Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ingeniería y competitividad
versão impressa ISSN 0123-3033
Ing. compet. vol.16 no.2 Cali jul./dez. 2014
Experimental and mathematical evaluation of molecular adsorption models for organic pollutants on TiO2-P25 particles
Evaluación experimental y matemática de modelos de adsorción molecular para contaminantes orgánicos en partículas de TiO2-P25
Karen S. Ochoa-Gutiérrez
E-mail: kochoag@unicartagena.edu.co
Miguel Angel Mueses
E-mail: mmueses@unicartagena.edu.co
Photocatalysis and Solar Photoreactors Engineering, Department of Chemical Engineering, Universidad de Cartagena, Cartagena, Colombia
Eje temático: CHEMICAL ENGINEERING / INGENIERíA QUíMICA
Recibido: 14 de mayo de 2014
Aceptado: 13 de octubre de 2014
Abstract
In this work we evaluated conventional models based on adsorption isotherms of L2 type for organic compounds: DCA, phenol and 4-chlorophenol over TiO2-P25 particles to different operational conditions. The models studied were Langmuir-Hinshelwood, Redlich-Petersen and Toth because these models can be applied as possible equations to improve the description of the reaction rate mechanism based on hydroxyl radical attack, and the degradation using heterogeneous photocatalysis. Finally the models were compared with experimental data for determination of the more appropriate isotherm. L-H model was the more appropriate and the results were high effective in the prediction of experimental data.
Keywords: TiO2-P25 photocatalyst, Organic compounds: DCA 4-Chlorophenol and Phenol, L2 isotherm.
Resumen
Se evaluaron modelos convencionales de adsorción molecular basados en isotermas del tipo L2 para compuestos orgánicos ácido dicloroacético, fenol y 4-clorofenol sobre partículas de TiO2-25 a diferentes condiciones de operación. Los modelos estudiados fueron Langmuir-Hinshelwood, Redlich-Petersen y Toth puesto que estos modelos pueden ser aplicados como posibles ecuaciones para mejorar la descripción de los mecanismos de velocidad de reacción basados en ataque de radicales hidroxilo y degradación usando fotocatálisis heterogénea. Los modelos fueron comparados con datos experimentales para determinar la isoterma más adecuada. El modelo L-H fue el mas apropiado y los resultados fueron de alta efectividad en la predicción de los datos experimentales.
Palabras Clave: Fotocatalizador TiO2-P25, Compuestos orgánicos: DCA, fenol y 4-clorofenol, Isoterma L2.
1. Introduction
The rapid development of industrialization has carried the increase of pollution of one of more important natural resources, the water. Many pollutants discharged inside hydric sources cannot be biological treatment by conventional methods. In the present, the Advanced Oxidation Technologies (AOT's) are an alternative for wastewater treatment due to the mineralization capacity of recalcitrant compounds (Chong, et al., 2012). The photocatalysis is contained in AOT's, which occur by electron-holes generation on the semiconductor surface for effect of radiation energy absorption with specific wavelength (Chong, et al., 2010).
The photocatalysis has five fundamental steps: i) molecular adsorption of: water, hydroxyl ions, oxygen and reactive compounds (molecules of substrate) [Ri], ii) radiation energy absorption for semiconductor and electron-holes generation (activation), iii) Recombination, iv) capture of photogenerated species (electron-holes), v) hydroxyl radical attack (see Table 1) (Turchi & Ollis, 1990).
In this mechanism the main way of the reaction is the oxidation for cracking of carbon-hydrogen bonds by hydroxyl radicals attack, which are generated for charge transfer to the holes h+ from semiconductor adsorbed species (hydroxyl ions or water molecules).
The hydroxyl radicals can interact in different ways: with the solid surface by irreversible reactions; generation of transition reactions with adsorbed molecules; or migrate to the interface inside Helmholtz layer and finally react with free compounds. The trapped holes by organic molecules are considered with low reactivity; therefore, the main attack is by effect to hydroxyl radicals in the Helmholtz layer. Meanwhile, the capture of electrons occur by interaction of active sites from solid with adsorbed molecules of oxygen to generate superoxide radical (Mueses, et al., 2013).
In this mechanism, the molecular adsorption (of water and hydroxyl ions) is the limiting factor of the hydroxyl radical generation process. This is due because the adsorbed species are charge carriers for photogenerated holes (Linsebigler, et al., 1995). Experiments have shown that the probability of organic compounds adsorption is low in comparison with the processes that involve water and hydroxyl ions (Fujishima, et al., 2008). However, although we considered that adsorption of contaminant molecules is low compared with adsorbed water and hydroxyl ions, it is essential to quantify amount of molecules from organic substrate which are adsorbed on the semiconductor surface. This consideration is very important because the molecules on the catalyst surface involve the active sites available loss for activation via photoexcitation. In addition we require knowing the real initial concentration after dark phase and before of photodegradation; this prevents erroneous estimates in quantification of the mineralization global yields.
Conventionally, the quantification of adsorbed molecules is made using isotherms based on mathematical model of empirical expressions with adjustment parameters. An adsorption model represents the amount of adsorbent adhered to the material surface in function of the initial concentration (in adsorption/desorption equilibrium) or concentration in function of the time (in a kinetic model). The literature presents different expressions for isotherms, which vary according to the number of theoretical or empirical parameters. Some of these are: Langmuir (Gomes da Silva, 2003) (Giraldo, 2010), Freundlich (Yousef, et al., 2011), Redlich-Peterson (Piccin, et al., 2009), Tempkin (Khaled, et al., 2009), Toth (Senthil Kumar, et al., 2010) and Dubinin-Radushkevich, among others. These equations can be used for prediction of fluid molecular adsorption over solid suspended particles inside of specific operational conditions (Allen, et al., 2003).
The concentration of adsorbed reactant has a relation with the concentration fluid and depends of temperature, pH, surface semiconductor area and reactive nature. The temperature is kept constant during the measurements of global equilibrium concentration in the determination of an adsorption isotherm.
For description of heterogeneous photocatalysis molecular adsorption, different isotherm models have been used in different compounds: nitric oxide NO, TCE in gas phase, herbicides, cresols, chlorobenzene, chlorophenols, methyl and dimethyl amines and dyes such as auramine-O, carmine indigo and black remazol-5 (RB5), among others (Hunger, et al., 2010) (Demeestere, et al., 2004) (Kaneco, et al., 2009) (Vulliet, et al., 2003) (Pulido Melián, et al., 2007) (Huang, et al., 2008) (Pino & Encinas, 2012) (Helali, et al., 2011) (Vasanth Kumar, et al., 2007) (Barka, et al., 2008). However, none of these models include molecular adsorption in the mathematical structure of the kinetic expression, thus the initial concentration quantification is not considered in the light phase. The main interest of this paper is to choose a semi-empirical mathematical model for description of the molecular adsorption for some organic pollutants in solution over TiO2-P25 particles, and find the theoretical relation between the adsorption process and the heterogeneous photocatalytic mechanism based on hydroxyl radical attack.
2. Methodology
Selection of the adsorption isotherm model
Langmuir-Hinshelwood, Redlich-Peterson and Toth models have been considered to be more appropriate for description of the molecular adsorption step in heterogeneous photocatalysis. Theorically, these models of equilibrium follow the molecular transport in the semiconductor/solution interface represented in Helmholtz layer model. In general the three models are consistent with thermodynamic and dynamic of the interface. The main mechanism of these processes includes: i) Diffusion of key compound from bulk unto boundary of Gouy-Chapman layer, ii) molecular transport through Helmholtz layer, iii) molecular transport from boundary Helmholtz layer unto the catalyst surface and iv) adsorption over the surface (Linsebigler, et al., 1995).
Langmuir-Hinshelwood model (L-H)
Langmuir-Hinshelwood model is commonly used to describe the heterogeneous photocatalytic kinetic (Kim, et al., 2008) (Guillard, et al., 2008) (Sagawe, et al., 2005), although the mathematical development has been formulated to conventional catalytic processes and not by photocatalysis. The model is applied in adsorption process over flat surface (homogeneous) and it is thermodynamically consistent in the equilibrium of adsorption/desorption for the minimization of Gibbs energy in the process of adhesion between the molecule and the catalyst surface. The model follows the Henry's law to dilution conditions of adsorbate, but it has a deviation at high concentration. Although this is the main limitation on the kinetic of heterogeneous photocatalytic processes (illuminated phase), however, the molecular adsorption in dark phase is described satisfactorily.
The model shown in the eq. 1 was originally developed by Irving Langmuir to represent the gas-solid adsorption, but subsequently extended to solid-liquid systems. It was assumed that the surface containing the adsorption sites is perfectly flat without corrugations (homogeneous surface), the adsorbent is in a stable state, all sites are equivalent, adsorption occurs in a single layer (monolayer) and no interaction between adsorbate molecules and adjacent sites (Allen, et al., 2004).
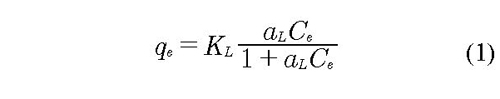
Where KL and aL are adsorption constant with units of (cm3/g) and (cm3/mg), respectively; qe is quantity of adsorbate in equilibrium by mass unit of adsorbent (mg/g) and Ce is the adsorbate concentration (mg/cm3).
Redlich-Peterson model
Redlich-Peterson model is also thermodynamically consistent in the phenomena of adsorption/desorption on the surface. The model represents a fictitious advantage in compared to Langmuir-Hinshelwood model. The predictions of the molecular adsorption can be effective both at high and low concentrations and therefore in principle corrects errors of Langmuir-Hinshelwood and Freundlich, however, the model has three parameters (see Eq. 2), one more that Langmuir-Hinshelwood, whereby it is not appropriate to establish a comparison numerically. The third parameter is an advantage in the adjustment of the experimental data but this parameter is not a representation of the physics the system. Redlich and Peterson introduced the three parameters to the isotherm for represent adsorption equilibriums in a wide range of concentrations (Pérez, et al., 2007). The mathematical expression is:
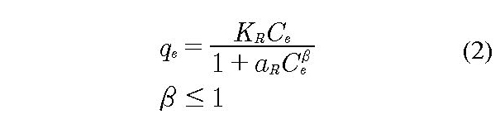
Where KR and aR are adsorption constants with units of (cm3/g) and (cm3/mg); qe is concentration of adsorbate in the equilibrium by mass unit of the adsorbent (mg/g); Ce is the initial concentration of adsorbate (mg/cm3) and β is adjustable parameter. This equation reduces to a lineal isotherm at low surface coverage, the Freundlich isotherm at a high adsorbate concentration and L-H isotherm if β = 1.
Toth model
Finally, Toth model is a combination of two models: one predicts well at low concentrations and another at high concentrations to obtain an acceptable adjustment in a wide range of concentrations. This model was obtained from energy potential theory, which is applicable to the adsorption over heterogeneous surface and satisfies numerically the limitations present to high and low concentrations of the adsorbate. The model provides a good description for several systems with coverage sub-monolayer because consider the dependency of the monolayer with the temperature and introduce an empirical constant in function of the temperature of the system (Arias, et al., 2009). The Eq. 3 describes the model:
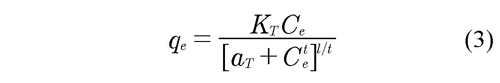
Where qe is the final concentration in equilibrium per mass of adsorbent (mg/g), Ce is initial concentration of adsorbate (mg/cm3); KT is a constant of the model in function of the temperature (mg/g), and aT and t are adjustment parameters of the model, which do not have physical meaning.
Experiments
Materials and equipment
The tests were done using phenol (PH), 4-chlorophenol (4-CP) and dichloroacetic acid (DCA) from Merck® analytic grade as substrates of the system. Titanium dioxide (TiO2-P25) from Degussa-Evonik (98%: 75% Anatase and 25% Rutile) was used as particle of adsorption. In addition HCl and NaOH from Merck® analytic grade were used to modify the pH in the solutions. The experimental data was obtained in a cylindrical glass vessel PYREX® inside of dark chamber to avoid contact between radiant light and catalyst particle. In addition the system was equipped with magnetic stirrer to guaranty the suspension of the particles (see Fig. 1). The analysis was done using a spectrophotometer SHIMADZU UV-1800.
Experimental procedures
The experimental test were done to ten values of initial concentration of the substrate (30, 50, 70, 90, 110, 130, 150, 180, 250, 300 mg/L) at pH 4, charge of catalyst of 0.1, 0.35, 0.5 and 1.0 g/L, and room temperature (29 ± 2°C) (Satuf et al., 2005; Mueses et al., 2013; Mueses & Machuca-Martínez, 2013). The spectrophotometer was calibrated following Beer-Lambert law with solutions of pure components each 10 mg/L in intervals between 20 and 300 mg/L. In a typical test for molecular adsorption we prepared the solution with the key compound, it was carried to previously defined operational conditions and it is stirred; subsequently the catalyst is added and the system keeps in stirrer during 12 hours. In the final time, the sample was filtered and it sent to analysis using the spectrophotometer (Mueses & Machuca-Martínez, 2013).
Parameter determination for molecular adsorption models
The model parameters were obtained using an optimization algorithm of nonlinear least squares coupled to modified Newton-Raphson algorithm with a damping parameter from Broyden (Mueses, et al., 2013). The method involves the use of ordinary derivatives of objective functions for each parameter of the model. The objective functions are obtained from experimental data and mathematical structure of the models. In this paper the derivatives function were quantified by application of elementary calculus from Taylor series. In the Table 2 we showed the mathematical expressions for residual functions (objective functions) for each model.
This mathematical optimization is used because the linearization techniques do not generate appropriate adjustments; in general, the linearization techniques have good performance only if the experimental data are lineal in the intervals where are applied. If the experimental data behaviors are of high order (high concentration for example) the adjustments are not correct. However a nonlinear model predicts more precisely the experimental data (Mueses et al., 2013).
The system solution was done using the multivariable Newton-Raphson method. For each residual function, fψ, we established non-lineal systems equations; for an iteration (m):
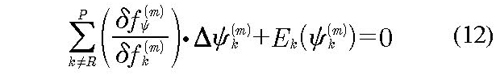
In the equation, fψ corresponds to derivative function from residual discrepancies functions with respect to each parameter ψ in the iteration (m), Δψk(m) is the solution for k parameter of the equation, Ek(ψk(m)) is evaluation of residual functions, fψ in the iteration (m) and the summation corresponds to the Jacobian of residual functions. The lineal solution is:
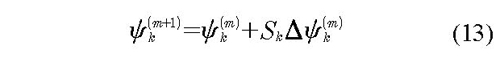
Here Sk is damping coefficient. In this paper we have considered a coefficient of Broyden type, which is generated by minimization of the Euclidian Norm obtained from the vector of residual discrepancies functions (Mueses & Machuca-Martínez, 2013). The value of Sk has two fundamental restrictions:
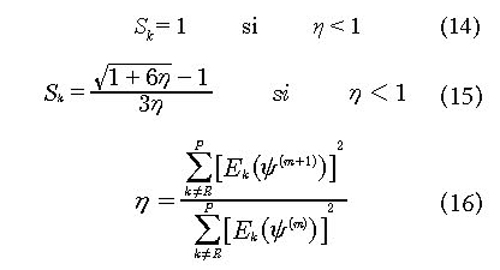
The use of the Broyden parameter Sk in this proposal allows continuous monitoring of the convergence path, besides having a direct mathematical functionality with the residual equations, reflecting high effective numerical stability and convergence to the solution of the system (Mueses & Machuca, 2013).
3. Results and discussion
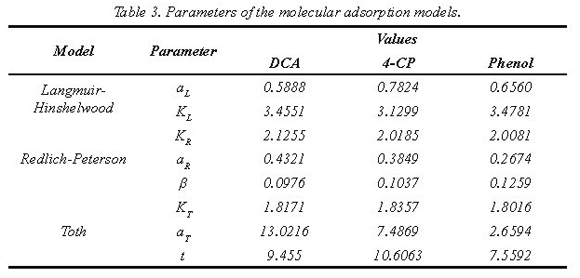
The parameters obtained for each molecular adsorption model are presented in the Table 3. The observations suggest that Langmuir-Hinshelwood & Redlich-Peterson have global constants with orders of magnitude less than the adsorption constants. This phenomenon involves high adsorption of organic compounds over TiO2-P25 particles.
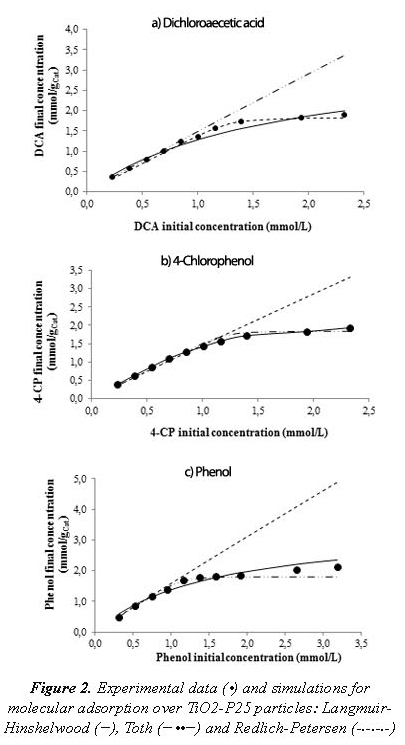
In the Figure 2 (a, b and c) we presented the experimental and simulation data obtained by application of the model and the optimization algorithm at optimal concentration of catalyst equal to 0.35 g/L of TiO2 used in solar heterogeneous photocatalytic processes, according reports by Mueses et al. (2013) & Colina et al. (2009).
In these results, the Langmuir-Hinshelwood isotherm and Toth described better the adsorption of the pollutants, but we considered that Langmuir-Hinshelwood is much appropriate because follows the transport molecular mechanisms inside the interface semiconductor/solution represented in the layer Helmholtz model, moreover this is amply used in the reaction rate expression of the heterogeneous photocatalytic processes (Mueses et al, 2013). Besides, Langmuir-Hinshelwood has one less parameter in comparison with Toth in the formulation.
Turchi & Ollis (1990) & Mueses et al (2013) showed that the photocatalytic mechanism more accepted is based on hydroxyl radical attack in the interface semiconductor/solution (Helmholtz layer). The generated mathematical procedure for application of pseudo-stable state involves as result the inclusion of a mathematical term similar to Langmuir-Hinshelwood function, with a modification by effects of luminous flux and synergic contribution of quantum yield and LVRPA (Mueses, et al., 2013). These results confirm the validity to use Langmuir-Hinshelwood model as a molecular adsorption mechanism for correct these effects over the kinetic of reaction rate in the photocatalysis process. This correction improves the description with respect to the organic compounds covering the free surface of the semiconductor and therefore improves the prediction of obtained experimental data, using only two parameters in a wide interval of the concentrations where photocatalysis is conventionally applied. The results support the validity of the Langmuir-Hinshelwood model in this processes type (Mueses & Machuca, 2013).
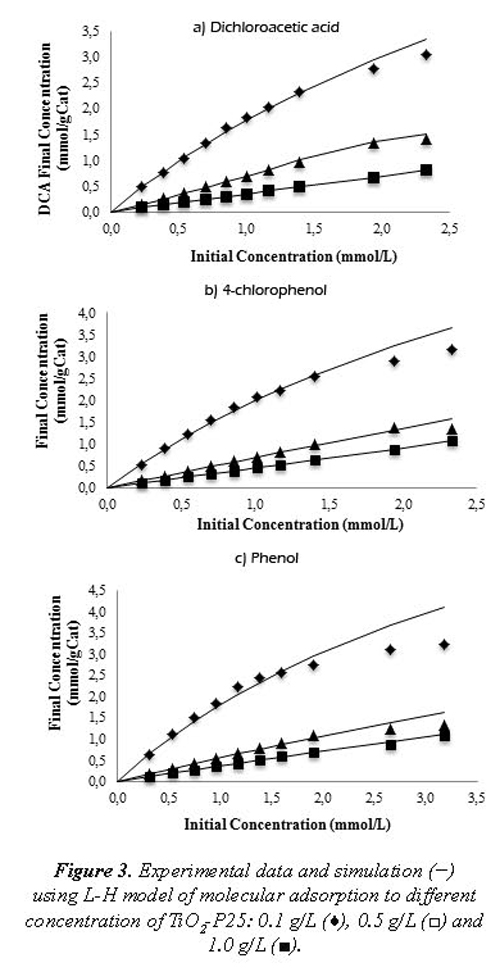
Furthermore, in the Figure 3 (a, b and c) we represented the performance of the molecular adsorption in function of the catalyst concentration both experimental data and the Langmuir (or Langmuir-Hinshelwood) model. We observed that the increase the catalyst concentration generates reductions on the molecular adsorption using a similar initial concentration of the pollutant and increases of the molecular adsorption with the increase of the substrate initial concentration. The predictions of the Langmuir-Hinshelwood model are effective for these operational conditions. It also is evidence a inflection point from the curve takes an asymptotic performance, which represents diminutions in the molecular adsorption due the active sites of the catalyst are occupied by water molecules, molecular oxygen, hydroxyl ions or organic compounds. These results are consistent with typical performance of L2 isotherms (Chen, et al., 1995). The adsorptions of halogenated organic compounds over particles (e. g. DCA) are represented with L2 isotherm, which is concave in relation with solute concentration axes. For this performance is consider appropriate the adsorption isotherms of the Langmuir type, Langmuir of double site, Langmuir-Freundlich and Tóth, among other, whereby even though the Langmuir model has limitations frequently at high concentrations, in this case the adjustment is effective. The adsorption at different values of the initial concentration of the catalyst showed that for high concentrations of TiO2 the adsorption is unfavorable due to the agglomeration phenomena and this cause a reduction of specific surface available to the activation in a photoreaction (Bekkouche, et al., 2004).
The obtained results showed that to concentrations less to 0.35 g/L, the adsorption presents asymptotic trends due to the saturation of active sites by high adsorption, while to concentration higher than 0.35 g/L, the performance of the curves presents lineal variation in function of catalyst concentration, even to the high initial concentration of substrate. These results support the saturation surface theory, which indicates that to high concentration of pollutants but low catalyst concentration the active sites are saturated; if now the catalyst concentration is increased these effects are not important due to the increase of available active sites. Finally, the results obtained in this research permit to confirm that Langmuir-Hinshelwood model can be used both molecular adsorption prediction in dark phase (before of photoreaction) and as a heterogeneous photocatalytic kinetic model using a modification with relation of photonic effect and quantum yield parameter (Mueses, et al., 2013).
4. Conclusions
The selection of a molecular adsorption model was made from semi-theoretical models based on adsorption isotherms of L2 type. The evaluation and validation of the selection were done with experimental data of molecular adsorption for DCA, phenol and 4-chlorophenol over TiO2-P25 particles (Degussa-Evonik titanium dioxide) to different operational conditions. We obtained that the -Hinshelwood model is the better because the model permits the prediction of experimental data with high effectiveness in dark phase using only two adjustment parameters, in addition the theory selection involves the consistent with the molecular transport around of Helmholtz layer close to the surface.
5. Nomenclature

6. Acknowledgments
The authors thanks to Universidad de Cartagena and Universidad del Valle for support the publication of this paper. Mueses thanks to COLCIENCIAS for financial of doctoral studies.
7. References
Allen, S., Gan, Q., Matthews, R., & Johnson, P. (2003). Comparison of optimised isotherm models for basic dye adsorption by kudzu. Bioresource Technology, 88, 143-152. [ Links ]
Allen, S., Mckay, G., & Porter, J. (2004). Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. Journal of Colloid and Interface Science, 280, 322-333. [ Links ]
Arias, J. M., Paternina, E., & Barragan, D. (2009). Adsorción física sobre sólidos: aspectos termodinámicos. Química Nova, 32, 1350-1355. [ Links ]
Barka, N., Assabbane, A., Nounah, A., & Aît Ichou, Y. (2008). Photocatalytic degradation of indigo carmine in aqueous solution by TiO2-coated non-woven fibres. Journal of Hazardous Materials, 152, 1054-1059. [ Links ]
Bekkouche, S., Bouhelassa, M., Hadj Salah, N., & Meghlaoui, F. (2004). Study of adsorption of phenol on titanium oxide (TiO2). Desalination, 166, 335-362. [ Links ]
Chen, H. Y., Zahraa, O., Bouchy, M., Thomas, F., & Bottero, J. Y. (1995). Adsorption properties of TiO2 related to the photocatalytic degradation of organic contaminants in water. Journal of Photochemistry and Photobiology A: Chemistry, 85, 179-186. [ Links ]
Chong, M. N., Jin, B., Chow, C. W., & Saint, C. (2010). Recent developments in photocatalytic water treatment technology: A review. Water research, 44, 2997-3027. [ Links ]
Chong, M. N., Sharma, A. K., Burn, S., & Saint, C. P. (2012). Feasibility study on the application of advanced oxidation technologies for decentralised wastewater treatment. Journal of Cleaner Production, 35, 230-238. [ Links ]
Demeestere, K., Visscher, A., Dewulf, J., Van Leeuwen, M. & Van Langenhove, H. (2004). A new kinetic model for titanium dioxide mediated heterogeneous photocatalytic degradation of trichloroethylene in gas-phase. Applied Catalysis B: Environmental, 54, 261-274. [ Links ]
Fujishima, A., Zhang, X., & Tryk, D. A. (2008). TiO2 photocatalysis and related surface phenomena. Surface Science Reports, 63, 515-582. [ Links ]
Giraldo, A. L., Peñuela, G. A., Torres-Palma, R. A., Pino, N. J., Palominos , R. A., & Mansilla, H. D. (2010). Degradation of the antibiotic oxolinic acid by photocatalysis with TiO2 in suspensión. Water research, 44, 5158-5167. [ Links ]
Gomes da Silva, C. & Faria, J. L. (2003). Photochemical and photocatalytic degradation of an azo dye in aqueous solution by UV irradiation. Journal of Photochemistry and Photobiology A: Chemistry, 155, 133-143. [ Links ]
Guillard, C., Bui, T. H., Felix, C., Moules, V., Lina, B., & Lejeune, P. (2008). Microbiological disinfection of water and air by photocatalysis. Comptes Rendus Chimie, 11, 107-113. [ Links ]
Helali, S., Puzenat, E., Perol, N., Safi, M. J., & Guillard, C. (2011). Methylamine and dimethylamine photocatalytic degradation-Adsorption isotherms and kinetics. Applied Catalysis A: General, 402, 201-207. [ Links ]
Huang, H., Tseng, D., & Juang, L. (2008). Heterogeneous photocatalytic degradation of monochlorobenzene in water. Journal of Hazardous Materials, 156, 186-193. [ Links ]
Hunger, M., Hüsken, G., & Brouwers, H. J. H. (2010). Photocatalytic degradation of air pollutants - From modeling to large scale application. Cement and Concrete Research, 40, 313-320. [ Links ]
Kaneco, S., Li, N., Itoh, K. K., Katsumata, H., Suzuki, T., & Ohta, K. (2009). Titanium dioxide mediated solar photocatalytic degradation of thiram in aqueous solution: Kinetics and mineralization. Chemical Engineering Journal, 148, 50-56. [ Links ]
Khaled, A., El Nemr, A., El-Sikaily, A., & Abdelwahab, O. (2009). Treatment of artificial textile dye effluent containing Direct Yellow 12 by orange peel carbon. Desalination, 238, 210-232. [ Links ]
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40, 1361-1403. [ Links ]
Linsebigler, A. L., Lu, G., & Yates, J. T. (1995). Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chemical Reviews, 95, 735-758. [ Links ]
Mueses, M., Machuca-Martínez, M., & Li Puma, G. (2013). Effective quantum yield and reaction rate model for evaluation of photocatalytic degradation of water contaminants in heterogeneous pilot-scale solar photoreactors. Chemical Engineering Journal, 215-216, 937-947. [ Links ]
Pérez, N., Sánchez, M., Rincón, G., & Delgado, L. (2007). Study of the behavior of metal adsorption in acid solutions on lignin using a comparison of different adsorption isotherms. Latin American Applied Research, 37, 157-162. [ Links ]
Piccin, J. S., Vieira, M. L. G., Gonçalves, J. O., Dotto, G. L., & Pinto, L. A. A. (2009). Adsorption of FD&C Red No. 40 by chitosan: Isotherms analysis. Journal of Food Engineering, 95, 16-20. [ Links ]
Pino, E., & Encinas, M. V. (2012). Photocatalytic degradation of chlorophenols on TiO2-325mesh and TiO2-P25. An extended kinetic study of photodegradation under competitive conditions. Journal of Photochemistry and Photobiology A: Chemistry, 242, 20-27. [ Links ]
Pulido-Melián, E., González-Díaz, O., Araña, J., Doña-Rodríguez, J. M., Tello-Rendón, E., & Herrera-Melián, J. A. (2007). Kinetics and adsorption comparative study on the photocatalytic degradation of o-, m- and p-cresol. Catalysis Today, 129, 256-262. [ Links ]
Sagawe, G., Brandi, R. J., Bahnemann, D., & Cassano, A. E. (2005). Photocatalytic reactors for treating water pollution with solar illumination: A simplified analysis for n-steps flow reactors with recirculation. Solar Energy, 79, 262-269. [ Links ]
Senthil-Kumar, P., Ramalingam, S., Senthamarai, C., Niranjanaa, M., Vijayalakshmi, P., & Sivanesan, S. (2010). Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination, 261, 52-60. [ Links ]
Kim, S.-H., Ngo, H. H., Shon, H. K., & Vigneswaran, S. (2008). Adsorption and photocatalysis kinetics of herbicide onto titanium oxide and powdered activated carbon. Separation and Purification Technology, 58, 335-342. [ Links ]
Turchi, C. S., & Ollis, D. F. (1990). Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. Journal of Catalysis, 122, 179-192. [ Links ]
Vasanth Kumar, K., Porkodi, K., & Selvaganapathi, A. (2007). Constrain in solving Langmuir Hinshelwood kinetic expression for the photocatalytic degradation of Auramine O aqueous solutions by ZnO catalyst. Dyes and Pigments, 75, 246-249. [ Links ]
Vulliet, E., Chovelon, J.-M., Guillard, C., & Herrmann, J.-M. (2003). Factors influencing the photocatalytic degradation of sulfonylurea herbicides by TiO2 aqueous suspension. Journal of Photochemistry and Photobiology A: Chemistry, 159, 71-79. [ Links ]
Yousef, R. I., El-Eswed, B., & Al-Muhtase, A. H. (2011). Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chemical Engineering Journal, 171, 1143-1149. [ Links ]

Revista Ingeniería y Competitividad por Universidad del Valle se encuentra bajo una licencia Creative Commons Reconocimiento - Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.