107453. https://doi.org/10.1016/j.jip.2020.107453
[ Links ]
Atherley, N.A.M., M.M. Dennis and M.A. Freeman. 2020b. Two species of Carcinonemertes Coe, 1902 (Nemertea: Carcinonemertidae) infesting the Caribbean spiny lobster, Panulirus argus (Latreille, 1804) (Decapoda: Achelata: Palinuridae), in Saint Kitts, West Indies. J. Crust. Biol., 40(6): 933-942. https://doi.org/10.1093/jcbiol/ruaa060
[ Links ]
Baeza, J.A., L. Simpson, L.J. Ambrosio, N. Mora, R. Guéron and M.J. Childress. 2016. Active parental care, reproductive performance, and a novel egg predator affecting reproductive investment in the Caribbean spiny lobster Panulirus argus. BMC Zool., 1(6). https://bmczool.biomedcentral.com/articles/10.1186/s40850-016-0006-6
[ Links ]
FAO. 2015. Report of the first meeting of the OSPESCA/WECAFC/ CRFM/CFMC Working group on Caribbean spiny lobster. FAO Fisheries and Aquaculture Report SLC/FIPS/ SLM/R1095 (Bi), Panama City, Panama. 112 p.
[ Links ]
FAO. 2019. Report of the second meeting of the OSPESCA/WECAFC/CRFM/CFMC Working Group on Caribbean Spiny Lobster. FAO Fisheries and Aquaculture Report, Santo Domingo. 68 p.
[ Links ]
Folmer. O., M. Black, W. Hoeh, R. Lutz and R. Vrijenhoek. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol., 3(5): 294-299.
[ Links ]
González-CuetoJ.A. and S. Quiroga. 2018. First record of Carcinonemertes conanobrieni Simpson, Ambrosio & Baeza, 2017 (Nemertea, Carcinonemertidae), an egg predator of the Caribbean spiny lobster Panulirus argus (Latreille, 1804), on the Caribbean coast of Colombia. Check List, 14(2): 425-429. https://doi.org/10.15560/14.2.425
[ Links ]
Gracia, A. y J. M. Díaz. 2002. Panulirus argus: 113-115. En Ardila, N., G. R. Navas y J. Reyes (Eds.). Libro rojo de los invertebrados marinos de Colombia. Invemar. Ministerio del Medio Ambiente. La serie Libros rojos de especies amenazadas de Colombia. Bogotá. 177 p.
[ Links ]
Hebert, P. D. N., S. Ratnasingham and J. R. de Waard. 2003. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. B. Biol Sci., 270 (Suppl 1): S96-S99. https://doi.org/10.1098/rsbl.2003.0025
[ Links ]
Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol., 16(2):111-120. https://doi.org/10.1007/BF01731581
[ Links ]
Minambiente. 2017. Resolución 1912 de 2017. Por la cual se establece el listado de las especies silvestres amenazadas de la diversidad biológica colombiana continental y marino costera que se encuentran en el territorio nacional, y se dictan otras disposiciones. Ministerio de Ambiente y Desarrollo Sostenible, fecha de expedición: 15/09/2017_ Diario Oficial No. 50364 del 22 de septiembre de 2017.
[ Links ]
Seijo, J.C. 2007. Considerations for management of metapopulations in small-scale fisheries of the Mesoamerican barrier reef ecosystem. Fish. Res., 87(1): 86-91. https://doi.org/10.1016/j.fishres.2007.06.016
[ Links ]
Shields, J. D. and Behringer, D. C. 2004. A new pathogenic virus in the Caribbean spiny lobster Panulirus argus from the Florida Keys. Dis. Aquat. Organ., 59(2):109-118. https://doi.org/10.3354/dao059109
[ Links ]
Simpson, L.A., L.J. Ambrosio and J.A. Baeza. 2017. A new species of Carcinonemertes, Carcinonemertes conanobrieni sp. nov. (Nemertea: Carcinonemertidae), an egg predator of the Caribbean spiny lobster, Panulirus argus. PLoS One, 12(5): e0177021. https://doi.org/10.1371/journal.pone.0177021
https://doi.org/10.1371/journal.pone.0155541
[ Links ]
Tamura, K., D. Peterson, N. Peterson, G. Stecher, M. Nei and S. Kumar. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol., 28(10): 2731-2739. https://doi.org/10.1093/molbev/msr121
[ Links ]












 text in
text in 



