Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ingeniería y competitividad
versão impressa ISSN 0123-3033
Ing. compet. vol.15 no.2 Cali jul./dez. 2013
Effect of air velocity, pH and frother concentration on vitrinite recovery using column flotation
Efecto de la velocidad del aire, pH y concentración de espumante sobre la recuperación de vitrinita usando flotación en columna
J. Piñeres
Programa de Ingeniería Química, Universidad del Atlántico, Barranquilla, Colombia
E-mail: jorgepineres@mail.uniatlantico.edu.co
J. Barraza
Escuela de Ingeniería Química, Universidad del Valle, Cali, Colombia
E-mail: juan.barraza@correounivalle.edu.co
A. Blandon
Universidad Nacional de Colombia, sede Medellín, Colombia
E-mail: asblando@unalmed.edu.co
Eje temático: Chemical engineering / Ingeniería química
Recibido: 16 de Abril de 2013
Aceptado: 10 de Septiembre de 2013
Abstract
Coal macerals can be separated using flotation process by taking advantage of differences in their surface properties. In this work, the effect of pH, air velocity (Jg) and frother concentration (FC) on the vitrinite separation of two coal samples from Colombia, Cerrejón and Jagua was studied. The experimental runs were carried out by flotation column at pH ranging from 4 to 10, FC from 2 to 6 ml of frother/kg of coal and Jg from 0.7 to 2.1 cm/s Jg. Results showed that both coals exhibited vitrinite recoveries above 60% at pH 5, Jg 1 cm/s and FC of 3 ml/kg of coal. For Cerrejón coal, the highest vitrinite recovery (63.55%) was obtained at pH 7, Jg 0.7 cm/s and FC of 4 ml de frother/kg of coal, whereas for Jagua coal, the highest vitrinite recovery (69.80%) was obtained at pH 4, Jg 1.4 cm/s, and 4 ml of frother/kg of coal respectively. It was also noted that both coals exhibited the highest value of vitrinite recovery at acid conditions compared with those obtained using basic conditions.
Keywords: Flotation column, mass yield, vitrinite concentrates.
Resumen
Los macerales del carbón se pueden separar usando procesos de flotación aprovechando las diferencias de sus propiedades superficiales. En este trabajo, se estudió el efecto del pH, la velocidad del aire (Jg) y la concentración de espumante (FC) sobre la separación de vitrinita de dos minas de carbones del Cerrejón y Jagua (Colombia). Las corridas experimentales se realizaron en una columna de flotación usando pH en el rango de 4 a 10; FC en el rango 2 a 6 ml de espumante/kg de carbón y Jg en el rango de 0.7 a 2.1 cm/s. Los resultados mostraron que ambos carbones presentaron recuperación de vitrinita superiores al 60% a pH 5, Jg 1 cm/s y FC 3 ml/kg de carbón. Para el carbón Cerrejón, la mayor recuperación de vitrinita (63.55%) se obtuvo a pH 7, Jg 0.7 cm/s and 4 ml de espumante/kg de carbón, mientras que para el carbón Jagua, la mayor recuperación de vitrinita (69.80%) se obtuvo a pH 4, Jg 1.4 cm/s and 4 ml de espumante/kg de carbón respectivamente. Se observó también que ambos carbones presentaron los más altos valores de recuperación del grupo vitrinita a condiciones acidas en comparación con las obtenidas a condiciones básicas.
Palabras claves: Columna de flotación, rendimiento másico, concentrados de vitrinita.
1. Introduction
The vitrinite maceral represents one of the most important maceral in coal, due to its high reactivity in coal transformation processes. Vitrinite has high reactivity towards carbonization and liquefaction process, where collotelinite submaceral would likely be responsible for this reactivity. Experimental studies on gasification and combustion show that both, ignition temperature and burning rate depend on vitrinite reflectance, ICCP (1998). Vitrinite is a maceral group composed of polycyclic aromatic compounds, which are very attractive as chemical feedstock to produce valuable chemical specialties and polymers, Schobert & Song (2002), and to be transformed into liquid through the coal-to-liquid (CTL) technology, Longwell et al., (1995).
The vitrinite maceral can be concentrated by flotation, heavy media separation and agglomeration process, flotation being highly selective in order to separate vitrinite due it utilizes very fine bubble, Sarkar (1984), Honaker & Mohanty (1996). Vitrinite can be separated by flotation process by taking advantage of differences in its hidrophobicity compared to liptinite and inertinite macerals, Sun (1954). The air velocity (Jg), pH, and frother concentration (FC) play an important role in vitrinite recovery using flotation column. It is well known that the slurry pH changes the hydrophobicity of maceral particles due to alter their electrostatic energies, Campbell et al., (1970). Jg has big effect on the maceral production and selectivity, whereas FC increases the mass yield, producing a more stable froth.
There are a few studies that have shown the effect of Jg, pH and FC on vitrinite recovery using flotation column at pilot scale in the last years. Honaker & Mohanty (1996) showed that pH has a great significant influence on the floatability of the individual macerals. Xinqian et al (2002) found differences between the properties of vitrinite and inertinite which rise to the possibility of separating single macerals by flotation process, Sarkar (1984). In our laboratory, Barraza & Piñeres (2005) studied the effect of pH and frother concentration on vitrinite maceral concentrates using Colombian coals from south west region of the country. It was noted that the vitrinite maceral in floats is higher than the feed coal, whereas liptinite, inertinite and mineral matter are lower. Vitrinite maceral concentration, dry-mineral matter free basis, obtained is close to 100%.
Information on vitrinite concentrates from Colombia coals in a wide range of pH is scarce to using flotation column. Some works (Honaker 1996, Barraza & Piñeres 2005) obtained float coal fractions having high concentration of vitrinite maceral using a flotation process in basic conditions. The objective of this flotation study was to study the effect of pH (acidic and basic conditions), air velocity (Jg) and frother concentration (FC) on vitrinite maceral concentration of two Colombian coal samples.
2. Experimental
Two Colombian coals, Cerrejón (Guajira) and Jagua (Cesar) were used in this study. Coals were crushed in a ball mill to a particle size of -38 μm to liberate vitrinite maceral (according to the results obtained of the liberation study). Creosote oil, sodium lauryl ether sulfate 28% w/w, sodium hydroxide (NaOH) 1.7M and hydrochloric acid (HCl) 3.3M reagent grade were used as a collector, frother and pH modifiers respectively.
A flotation column of 5 m height and 0.05 m diameter was used. A single separation stage was carried out using a slurry concentration of 2.5 % w/w and a collector concentration of 3.4 kg/t of coal. Air velocities ranging from 0.7 to 2.1 cm/s were used. The pH and the frother concentrations were used in the range 4 to 10 and 2 to 6 ml of frother/kg of coal respectively. All runs were carried out using a single separation stage. The tails flow was adjusted according to air flow, guaranteed a constant interface level at 23 cm above the feeder position, while the frother mixed with air, was added by means of a peristaltic pump at a flow of 42.2 ml/min. Details of the flotation column operation are reported by Piñeres & Barraza (2012). A 23factorial central composite experimental design, Montgomery (1999); was used in this work, where the effect of the conditions used in column flotation on vitrinite recovery for two coal samples were evaluated. Independent variables selected for the experimental design were the pH of the slurry, frother concentration and the air velocity (with which it adjusts of air flow). Details of the experimental design are reported by Piñeres & Barraza (2012).
The pH was chosen as the independent variable by its influence on coal surface properties, while the frother concentration and air velocity were selected because of their influence on the hydraulic of the column flotation and the bubble diameter. The experimental error is based on repeated experiments at the central points of experimental design and was ± 5%.
3. Results and discussion
3.1 Coal analysis
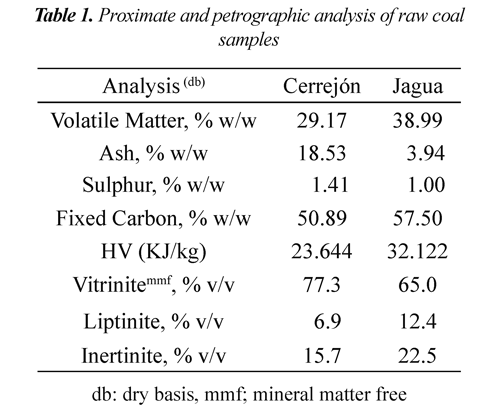
Table 1 shows the proximate and petrographic analysis of raw coals. Cerrejón coal presented higher ash and sulphur content, whereas Jagua coal showed higher heat high value. In general, both coals have high vitrinite content (<65% v/v) and low liptinite content (>12.4% v/v). It is observed that the heat value is related to ash content. The Jagua coal had higher heat value due to its lower ash percentage.
3.2 Effect of pH, Jg and FC on mass yield, ash and maceral content of float
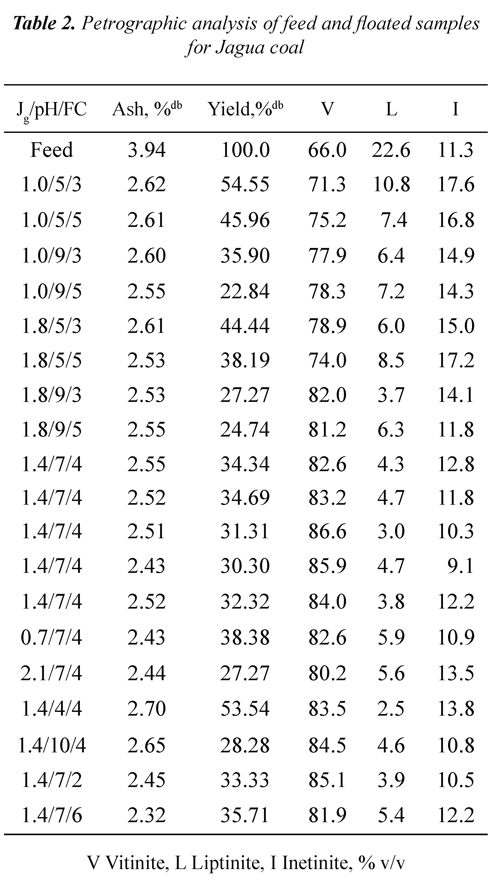
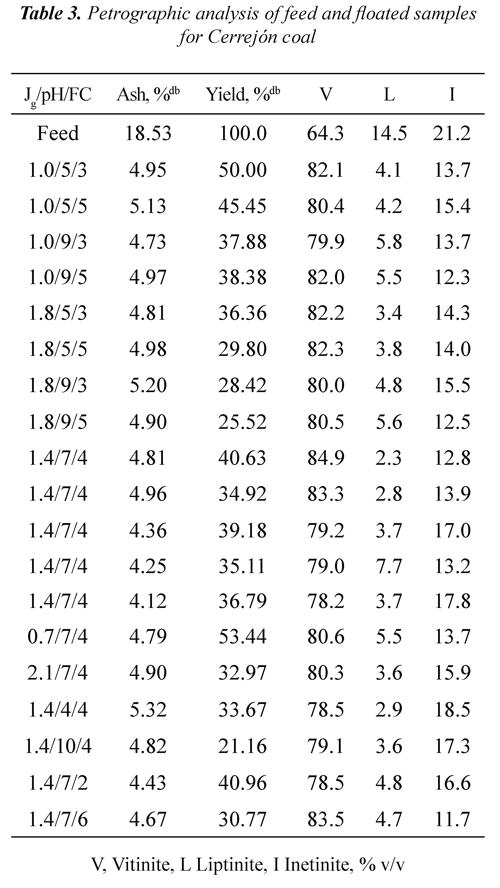
Tables 2 and 3 show ash, mass yields and petrographic analysis of feed and concentrates (floated) coals at different Jg, pH and FC for both coals. The results showed that in general the ash contents of the concentrates are lower than the ash percentage of the feed coal (3.94% for Jagua and 18.53% for Cerrejón coal), indicating that the db: dry basis, mmf; mineral matter freeflotation process used is selective for separating the mineral matter of the organic matter. It can be seen that the lowest ash percentage (2.32% for Jagua and 4.12% for Cerrejón) was obtained at pH 7, Jg of 1.4 cm/s, and FC of 6 and 4 ml of frother/kg of coal respectively.
It can be observed from Tables 2 and 3 that for both coals the mass yield was obtained in the range of 21 to 54%. For the Jagua coal, the highest mass yield it was 54.55% and for Cerrejón coal was 53.44%, both obtained at pH 5 and 7, Jg 1.0 and 0.7 cm/s, and FC 3 and 4 ml of frother/kg of coal respectively. Those results may be associated with an increased coal hidrophobicity obtained at these pH conditions, improving the rate of particle - bubble adhesion. It can be also be seen that for the two coals, the mass yield decreases as the pH increases, which may be associated with the ionization of the oxygen functional groups, increasing the wettability through hydrogen bonds with water molecules and the precipitation of some mineral species.
It is worth pointing out that the obtained mass yield for the two coals is affected by oxidation on the coal surface, as a result of the formation of a coal - oxygen - water complex, which has acidic properties, Xu & Yoon (1989), Muse & Gulhan (1995), Stachurski & Tora (2001), Vamvuka & Agridiotis (2001). Mineral species, occurrence mode (syngenetic or epigenetic) and mineral matter liberations during the milling process may produce significant differences in the ash content of the coals, Fuerstenau et al., (1983).
Tables 2 and 3 show that, in general for all range of Jg, pH, FC and the two coals used, the floated presented higher vitrinite concentration compared to the feed coal. At pH 7, air velocity of 1.4 cm/s and 4 ml of frother/kg of coal, the Jagua coal showed an increase in vitrinite concentration from 66.0 % v/v in the feed to 86.6% v/v in the floated, whereas the Cerrejón coal raised its vitrinite concentration from 64.3 % v/v to 84.9% v/v. Data in Tables 2 and 3 were used to determine the vitrinite recovery as a function of Jg, pH and FC.
3.3 Effect of pH, Jg and FC on vitrinite recovery
Vitrinite recovery (VR, % w/w) was obtained by using the following equation:

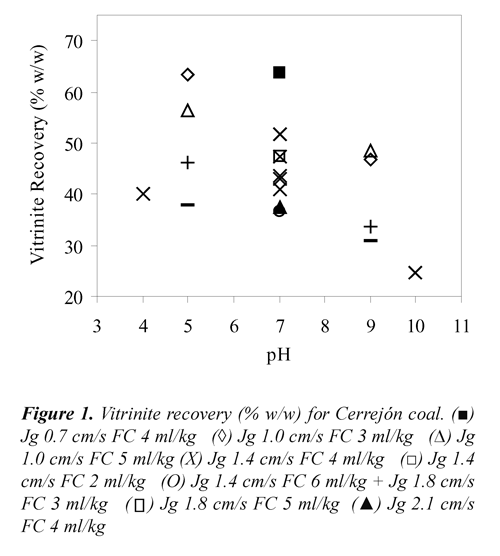
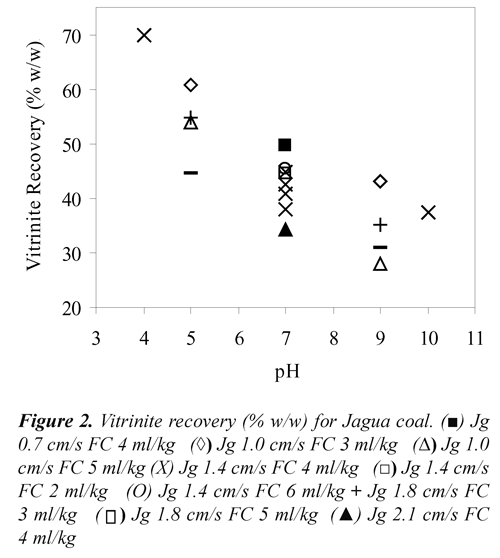
Where, Yflaot, MMfloat, MMfeed, Vfloat and Vfeed stand for mass yield (% w/w), mineral matter (% v/v) and vitrinite percentage (% v/v) of floated and feed coals. To convert the percentage of vitrinite density of 1.3 g/cc was used, Kizgut et al.,(1995). The vitrinite recovery as a function of Jg, pH and FC for the two coal samples are presented in Figures 1 and 2. These results showed that both, Cerrejón and Jagua coals have vitrinite recovery higher than 60% at pH 5, Jg 1.0 cm/s and 3 ml of frother/kg of coal.
The highest vitrinite recovery for Cerrejón coal (63.6%) was obtained at pH 7, Jg 0.7 cm/s and 4 ml of frother/kg of coal, whereas for Jagua coal was 69.80% at pH 4, Jg 1.4 cm/s and the same FC. As shown in Figures 1 and 2, both coals presented high vitrinite recovery under acidic condition (pH 4 and 5) compared to those obtained under basic conditions (pH 9 and 10). This means that under acidic conditions, vitrinite maceral is more hydrophobic, producing both high adhesion coal particle - bubble and vitrinite recovery. Under basic conditions, a strong adsorption of water (electrostatic interaction) would be formed, due to the presence of carboxyl, carbonyl and phenolic polar groups, with the water molecules producing a stable wet film on the coal surface (maceral), and therefore, the coal surface becomes more hydrophilic. Xu & Yoon (1989), Muse & Gulhan (1995), Stachurski & Tora (2001), Vamvuka & Agridiotis (2001), Miller et al., (1992), Miller et al (1993), Tao et al., (2002), Polat et al., (2003), Jena et al., (2008), Piñeres & Barraza (2011).
On the other hand, FC also has an important effect on the vitrinite recovery. As can be seen in Figures 1 and 2, high values of vitrinite recovery were obtained at 3 and 4 ml of frother/kg of coal, which may be due to the interaction between functional groups such as [-OH],[-COOH], [C=O], [-C-O-C], and the positive charge of the Na+ ion that belong to the frother. It has been reported, Bozena (1987), Arnold & Aplan (1989) that highvitrinite recovery is caused by interaction between the oxygen of the negatively charged frother and the cations of mineral matter associated with the vitrinite maceral.
Regarding the effect of Jg, Figures 1 and 2 shows that, for both coals high vitrinite recovery is obtained at low air velocity (1.0 cm/s). This may be due to the small bubbles produced, which have high surface area, increasing the vitrinite - bubbles collision probability, Yoon, (1993), Diaz-Penafiel & Dobby (1994).
3.4 Statistical analysis
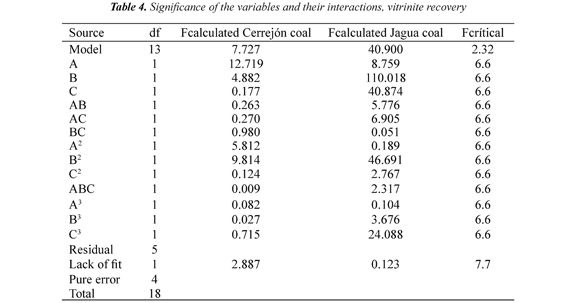
Experiments in the flotation column were carried out according to the experimental design. Nineteen experimental runs were conducted for each coal sample. The data vitrinite recovery was analyzed statistically using Design Expert software. The results of the significance of the variables and their interactions are presented in Table 4. The nomenclature is: Vitrinite recovery RV, A = air velocity (Jg), B = pH, C = frother concentration (FC). The model equations obtained are:

Tables 4 show that the model has a significant (95% confidence level) effect (Fcalculated> Fcritic), while for the lack of fit shows no significant effect (Fcalculated< Fcritic), indicating that the regression models were found to be highly adequate for accurate prediction of the response variables, Montgomery (1999).
Vitrinite recovery: Table 4 shows that for vitrinite recovery, pH has a significant effect and negative on Jagua coal. Likewise, pH has significant effect for the quadratic terms in the two coal samples, for Jagua coal have a positive effect, while for the Cerrejón coal show a negative effect, Montgomery (1999).
These results indicate that changes in pH (acidic or basic) directly affect the vitrinite recovery. For Jagua and Cerrejón coals, with pH increase decrease the vitrinite recovery. These results may be attributed to the coal surface charge changes with the change in pH; this discussion refers to the electrokinetic study of the samples, Piñeres & Barraza (2011). The curvature effects (quadratic) for Jagua and Cerrejón coals shows that increasing pH decreases the vitrinite recovery, this decreases is possibly due to the depression of the coal particles with high content of vitrinite at high pH values, this indicates that there a pH value, where the vitrinite recovery is maximum, as shown in Figure 2, Piñeres & Barraza (2011), Piñeres & Barraza (2012).
The air flow is significant and negative in Cerrejón and Jagua coals, indicating that high air flow it generates higher bubble sizes, which change the flow regime and deteriorate the flotation process, decreasing the vitrinite recovery. Finally, the frother concentration has a significant effect and negative in the Jagua coal, showing that increasing the frother concentration decreases the vitrinite recovery, Montgomery (1999).
The frother concentration is significant and positive in the cubic term in Jagua coal, indicating an effective interaction between the frother and the surfaces of the bubble and the coal, which increases the floatability. The significance of pH, Jg and FC can be seen from the values of Fcalculated and Fcritic with 95% confidence level, Montgomery (1999).
4. Conclusions
pH, air velocity and frother concentration had a significant effect on the vitrinite recovery by flotation column. Both coals, Jagua and Cerrejón, showed high vitrinite recovery at low values of Jg, FC under acidic conditions, which is due to the interaction between the oxygen negatively charged of the frother and the electrostatic energy attraction between the vitrinite maceral and the air bubble. For Cerrejón and Jagua coals the largest increases vitrinite maceral occurred in a pH 7, air velocity of 1.4 cm /s (1.27 mm for bubble diameter) and frother concentration of 4 ml/kg of coal. Both coals, Jagua and Cerrejón the liptinite maceral content was increased under basic conditions.
5. Acknowledgements
The authors would like to acknowledge the support of Colombian Institute of Science and Technology (Colciencias) through the Project "Separation of Colombian coal maceral using flotation column," 1106-06-17318 and financial support given to the author within doctoral training program Colombian students.
6. References
Arnold, B., & Aplan, F. (1989). Hydrophobicity of coal macerals. Fuel, 68, 651-658. [ Links ]
Barraza, J., & Piñeres, J., (2005). Apilot-scale flotation column to produce beneficiated coal fractions having high concentration of vitrinite maceral. Fuel, 84, 1879-1883. [ Links ]
Bozena, B. (1987). Studies on floatability of maceral of bituminous coal of various coali.cation degrees. Fuel Processing Technology, 16, 191 - 204. [ Links ]
Campbell, J.A.L. & Sun, S.C., (1970). Bituminous coal electrokinetics. Trans. AIME, 247, 111. [ Links ]
Diaz-Penafiel, P., & Dobby, G. (1994). Kinetic studies in flotation columns: bubble size effect. Mineral Engineering, 7, 465 - 478. [ Links ]
Fuerstenau, D., Rosenbaum, J., & Laskowski J. (1983). Effect of surface functional groups on the flotation of coal. Colloid and Surface, 8, 153- 174. [ Links ]
Honaker, R., & Mohanty, M. (1996). Enhanced column flotation performance for fine coal cleaning. Mineral Engineering, 9, 931 - 945. [ Links ]
International Committee for Coal and Organic Petrology (ICCP). (1998). The new vitrinite classi.cation (ICCP System 1994). Fuel, 77, 349 - 358. [ Links ]
Jena, M., Biswal, S., & Rudramuniyappa, M. (2008). Study on flotation characteristics of oxidized Indian high ash sub-bituminous coal. International Journal of Mineral Processing, 87, 42 - 50. [ Links ]
Kizgut, S., Miles, N., & Cloke, M (1995). Production of coal maceral concentrates by flotation. Coal Science and Technolog y, 24, 1553 - 1556. [ Links ]
Longwell, J., Rubin, E., & Wilson, J. (1995). Coal: Energy for the future. Progress Energy Combustion Science, 21, 269- 360. [ Links ]
Miller, J., Jensen, G., Yu, Q., & Yea, Y. (1992). Selective flotation of fossil resins from Western coal, Final Report, U.S. Department of Energy. Report No. DEACE907822-90PC90178. Washington D.C.: U.S. Department of Energy. [ Links ]
Miller, J., Yu, Q., Buyya, K., & Ye, Y. (1993). Analysis of selective resinite flotation from Wasatch plateau coal by pH control. Coal Preparation, 13, 31 - 51. [ Links ]
Montgomery, D. C. (1999). Diseño y análisis de experimentos. México D.F.: Grupo Editorial Iberoamérica. [ Links ]
Muse, S., & Gulhan, O. (1995). Flotation characteristics of oxidized coal. Fuel, 74, 2, 291 - 294. [ Links ]
Piñeres, J., Barraza, J. (2011). Energy barrier of aggregates coal particle-bubble through the extended DLVO theory. International Journal of Mineral Processing, 100, 14-20. [ Links ]
Piñeres, J., Barraza, J. (2012). Effect of pH, air velocity and frother concentration on combustible recovery, ash and sulphur rejection using column flotation. Fuel Processing Technology, 97, 30-37. [ Links ]
Polat, M., Polat, H., & Chander, S. (2003). Physical and chemical interactions in coal flotation. International Journal of Mineral Processing, 72, 199 - 213. [ Links ]
Sarkar, G. (1984). Selective of coal macerals during flotation and oil agglomeration: A case study. Coal Preparation, 1, 39 - 52. [ Links ]
Schobert, H., & Song, C. (2002). Chemical and materials from coal in the 21st century. Fuel, 81, 15-32. [ Links ]
Stachurski, J., & Tora, B. (2001). The investigation on coals flotation. Memorias V Congreso Nacional de Ciencias y Tecnología del Carbón (pp. 325-334). Valledupar, Colombia. [ Links ]
Sun, S. (1954). Hypothesis for different floatabilities of coals, carbons, and hydrocarbon minerals. Transactions AIME, 199, 67 - 75. [ Links ]
Tao, D., Li, B., Johnson, S., Parehk B. (2002). A flotation study of refuse pond coal slurry. Fuel Processing Technology, (76), 201-210. [ Links ]
Vamvuka, D., & Agridiotis, V. (2001). The effect of chemical reagents on lignite flotation. International Journal of Mineral Processing, 61, 209-224. [ Links ]
Xinqian, S., Zuna, W., & Jingqiu. (2002). Separation and preparation of macerals in Shenfu coals by flotation. Fuel, 81, 495 - 501. [ Links ]
Xu, Z., & Yoon, R. (1989). The role of hydrophobic interactions in coagulation. Journal of colloid and interface science, 132, 532 - 541. [ Links ]
Yoon, R. (1993). Microbubble flotation. Mineral Engineering, 6, 619-630. [ Links ]

Revista Ingeniería y Competitividad por Universidad del Valle se encuentra bajo una licencia Creative Commons Reconocimiento - Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.