Acropora palmata is a coral species considered globally threatened (Sullivan et al., 1993), included in the Appendix II of CITES. In Colombia, it is included in the Red Book of Threatened Species, cataloged as an endangered species (Ardila et al., 2002; Reyes and Santodomingo, 2002; García-Urueña and Garzón-Machado, 2020). This species has been found to be susceptible to bleaching and diverse coral diseases (Holden, 1996; Smith et al., 1996; Goreau et al., 1998; Green and Bruckner, 2000; Bruckner, 2002; Rosenberg and Bem-Haim, 2002; Sutherland et al., 2004, 2011; Cróquer et al., 2006; Weil et al., 2006; Gil-Agudelo et al., 2009; Navas-Camacho et al., 2010; Vega et al., 2014), particularly white pox disease, which only affects A. palmata (Patterson et al., 2002; Casadevall and Pirofski, 2014; Lamb et al., 2014 ; Muller and van Woesik, 2014; Pollock et al., 2014; Joyner et al., 2015; Sutherland et al., 2016; Young et al., 2020)
In 2005, as part of the National Coral Reef Monitoring System of Colombia (SIMAC in Spanish), in Cinto Bay (Tayrona National Park) (Díaz et al., 2000) a patch of A. palmata (11º 20’ 34.78” N and 74º 03’ 51.21” W), affected by a disease similar in appearance to White Pox (Figure 1). During three occasions, in May and August 2006 and March 2007, photographic monitoring of the lesions and estimation of the associated loss of living coral tissue was carried out. Fifteen (15) lesions were marked with an alphanumeric code and photographs were taken of each one on each date to analyze the area of the internal and external borders of each lesion with the CPC’e program (Kohler and Gill, 2006). Each measurement was repeated three times during each follow-up period. Descriptive statistics were calculated. Shapiro-Wilk’s test was used to test for normality in the distribution of the data and a Kruskal-Wallis test was used to determine if there were significant differences between the three study periods. A Dunn´s multiple comparison test was carried out to determine between which of the three data collection moments the significant differences were present. To characterize the main stages of the lesions, 302 lesions were additionally marked and described in detail.
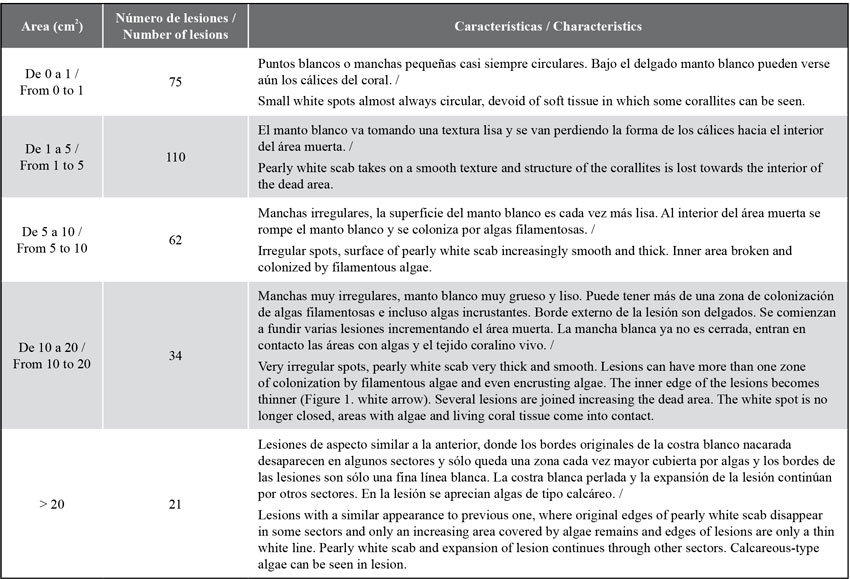
The lesions observed are irregular white spots, with a smooth hard texture like a pearly scab and, as they grow, the center erodes and is covered by algal turfs. As they grow, the lesions change their appearance, join one another and increase in size (see Table 1). Out of the 302 lesions found, 110 (36.4 %) had sizes between 1 and 5 cm2 (Table 1), followed by those with less than 1 cm2, 75 (24.8 %). Lesions larger than 5 cm2 showed the loss of the calyces and the presence of a smooth and pearly layer was evident; lesions larger than10 cm2 showed the loss of the internal area of the coral and its replacement by an algal turf (Figure 1b).
Table 1 Main morphological characteristics, size ranges, and number of lesions per range, observed in the A. palmata formation in Cinto Bay, PNN Tayrona
A maximum growth rate of 0.8 cm2/day (0.4 ± 0.3 cm2/day ) was found. The outer and inner edges of the lesions (Figure 1b) were permanently expanding during the study period. While there were no significant differences in the growth of the inner edges, the outer edges showed differences in the average growth of the lesions (H = 6.87, gl = 2, p < 0.032) between the three evaluation times, (H = 3.89, gl = 2, p = 0.14).
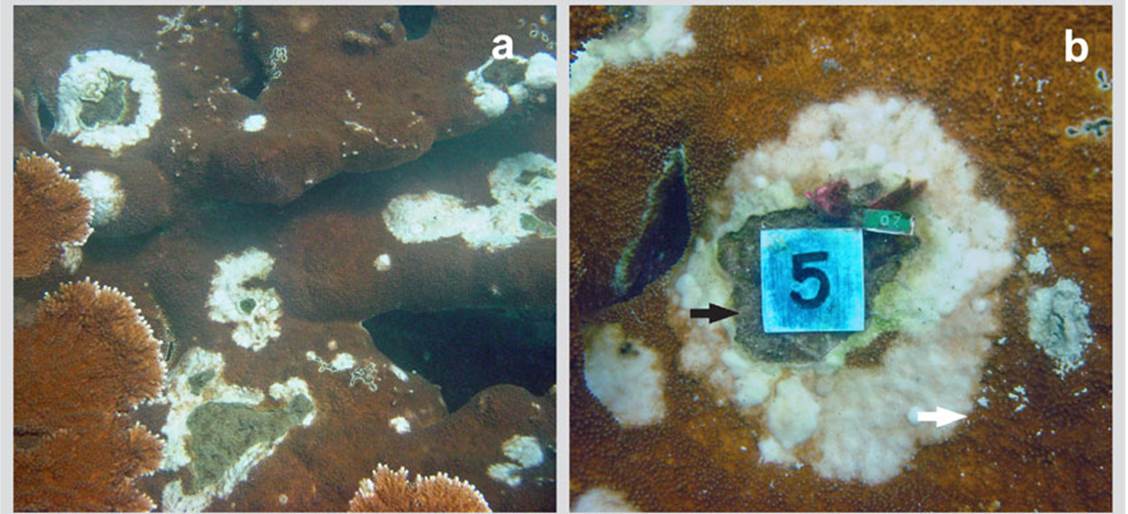
Figure 1 a) Colony of Acropora palmata in the bay of Cinto in 2005 invaded by lesions of a deterioration agent with an appearance similar to that of White Pox. b) Close-up of a lesion with thickening and pearl texture characteristic of these lesions and internal area totally eroded and invaded by turf algae. Red arrow = outer edges; White arrow = inner edges
Initially, the pearly area was wider than the internal area populated by algae; in large lesions the pearly scab area was less wide and the area with algae much larger. If the lesion advances rapidly, the width of the band is larger, if its deterioration speed decreases, the area eroded with algae advance faster narrowing the pearly scab area.
The decrease in temperature in the first months of the year, which lowers to 22 ° C, does not stop or eliminate the disease as seen in other coral diseases. On the contrary, in March 2007 the area of the lesions increased significantly (H = 6.87, p < 0.032) compared to May and August 2006. The multiple range test illustrates the difference between the average growths data recorded in the three moments of data collection (Figure 2). During the study, the lesions continued to grow and spread throughout the coral formation. Even, as observed in the last photo of the sequence (Figure 3), different growing lesions merged, increasing the affected area.
Through the LSD multiple range test, differences between March 2007 and May and August 2006 were observed.
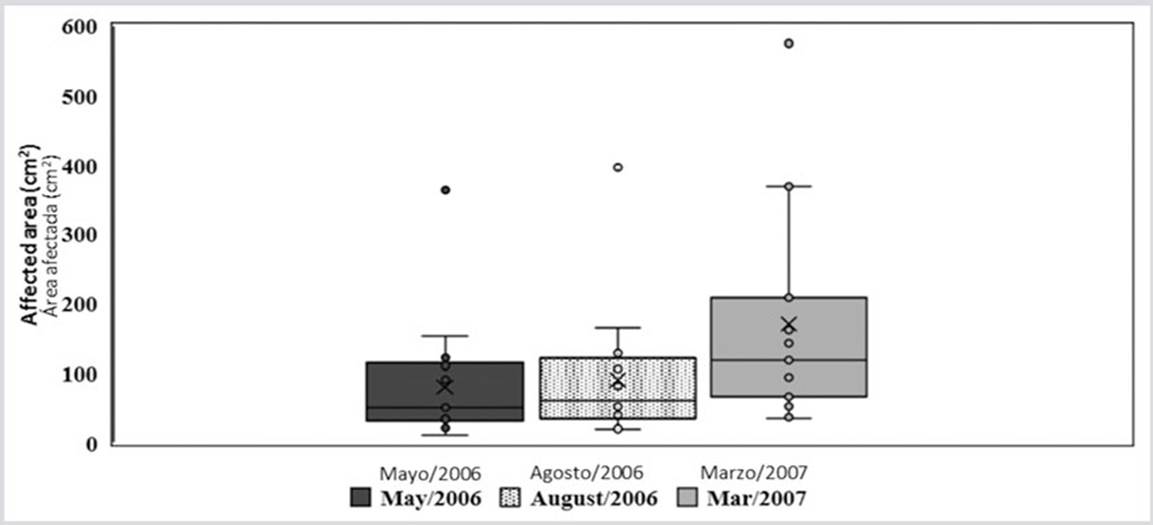
Figure 2 Box and whisker plot showing the averages (x), medium (line inside the box), quartiles (border of the boxes) and the maximum and minimum values (upper and lower points) at the outer edges of the affected areas for each of the sampling periods.
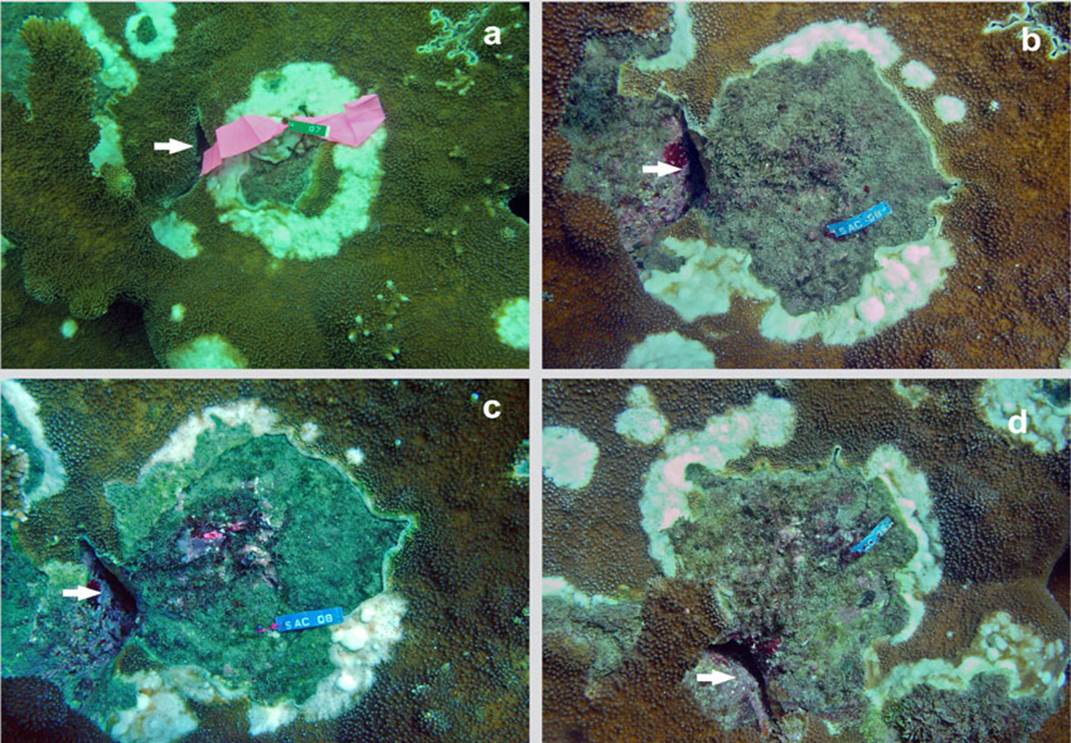
Figure 3 Time series of an injury in Acropora palmata in Cinto, (in 2005 it was registered with tag No. 7. Later it was renamed with tag No. 8. It is recognized by the presence of the crack indicated by the arrow): a) October 2005; b) May 2006; c) August 2006 and d) March 2007.
The difficulty isolating marine pathogens in pure culture (Sutherland et al., 2016), did not allow this disease to be identified. However, due to its appearance, growth form, and the fact that it only affected A. palmata despite the presence of other coral species, we believe that it is likely to be a form of White Pox. White Pox only affects A. palmata while white plague and other white diseases affect several other species, particularly P. strigosa and M. cavernosa.
The prevalence and infectivity of this disease, which did not stop spreading throughout the reef and whose lesions did not stop growing throughout the year during the study period, was evidenced by the large number of lesions with small sizes (N = 185), even in the first months of the year that present colder waters (18-22º C). Usually in the NNP Tayrona, diseases such as the black band and the white plague stop and even disappear during those months of colder waters (R. Navas-Camacho, unpublished data), but the lesions of the disease reported in this study did not stop.
The observed growth rate of this disease at Cinto is low when compared to previous studies of white pox disease in Florida with a growth of up to 10.5 cm2/day (Patterson et al., 2002) and a reported rate of tissue loss of 2.5 cm2/day (Sutherland et al., 2004). The limited growth of lesions in Cinto could be related to the prevailing temperatures (27 °C on average) and that between December and April temperature drops down to 20-22 °C due to local upwelling (Fajardo, 1978; Andrade and Barton, 2005; Petus et al., 2007), even if such drop fails to deactivate the responsible pathogen. A greater number of lesions (46.7 %) presented slow growth (0.1 cm2/day), while only 6.7 % of the lesions grew faster (0.8 cm2/day).
Finally, the prevalence of injury-like coral diseases has been associated with sewage discharges and septic systems (Patterson et al., 2002; Sutherland et al., 2004, 2010; Lesser and Jarett, 2014). During the months of August and September, currents change in the Tayrona NNP (Dimar-CIOH, 2009), observing the plume of the Magdalena River, a probable source of domestic wastewater from the upper Andean basin. Serratia marcescens, the bacteria responsible for the disease most like to what was observed in this study (White Pox disease), is associated with domestic wastewater, so the plumes of the Magdalena River could be a source of that organism. Additionally, the shared responsibility with the parrotfish Sparisoma viride in the incidence of the disease reported here cannot be ruled out, since its feces contain the bacteria S. marcescens (Bruckner, 2002) and it is a fish that was commonly recorded throughout the study, discharging feces close to the branches of the coral Acropora palmata.











 texto en
texto en 



