Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ingeniería y competitividad
versão impressa ISSN 0123-3033
Ing. compet. vol.17 no.1 Cali jan./un. 2015
Microbiology cell-staining wastewater treatment using TiO2 thin films
Tratamiento de agua residual de microbiología usando películas delgadas de TiO2
Jorge A. Fernández*,**
E-mail: jorge.fernandez@javeriana.edu.co
Magda G. Cardozo*
E-mail: guadalupecardozo@gmail.com
Ana K. Carrascal*
E-mail: acarrasc@javeriana.edu.co
Juan C. Salcedo***
E-mail: salcedo.juan@javeriana.edu.co
Aura M. Pedroza*
E-mail: apedroza@javeriana.edu.co
Carlos E. Daza****
E-mail: cedazav@unal.edu.co
* Grupo de Biotecnología Ambiental e Industrial, Pontificia Universidad Javeriana, Bogotá DC, Colombia.
** Grupo de Fitoquímica, Pontificia Universidad Javeriana, Bogotá, DC, Colombia.
*** Grupo de Películas Delgadas y Nanofotónica, Pontificia Universidad Javeriana, Bogotá DC, Colombia.
**** Departamento de Química. Universidad Nacional de Colombia, Bogotá DC, Colombia.
Eje temático: CHEMICAL ENGINEERING/INGENIERIA QUíMICA
Recibido: febrero 02 de 2014
Aceptado: febrero 18 de 2015
Abstract
Microbiology cell-staining wastewater was treated by UV/TiO2 thin films photocatalysis. A simple method of applying gravity sedimentation over glass-type substrate was used for the preparation of the films. The use of Na4P2O7, microwaves, and ultrasounds were studied for decreasing the TiO2 grain sizes on the films. It was established that the best method for reducing grain size resulted from a combination of Na4P2O7 (0.01M) and microwave radiation (700 W, 20 min). The Films were characterized by several microscopic and spectroscopic methods. Anatase phase (gap energy of 3.2 eV) and grain sizes averaging 83 nm were achieved. Photocatalysis tests using TiO2-films showed 99.5 % of decolorization, 79 % TOC abatement, and total microbial inactivation after 14 h of treatment. No bacteria re-growth was found 48 h after the treatment was completed.
Key words: Bacteria, decolorization, mineralization, photocatalysis, wastewater.
Resumen
La fotocatálisis con UV/TiO2 usando películas delgadas fue empleada para el tratamiento de agua residual de microbiología. Se empleó un método simple de sedimentación por gravedad sobre sustrato de vidrio para la preparación de las películas. El uso de Na4P2O7, microondas y ultrasonido fue estudiado para la disminución de los granos de TiO2 en las películas. Se estableció que el mejor método para disminuir los agregados resultó de una combinación de Na4P2O7 (0.01M) y radiación de microondas (700 W, 20 min). Las películas fueron caracterizadas por métodos microscópicos y espectroscópicos. Se obtuvo la fase anatasa (brecha de energía de 3.2 e.V) y tamaños de grano de 83nm. Los ensayos fotocatalíticos utilizando las películas de TiO2 generaron un 99.5% de decoloración, 79% de remoción de COT y una inactivación microbiana total luego de 14 h de tratamiento. No se encontró reactivación microbiana luego de 48 h de finalizado el tratamiento.
Palabras clave: Agua residual, bacteria, decoloración, fotocatálisis, mineralización.
1. Introduction
Alternative water treatment technologies such as Advanced Oxidation Processes (AOPs) have attracted worldwide attention as sustainable technologies, widely used for the removal of organic pollutants not treatable by conventional techniques due to their high chemical stability and/or low biodegradability (Bandala et al., 2008, Oller et al., 2011). Among AOPs, photocatalysis aligns with the "zero" waste scheme in water/wastewater treatments, with a number of important features that have extended their feasible applications in water treatment including mineralization of refractory organic compounds, water pathogens and disinfection by-products (Chong et al., 2010, Foster et al., 2011). TiO2 as heterogeneous semiconductor is the most active photocatalyst under the photon energy of 300 nm < λ < 390 nm, with high efficiency, high physical and chemical stability, low toxicity, commercial availability and low cost (Carp et al., 2004, Chong et al., 2010, Gümüş and Akbal, 2011). The degradation of pollutants is achieved by the formation of hydroxyl radicals (•OH) highly reactive and non-selective and other reactive oxygen species (ROS) that mineralize compounds to carbon dioxide and water (Oller et al., 2011).
New achievements in TiO2 thin films have promoted its extensive application in photocatalytic water treatment mainly by avoiding agglomeration of the catalysis during operation. This methodology proved to more efficient, practical and with better disinfection efficiency that colloidal or particulate TiO2 suspension (Benabbou et al., 2007). At present, a limited number of methods are used to prepare TiO2 thin films that include chemical and physical techniques (Carp et al., 2004, Chong et al., 2010, Gümüş and Akbal, 2011). The sedimentation technique represents a feasible way to prepare TiO2 thin films due to its simplicity and low cost. However, a non-uniform substrate coating is obtained due to aggregate formation and uncontrolled surface morphologies caused by electrostatic attraction forces, which is highly detrimental in terms of particles size preservation, surface-area reduction, its reusable lifespan and a decrease in overall efficiency (Chong et al., 2010, Jiang et al., 2009). In view of this, a simple procedure of TiO2 thin films preparation was designed in order to reduce the formation of aggregates by combining certain parameters that could increase particle repulsion and stimulates mechanical destruction of agglomerates.
On the other hands, biological stains are used in laboratories. These chemical compounds are used continually in tests performed for the identification and diagnosis proposes. The dyes used in the staining process include triphenyl-methane and azo-dyes such. Mordants and solvents are also used in different techniques like Gram staining. However, the collected liquid residuals after staining processes results in the formation of wastewater of high toxicity, presence of microorganisms, low light transparency and high organic carbon content (Sioi et al., 2006). Some of those compounds such as crystal violet and malachite green are known for being toxic and carcinogenic, causing health disorders like mutations, cytotoxicity, neurotoxicity, and chromosomal aberrations (Oplatowska et al., 2011). Due to the high toxicity and mutagenicity of these reagents, its degradation is an indispensable step before discharging or even recycling. This study focuses on the evaluation of a novel TiO2 thin film elaborated by a simple and inexpensive method and the degradation and disinfection efficiency in photocatalysis in real cell-staining microbiology wastewater. The degradation of organic matter reflected in total organic carbon (TOC) and color removal as well as bacterial inactivation of several existing microbial populations in the wastewater and the durability of the systems in post-irradiation events are evaluated.
2. Experimental
2.1 Wastewater samples and characterization
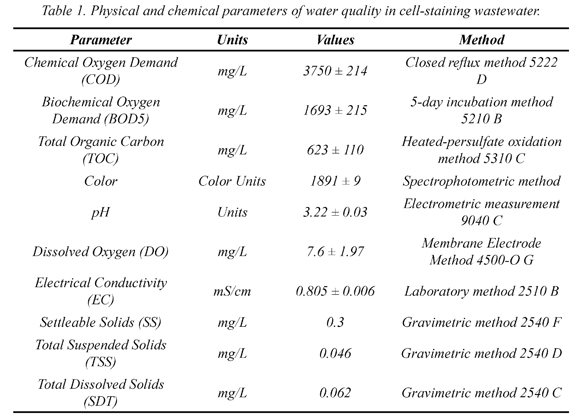
The wastewater comes from different microbiology laboratories where cell-staining techniques are performed constantly. It might consist of a combination of different triphenyl-methane dyes such as fuchsine and crystal violet, solvents and mordants like ethanol, acetone and lugol's iodine as reagents of Gram staining as the most extensively performed test in microbiology. Also, other organic compounds like malachite green, Safranin O, Congo red and methylene blue might also be present. Physical and chemical characteristics of the wastewater were determined. The analyses were carried out following the procedures outlined in Standard Methods (Table 1).
Furthermore, in order to approach the bacterial community structure of the wastewater, isolations in plating media were performed. The samples were analyzed for heterotrophic and coliform bacteria in Plate Count Agar and Violet Red Bile agar (Scharlau, Spain), Gram-positive cocci in Baird-Parker (Merk, Germany), bacilli in Starch Media supplemented with polymyxin B (Oxoid, England), and Pseudomonas spp. in Cetrimide (Scharlau, Spain). The colonies were counted after incubation at 35 °C for 24 h and the populations were enumerated and expressed as log 10 (CFU/mL). In addition, to estimate the presumptive evidence of the presence of Pseudomonas spp,. typical Pseudomonas-like colonies were examined under 360 nm ultraviolet radiation and a oxidase test strip was performed to the colonies that showed fluorescence (Oxoid, England). Likewise, representative colonies in Starch Media showing hydrolysis in the plates were selected and evaluated by Gram staining. Gram-positive amylolytic colonies were estimated to potentially belong to the Bacillus spp. genus. Finally, an analysis of aerobic endospores was performed (Standard Methods 9218B). All samples were plated in duplicate and a microscopical characterization was carried out on the basis of Gram staining.
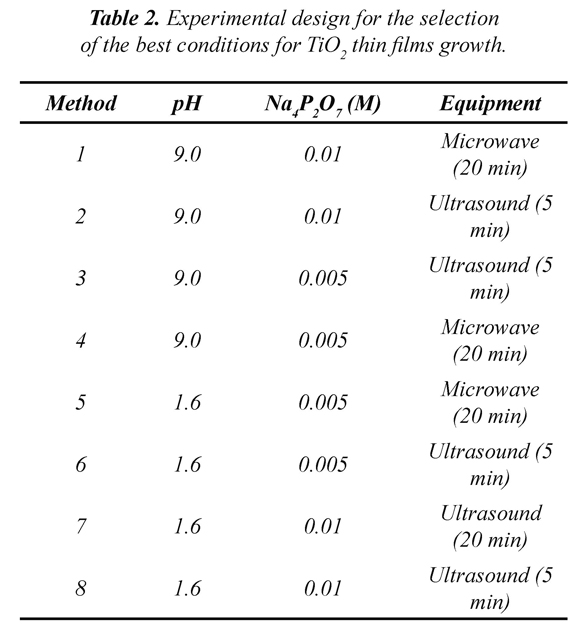
2.2 TiO2 thin films preparation and characterization
TiO2 USP Industrial grade was employed. In order to filter grain sizes (100 nm), this material was settled by gravity fourfold before use. In order to improve the effective surface of the films, in terms of aggregates formation (grain sizes), a number of experiments were performed establishing the best method for the films preparation (Table 2). A TiO2 suspension in deionized H2O was prepared (0.05 % w/v). The suspension was adjusted to different pH values (1.6 and 9.0) and Na4P2O7 (0.01 or 0.005 M) was added. Then, the suspension was either microwave irradiated for 20 min (conventional microwave, 700 W) or sonicated for 5 min (90 W, Fisher-Scientific FS20) (Jiang et al., 2009, Paušová et al., 2011). Films were elaborated using soda lime glasses (26 X 20 mm) as substrate, which were previously immersed in a solution of H2SO4/H2O2 (3:1 v/v) in order to clean the surface. Two layers of TiO2 were deposited on the substrate by simple gravity sedimentation. Films were thermally treated at 450 °C for 1 h.
The films topography was analyzed using Atomic Force Microscopy (AFM) (Nanosurf Easy Scan 2 AG) in contact mode, in order to establish the best conditions for photocatalyst preparation according to homogeneity of film surface. TiO2 thin films obtained by the chosen method were characterized by Scanning Electron Microscopy (SEM) in a JEOL JSM 6490-LV electron microscope. X-ray diffraction was measured in a Siemens D-5000 diffractometer, employing an anode of Cu Kα. UV-VIS spectra was determined using a quartz tungsten-halogen lamp (240-800 nm) coupled with a mono-chromator (Acton Research spectral pro 775), interface Lab view software® was used to process the data. FT-IR measurements were performed in Shimadzu IR prestige 21 equipment using the KBr pellets method. Raman analysis was performed in Labram equipment (Internal laser of He-Ne of 20 mW, λ = 400 nm).
2.3 Description of the reactor and experimental procedure
All photocatalytic experiments were carried out in quartz glasses (24.7 X 4.7 cm) used as batch-type reactors with 0.250 L of effective volume. Two 15 W UV lamps emitting radiation at 254 nm were used as the light source. The lamps were placed 5 cm away from the glasses. Each quartz glass contained 12 films of the selected method (12 mg of TiO2 each), the films were placed vertically and kept in dark for 30 min to reach adsorption-desorption equilibrium. The system operated at 120 rpm at room temperature during 14 h. Three control experiments were performed for the photocatalytic experiments: UV photolysis, TiO2/adsorption in dark and absolute control by triplicate.
2.4 Bacterial reappearance experiments
The disinfection durability of the photocatalysis treatment was tested post-irradiation for total bacterial inactivation. After irradiation time, the water was kept in the dark and samples were taken at 24 h and 48 h and exanimated for the presence of heterotrophic bacteria, total coliforms and Gram-positive bacilli.
2.5 Analysis and statistical analysis
In order to evaluate the efficiency of the treatments, the % removal of organic matter; Chemical Oxygen Demand (COD), Total Organic Carbon (TOC) and Color Units (CU) were calculated. Pseudo-first-order rate constants k in min-1 were calculated for CU decrease and TOC abatement. The mineralization rate was characterized qualitatively in terms of TOC removal.
Absorbance spectra were obtained to determine the level of degradation of the wastewater. A UV-Vis spectrophotometer (Thermo Scientific Evolution 60) was used with a 1 cm quartz cell. To evaluate disinfection efficiency, the bacterial count was monitored in the three bacterial populations with the highest (Colonies Forming Units) CFU/mL isolated from the wastewater: heterotrophs, total coliforms and Gram-positive bacilli. Microbial inactivation is presented as percentage of inactivation. Assuming that the bacterial inactivation efficiency followed a pseudo-first order kinetics with respect to bacterial colony count (Nt), inactivation rate constants were analyzed.
The results obtained are presented in averages and standard deviations. A Shapiro-Wilk test for normality and Levene tests for homogeneity of variance were performed to verify data normality. Differences among measurements of removal and inactivation achieved by both treatments were assessed with One-way ANOVA (IBM SPSS® Statistics software version 20, Statistix 9.0®) and a post-hoc HSD Tukey test. P < 0.05 was used to determine statistical significance of the results obtained.
3. Results and discussion
3.1 Wastewater characterization
3.1.1 Physical and chemical parameters
The wastewater was intensely colored (1890.7 CU), presented a considerable high organic content: the mean COD, BOD5 and TOC in the wastewater were 3750.8 mg/L, 1693 mg/L, and 623.2 mg/L respectively. Other characteristics of the wastewater are included in Table 1. The results indicate that chemical substances such as dyes, mordants and solvents and others used in biological staining processes contribute highly to the pollution of this water. Color and COD/BOD are the most obvious indicators of water pollution and the discharge of highly colored dye effluents is aesthetically displeasing and cause severe environmental problems (Chong et al., 2010). Together, with acidic pH makes this wastewater imperative to treatment before being discharged into sewage systems or consider its reuse.
3.1.2 Microbial analysis
The substances present in the wastewater are generally considered antimicrobial agents (i.e. Crystal violet, malachite green, ethanol, iodine etc.). However, a high bacterial population was found. Only four groups of bacteria were successfully identified. Overall the number of bacteria in each group was relatively high. Heterotrophic bacteria were the largest microbial population isolated (7.86 log CFU/mL), followed by total coliforms (6.56 log CFU/mL), Gram-positive bacilli (6.37 log CFU/mL) and Pseudomonas spp (4.71 log CFU/mL). The isolation of theses microorganisms from the wastewater indicates natural adaptation to survive to the conditions of this particular wastewater, toxic bactericidal substances including dyes and solvents, and acidic pH. The higher concentration of bacteria in the wastewater could be explained either by the formation of bacterial spores or by biofilms at the surface of the containers where the water is kept (Jefferson, 2004).
However, no colonies were observed from all dilutions for spore analysis (< 10 spores/mL were obtained). Gram-positive bacilli spore-forming are the species of Bacillus spp. and Clostridium spp. Even though aerobic amylolytic Gram-positive bacilli were observed, it is likely that certain compounds present in the cell-staining wastewater like ethanol, acid, iodine and dyes as methylene blue inhibited its sporulation activity (Bohin et al., 1976, Kearns et al., 2005, Setlow et al., 2002). It has shown that in the spore forming bacterium Bacillus subtilis the efficiency of spore formation can be reduced more than 100-fold by alcohol stress (Bohin et al., 1976). The general stress genes and the molecular mechanisms considered to be involved in the inhibitory effect of alcohol stress on sporulation in B. subtilis, has been previously studied (Gottig et al., 2005, Reder et al., 2012). Likewise, spores can be photoinactivated by phenothizinium dyes such as methylene blue, toluidine blue O and dimethylmethylene (Demidova and Hamblin, 2005).
On the other hand, survival mechanisms including the formation of biofilm in Gram-positive and Gram-negative bacteria have been well studied (Andersson et al., 2008, Kearns et al., 2005). Organisms within biofilms can withstand nutrient deprivation, pH changes, oxygen radicals, disinfectants, and antibiotics making it possible to microorganisms to survive in given conditions of the cell-staining wastewater (Andersson et al., 2008, Jefferson, 2004). It has been reported that certain species can induce biofilm formation of other bacteria without out-competing them, like B. cereus strong EPS producers could be used as anchors for attachment of adherence-deficit strains. Thus a wide range of species can survive in environmental challenging conditions that would not grow as pure cultures. A synergistic effect on metabolism and biosynthesis of non- or weak EPS formers such as E. coli can be seen when together formed a strong biofilm in wastewater medium (Andersson et al., 2008). Similarly, Pseudomonas aeruginosa is reported to form biofilm in dual cultures and usually found to be one of the dominating species, which could explains its relatively high concentration in the wastewater.
The profile of isolated bacteria in cell-staining wastewater must be taken into consideration as these results can serve as a tool for the design of efficient photocatalytic systems in the disinfection and/or degradation of wastewater.
3.2 TiO2 thin film preparation and characterization
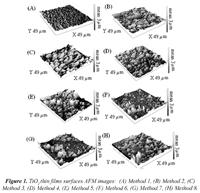
The AFM images of the evaluated combinations of variables (Fig. 1), suggest that method 1 (pH 9.0, Na4P2O7 0.01 M, 20 min of Microwave radiation) showed the best results in terms of substrate coating and decrease of aggregation of TiO2 particles over the substrate. This was due to the factors combined in the experiment. Because of its isoelectric point, TiO2 is charged negatively at alkaline conditions, this caused charge repulsion between TiO2 particles (Konstantinou and Albanis, 2004). Additionally, at this pH, sodium pyrophosphate is ionized, increasing the medium ionic strength, which interferes with particle aggregation (Jiang et al., 2009). Moreover, the electromagnetic field created by microwave radiation caused collisions between TiO2 aggregates leading to their fragmentation (Al-Harahsheh and Kingman, 2004).
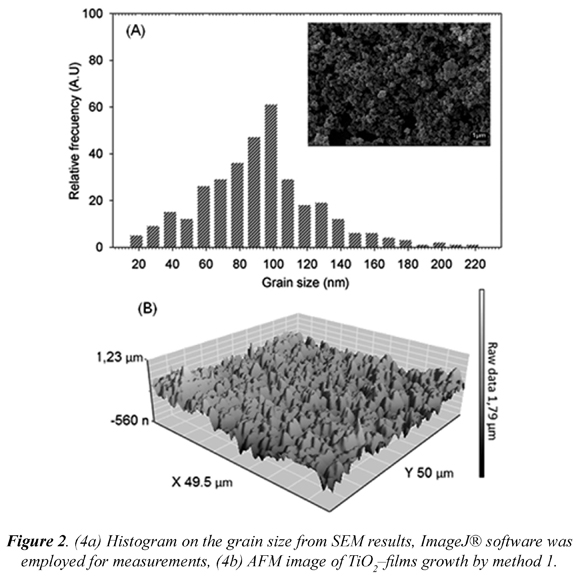
Once the method 1 for TiO2 thin films preparation was established, the films were characterized by microscopic and spectroscopic techniques. From Fig. 2-A it is observed that the higher frequencies of grain size ranged between 90 and 100 nm. The imperfection formed by the material can improve microbial inactivation since it can act as microscopic cavities, microorganisms, such as bacteria and yeast which average size are between 2 and 10 µm, could be trapped and potentially inactivated by the photocatalysis process. Affirmed that material structural defects associated with forms and different grain sizes can increase the number of catalytic sites (Acosta et al., 2005).
AFM high-resolution images showed that TiO2 grains formed small aggregates between 4 and 5 μm in size. The films obtained from this methodology appear to be rough (136,34 nm ± 29,79, 39,18 µm2) (Fig. 2-B). This is an important aspect since it can facilitate bacteria adherence to the film allowing bacterial inactivation (Lin et al., 2008).
3.2.1 Spectroscopic analysis
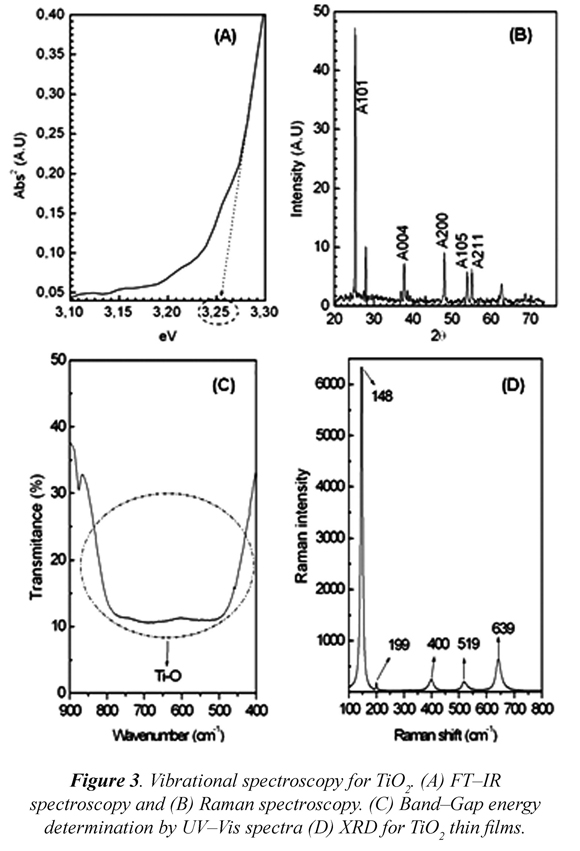
Spectroscopic analysis was carried out in order to determine band gap energy and crystalline phase of photocatalysts. UV-Vis spectroscopy (Fig. 3-A) indicated that energy band gap corresponds to a 3.2 e.V. (387 nm), which is related to anatase band gap energy (Gaya et al., 2009, Jin-hui, 2012). From XRD profiles one can observe diffraction signals for 101, 004, 200, 105 and 211 crystallographic planes, indicating that anatase phase was predominantly found (Fig. 3-B). A broad IR signal between 500 and 700 cm-1 has been related with Ti-O tension and associated with the presence of anatase phase (Fig. 3-C). Moreover, in Fig. 3-D, Raman Spectra showed vibrational modes, which are assigned to the previously mentioned crystalline phase, in 148, 199, 400, 519, and 639 cm-1 (Wang et al., 2011).
3.3 UV/TiO2 photocatalysis tests
3.3.1 Color removal and UV-Visible absorption changes
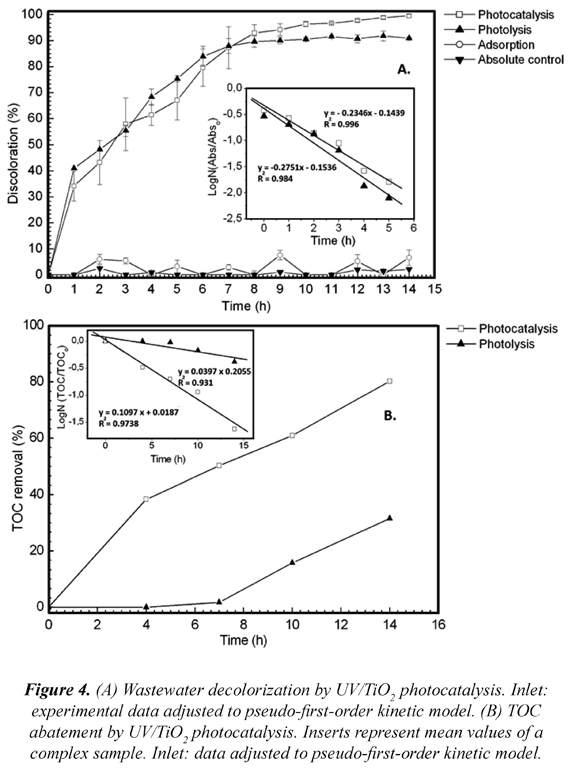
The photocatalysis treatment achieved 99.5 % (8.8 CU) of decolorization, while the UV photolysis control only 91.3 % (152.8 CU) and TiO2/adsorption in dark control 6.8 % (1,623.7 CU) of color removal after 14 h. During the first 7 h of treatment decolorization percentages were similar for photocatalysis and photolysis (87.3 %). Decolorization kinetic rate constants obtained were similar as well (k = 0.2346 min-1 R2 = 0.9963 and k = 0.2751 min-1 R2 = 0.9846, respectively) (Fig. 4-A, inlet). From Fig. 4-A, it is easy to show that photolysis is as effective as photocatalysis (p < 0.05) and, therefore, plays an important role in the degradation of dyes in the wastewater, similar results are found in the literature (Barka et al., 2008). Adsorption control experiment showed no significant adsorption of the dyes onto the TiO2 surface
Comparing UV/TiO2 photocatalysis and photolysis from 7 h to 14 h, an increase in the percentage of color removal for photocatalysis can be observed. In agreement with TiO2 photocatalysis systems in highly colored wastewater such as dye and textile wastewater, an efficient photocatalytic degradation and color removal can be achieved between UV/TiO2 and photolysis. However, photo-oxidation and degradation efficiency in real dye wastewater depends strongly on the characteristics of the wastewater such as dye structure and other present organic compounds, irradiation time and UV lamp, etc (Al-Momani et al., 2002). It can be suggested that after 7 h of treatment, UV radiation could not penetrate effectively through the highly colored wastewater; hence, films were not successfully irradiated. Once the CU decreased and UV light reached the film surface, the wastewater was 100 % discolored by the photocatalysis treatment. It should be emphasized, that photolytic treatment showed high percentages of decolorization. This is an interesting fact since, to the author's knowledge; triphenyl-methane dyes such as crystal violet in dilution has not been reported high decolorization rates through direct photolysis. The presence of other compounds such as solvents possibly contributed as electron donors under UV light irradiation.
3.3.2 TOC abatement
According to analysis of TOC abatement measurements, a clear difference between treatments and controls can be observed in Fig. 4-B. TiO2 photocatalysis resulted in a significant 79.5 % of TOC mineralization, compared to 31.6 % by the use of UV light after 14 h. However, the complexity of real wastewater, as the cell-staining wastewater here studied, containing several compounds and high microbial population results in a longer treatment time. It is important to mention that TOC abatement was slower in comparison to decolorization, possibly the chromophore rupture and by-product formation could be involved in a slow TOC abatement. Similar results were reported by Li (Li and Li, 2001), who degrade methylene blue, reaching 43.7 % of TOC abatement after 2 h of treatment. The process followed a pseudo-first-order kinetic (Fig. 4-B inlet). In photocatalysis, the k obtained value was higher (k = 0.197 min-1 R2 = 0.9738) in comparison to photolysis (k = 0.0397 min-1 R2 = 0.931) therefore; a faster organic matter mineralization was obtained with UV/TiO2 photocatalysis.
3.4 Bacterial inactivation and post-irradiation events
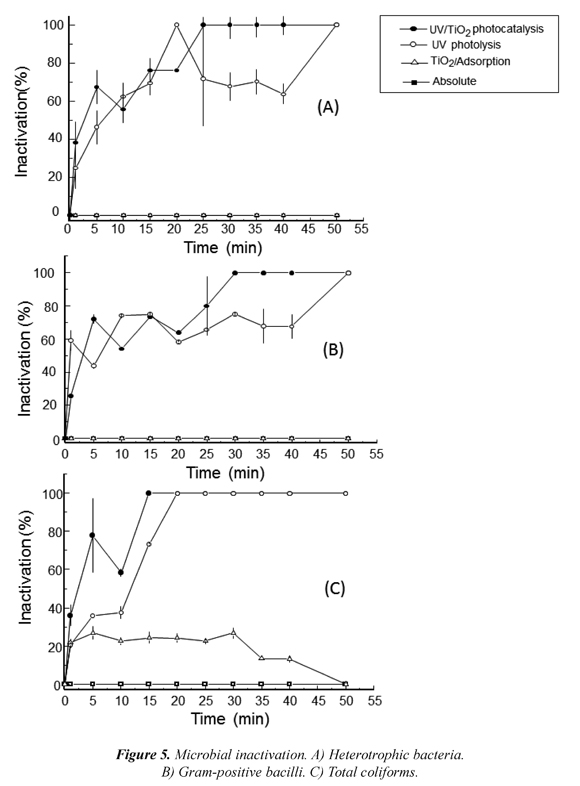
The efficiency of the treatment in the inactivation of three mayor populations found in the water was evaluated. Photocatalysis inactivated 100 % of all population after 14 h of treatment. Heterotrophic bacteria were completely inactivated after 25 min (Fig. 5-A), total coliforms after 20 minutes (Fig. 5-B) and Gram-positive bacilli were more resistant after 50 minutes of treatment (Fig. 5-C). The results showed the so-called shoulders in which bacteria repair the damage caused by reactive oxygen species (ROS). As the generation of hydroxyl radical progresses, is followed by an exponential decrease named tail, in which bacteria are no longer capable of self-repairing and eliminate intracellular ROS called "oxidative stress" leading to lysis of the cell and release of intracellular components (Chen et al., 2010).
The analysis of inactivation rate constants determined Gram-positive bacilli (k = 0.2677 min−1, R2 = 0.9240) as the more resistant group to degradation by hydroxyl radicals, compared to total coliforms (k = 0.8744 min−1, R2 = 0.9946). The photocatalytic inactivation of bacterial groups has been widely reported; where the inactivation rate increases with increasing cell wall thickness thus hydroxyl radicals and other ROS are absorbed in the peptidoglycan layer lowering the number of radicals that damage the DNA (Pal et al., 2007, Rincón and Pulgarin, 2006). It has been reported that Gram-negative bacteria can be easily affected by ROS. Water disinfection studies using TiO2 affirmed that comparing the Gram-negative bacteria E. coli with Staphylococcus aureus (Gram-positive), the latter appeared to be more resistant to the photocatalysis process (Kühn et al., 2003).
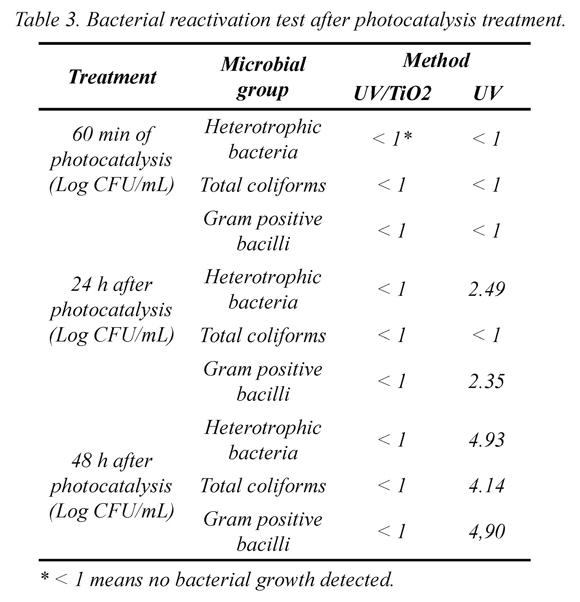
Results of secondary reactivation are shown in Table 3. The photocatalysis experiment proved to be efficient in the degradation of microorganism since no bacterial growth was detected after 24 h and 48 h in dark. Reactivation occur only after photolysis due to bacteria that lost their cultivability during irradiation time but activated resistance to stressful conditions and recover their cultivability when they were introduced into more favorable conditions (Benabbou et al., 2007). This effect has been reported by other researchers and attributed to bacterial UV-induced self-defense and activation of post-treatment recovery mechanisms. Hence, a bacteriostatic effect and activation of dark repair systems could be observed, reconstitution of cellular components and DNA such as those found in E. coli could occur (Benabbou et al., 2007, Rincón and Pulgarin, 2004).
4. Conclusion
A simple TiO2 thin films preparation based on sedimentation and a combination of pH 9.0, Na4P2O7 0,01 M, and microwave radiation for 20 min gave the best results with a uniform deposition on the substrate. Additionally, microscopic analysis showed that the higher frequencies of grain size ranged between 90 and 100 nm, and spectroscopic analysis indicates that the predominant crystalline phase in the material is anatase with gap value of 3.2 eV. The photocatalysis treatment leaded to 99.50 % of color removal and 79 % of TOC abatement in 14 h. As a result, mineralization was a slow process compared to decolorization, as it could be associated with the formation of transformation by-products. Likewise, the microbial population was totally inactivated in less than 1 h and no re-growth was found after 48 h of treatment. This result indicates that the oxidative species developed at the TiO2 surface caused severe damage to the cells. Therefore, disinfection durability of the photocatalysis treatment was attained.
5. Acknowledgements
This research was supported by the Vicerrectoría de Investigación (Project ID 4354), at Pontificia Universidad Javeriana (Bogota, Colombia).
6. References
Acosta, D. R., Martínez, A., Magana, C. R., & Ortega, J. M. (2005). Electron and Atomic Force Microscopy studies of photocatalytic titanium dioxide thin films deposited by DC magnetron sputtering. Thin solid films, 490 (2), 112-117. [ Links ]
Al-Harahsheh, M., & Kingman, S. (2004). Microwave-assisted leaching-a review. Hydrometallurgy, 73 (3-4), 189-203. [ Links ]
Al-Momani, F., Touraud, E., Degorce-Dumas, J., Roussy, J., & Thomas, O. (2002). Biodegradability enhancement of textile dyes and textile wastewater by VUV photolysis. Journal of Photochemistry and Photobiology A: Chemistry, 153 (1-3), 191-197. [ Links ]
Andersson, S., Kuttuva Rajarao, G., Land, C. J., & Dalhammar, G. (2008). Biofilm formation and interactions of bacterial strains found in wastewater treatment systems. FEMS microbiology letters, 283 (1), 83-90. [ Links ]
Bandala, E. R., Velasco, Y., & Torres, L. G. (2008). Decontamination of soil washing wastewater using solar driven advanced oxidation processes. Journal of hazardous materials, 160 (2-3), 402-407. [ Links ]
Barka, N., Assabbane, A., Nounah, A., & Ichou, Y. A. (2008). Photocatalytic degradation of indigo carmine in aqueous solution by TiO2-coated non-woven fibres. Journal of hazardous materials, 152 (3), 1054-1059. [ Links ]
Benabbou, A., Derriche, Z., Felix, C., Lejeune, P., & Guillard, C. (2007). Photocatalytic inactivation of Escherischia coli: Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Applied Catalysis B: Environmental, 76 (3), 257-263. [ Links ]
Bohin, J., Rigomier, D. & Schaeffer, P. (1976). Ethanol sensitivity of sporulation in Bacillus subtilis: a new tool for the analysis of the sporulation process. Journal of bacteriology, 127 (2), 934-940. [ Links ]
Carp, O., Huisman, C. & Reller, A. (2004). Photoinduced reactivity of titanium dioxide. Progress in solid state chemistry, 32 (1-2), 33-177. [ Links ]
Chen, F., Yang, X., Mak, H. K., & Chan, D. W. (2010). Photocatalytic oxidation for antimicrobial control in built environment: A brief literature overview. Building and Environment, 45 (8), 1747-1754. [ Links ]
Chong, M. N., Jin, B., Chow, C. W., & Saint, C. (2010). Recent developments in photocatalytic water treatment technology: a review. Water Research, 44 (10), 2997-3027. [ Links ]
Demidova, T. N., & Hamblin, M. R. (2005). Photodynamic inactivation of Bacillus spores, mediated by phenothiazinium dyes. Applied and environmental microbiology, 71 (11), 6918-6925. [ Links ]
Foster, H. A., Ditta, I. B., Varghese, S., & Steele, A. (2011). Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Applied microbiology and biotechnology, 90 (6), 1847-1868. [ Links ]
Gaya, U. I., Abdullah, A. H., Zainal, Z., & Hussein, M. Z. (2009). Photocatalytic treatment of 4-chlorophenol in aqueous ZnO suspensions: Intermediates, influence of dosage and inorganic anions. Journal of hazardous materials, 168 (1), 57-63. [ Links ]
Gottig, N., Pedrido, M. E., Méndez, M., Lombardía, E., Rovetto, A., Philippe, V., Orsaria, L., & Grau, R. (2005). The Bacillus subtilis SinR and RapA developmental regulators are responsible for inhibition of spore development by alcohol. Journal of bacteriology, 187 (8), 2662-2672. [ Links ]
Gümüs, D., & Akbal, F. (2011). Photocatalytic degradation of textile dye and wastewater. Water, Air, & Soil Pollution, 216 (1-4), 117-124. [ Links ]
Jefferson, K. K. (2004). What drives bacteria to produce a biofilm? FEMS microbiology letters, 236 (2), 163-173. [ Links ]
Jiang, J., Oberdörster, G., & Biswas, P. (2009). Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. Journal of Nanoparticle Research, 11 (1), 77-89. [ Links ]
Jin-Hui, Z. (2012). Research on UV/TiO2 Photocatalytic Oxidation of Organic Matter in Drinking Water and Its Influencing Factors. Procedia Environmental Sciences, 12 (A), 445-452. [ Links ]
Kearns, D. B., Chu, F., Branda, S. S., Kolter, R., & Losick, R. (2005). A master regulator for biofilm formation by Bacillus subtilis. Molecular microbiology, 55 (3), 739-749. [ Links ]
Konstantinou, I. K., & Albanis, T. A. (2004). TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: A review. Applied Catalysis B: Environmental, 49 (1), 1-14. [ Links ]
Kühn, K. P., Chaberny, I. F., Massholder, K., Stickler, M., Benz, V. W., Sonntag, H.-G., & Erdinger, L. (2003). Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere, 53 (1), 71-77. [ Links ]
Li, X., & Li, F. (2001). Study of Au/Au3+-TiO2 photocatalysts toward visible photooxidation for water and wastewater treatment. Environmental science & technology, 35 (11), 2381-2387. [ Links ]
Lin, H., Xu, Z., Wang, X., Long, J., Su, W., Fu, X., & Lin, Q. (2008). Photocatalytic and antibacterial properties of medical grade PVC material coated with TiO2 film. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 87 (2), 425-431. [ Links ]
Oller, I., Malato, S. & Sánchez-Pérez, J. (2011). Combination of advanced oxidation processes and biological treatments for wastewater decontamination-a review. Science of the total environment, 409 (20), 4141-4166. [ Links ]
Oplatowska, M., Donnelly, R. F., Majithiya, R. J., Glenn Kennedy, D., & Elliott, C. T. (2011). The potential for human exposure, direct and indirect, to the suspected carcinogenic triphenylmethane dye Brilliant Green from green paper towels. Food and Chemical Toxicology, 49 (8), 1870-1876. [ Links ]
Pal, A., Pehkonen, S. O., Yu, L. E., & Ray, M. B. (2007). Photocatalytic inactivation of Gram-positive and Gram-negative bacteria using fluorescent light. Journal of Photochemistry and Photobiology A: Chemistry, 186 (2-3), 335-341. [ Links ]
Pausová, S., Krýsa, J., Jirkovský, J., Forano, C., Prevot, V., & Mailhot, G. (2011). Photocatalytic properties of aqueous systems containing TiO2 nanoparticles. Catalysis Today, 161 (1), 140-146. [ Links ]
Reder, A., Albrecht, D., Gerth, U., & Hecker, M. (2012). Cross-talk between the general stress response and sporulation initiation in Bacillus subtilis - the sB promoter of spo0E represents an AND-gate. Environmental Microbiology, 14 (10), 2741-2756. [ Links ]
Rincón, A.G., & Pulgarin, C. (2004). Field solar E. coli inactivation in the absence and presence of TiO2: Is UV solar dose an appropriate parameter for standardization of water solar disinfection? Solar Energy, 77 (5), 635-648. [ Links ]
Rincón, A.G., & Pulgarin, C. (2006). Comparative evaluation of Fe3+ and TiO2 photoassisted processes in solar photocatalytic disinfection of water. Applied Catalysis B: Environmental, 63 (3-4), 222-231. [ Links ]
Setlow, B., Loshon, C., Genest, P., Cowan, A., Setlow, C., & Setlow, P. (2002). Mechanisms of killing spores of Bacillus subtilis by acid, alkali and ethanol. Journal of applied microbiology, 92 (2), 362-375. [ Links ]
Sioi, M., Bolosis, A., Kostopoulou, E., & Poulios, I. (2006). Photocatalytic treatment of colored wastewater from medical laboratories: photocatalytic oxidation of hematoxylin. Journal of Photochemistry and Photobiology A: Chemistry, 184 (1-2), 18-25. [ Links ]
Wang, J., Zhao, G., Zhang, Z., Zhang, X., Zhang, G., Ma, T., Jiang, Y., Zhang, P., & Li, Y. (2007). Investigation on degradation of azo fuchsine using visible light in the presence of heat-treated anatase TiO2 powder. Dyes and Pigments, 75 (2), 335-343. [ Links ]
Wang, X., Shen, J., & Pan, Q. (2011). Raman spectroscopy of sol-gel derived titanium oxide thin films. Journal of Raman Spectroscopy, 42 (7), 1578-1582. [ Links ]

Revista Ingeniería y Competitividad por Universidad del Valle se encuentra bajo una licencia Creative Commons Reconocimiento - Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.