Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería y competitividad
Print version ISSN 0123-3033
Ing. compet. vol.17 no.1 Cali Jan./June 2015
Block freeze concentration intensification by means of vacuum and microwave pulses
Intensificación de la crioconcentración en bloque por medio de pulsos de microondas y vacío
J. Mauricio Pardo*
E-mail: jpardo@up.edu.mx
Ricardo Sánchez*
E-mail: 0126622@up.edu.mx
*School of Engineering, Universidad Panamericana, Campus México D.F., México
Eje temático: FOOD ENGINEERING/INGENIERÍA DE ALIMENTOS
Recibido: Febrero 22 de 2015
Aceptado: Marzo 20 de 2015
Abstract
Block freeze concentration has gained interest as a separation technology since it is simpler than the suspension process. However, it has not been implemented at an industrial level because its separation efficiency is lower than that of the traditional freeze concentration process. Therefore, numerous assisting techniques are being studied around the world to overcome this deficiency. In this paper, the combination of microwave heating and vacuum separation is being studied and compared with convection heating and gravity separation. Separation efficiency was measured using the cumulative concentration factor (CumCI) and the area under the curve defined by yield (Y) as a function of the thawed fraction (f). It has been observed that all treatments that used vacuum as separation method showed a higher efficiency than those in similar processing conditions that used gravity as the separation method. Regarding the effect of microwave pulses, it has been observed that pulses below 2.4 kJ increased the separation efficiency when combined with the hydrodynamic effect generated by the vacuum pulses.
Keywords: Cryoconcentration, microwave heating, separation efficiency, vacuum.
Resumen
La crioconcentración en bloque ha ganado interés como una tecnología de separación, ya que es más sencilla que el proceso de suspensión. Sin embargo, no ha sido implementada en una escala industrial ya que su eficiencia de separación es menor que la del proceso de crioconcentración tradicional. Por lo tanto, numerosas técnicas de apoyo están siendo estudiadas en todo el mundo para superar esta deficiencia. En este trabajo, se estudia la combinación de calentamiento por microondas y la separación con vacío y se compara con el calentamiento por convección y la separación con gravedad. La eficiencia de separación se midió utilizando el factor de concentración acumulativa (CumCI ) y el área bajo la curva definida por el rendimiento (Y) como una función de la fracción descongelada (f ). Se ha observado que todos los tratamientos que utilizan vacío como método de separación mostraron una eficiencia superior a los de condiciones de procesamiento similares que utilizan a la gravedad como el método de separación. En cuanto al efecto de los pulsos de microondas, se ha observado que aquellos que están por debajo de 2,4 kJ aumentaron la eficiencia de separación cuando se combinaron con el efecto hidrodinámico generado por los pulsos de vacío.
Palabras clave: Calentamiento por microondas, crioconcentración, eficiencia de separación, vacío.
1. Introduction
Freeze concentration (FC) has been regarded as a reliable separation method for biomaterials since it preserves their original organoleptic and functional properties (Aider & de Halleux, 2009; Escalante-Minakata et al., 2013; Moreno, et al., 2014; Sanchez, et al., 2009; Virgen-Ortiz et al., 2012). Additionally, as the latent heat of solidification is around 7 times lower than that of evaporation, its energy consumption and environmental impact is low when compared with traditional evaporation technologies (Fernández-Torres, et al. 2012). Therefore, it is common to find that FC is applied previous to spray drying or freeze drying in the processing plants in order to reduce costs without affecting quality. There are also applications for waste water treatment and desalination (Belen, et al., 2012; Kuo & Lee, 2010; Rich et al., 2012).
FC may be classified into three groups: suspension, film and block (Moreno, et al., 2013). Suspension FC is based on the formation and growth of multiple crystals which are separated from the concentrated solution by filters or centrifuges. Meanwhile, the film technique aims to facilitate the separation by forming a single crystal that can grow on plates (Auleda, Raventos, & Hernandez, 2011) or cylinders (Miyawaki, et al., 2005). Finally, the block technique is a freeze-thaw process where the entire sample is frozen and afterwards partially thawed to obtain liquid fractions with decreasing concentration (Miyawaki, et al., 2012; Nakagawa, et al., 2010). In recent years, some research groups have been interested in the study of this technique (Aider & de Halleux, 2009; Moreno et al., 2013; Petzold, et al., 2013) since it allows the use of simple equipment with few moving parts that reduce investment and maintenance costs enabling FC to be used in minor scales than currently employed.
As mentioned before, block FC is based on the freeze - thaw principle and is composed of three major steps: freezing, thawing and separation. Each of these has an influence on the process quality and kinetics. The separation begins during freezing and as crystals grow the ice front expels solutes to its surroundings. However, there are crystalline imperfections which entrap solids and also the growth of various crystals inevitably confines concentrated fluid in the inter-crystalline zones (Iritani, et al., 2013).
Once the whole sample is frozen it becomes a crystalline matrix with a concentrated solution dispersed in it. There are concentration gradients of the unfrozen solution generated according to the crystal growth direction (Okawa, et al., 2009) and according to the temperature of the cooling medium (van der Sman, et al., 2013).
It is common to partially thaw the sample in order to separate solids from the frozen matrix, and then collect the liquid drops that diffuse out and fall due to gravity. In this step, the amount of heat should be controlled to avoid excessive melting of ice. This process may take from minutes to days depending on how energy is added to the sample (Nakagawa, et al., 2010). In recent years, various studies focused on support techniques to increase the separation efficiency. Some authors are interested in the crystallization step and others in the heating and separation steps. For example, Iritani, et al., (2013) stirred samples during freezing and also noticed a positive effect on the separation of solids. Other authors have focused on support techniques to facilitate the elution of the solutes from the frozen matrix. Aider and Ben Ounis (2012) used microwave as a heating medium and didn't find a significant difference in separation efficiency when compared to the convection method, but found that time is drastically reduced by using microwaves. On the opposite, Moreno, et al. (2013) observed a positive effect of microwave heating when combined with vacuum suction on the separation efficiency and on processing time. Petzold et al. (2013), used vacuum and obtained an improvement in the amount of solids recovered and also a reduction of the processing time. Finally, Petzold and Aguilera (2013) observed a positive effect of centrifuging on the separation efficiency.
This article focuses on the effect of microwave (MW) and vacuum (VA) pulses on the separation efficiency and on the separation time. It differs from previous studies because it uses subsequent microwave and vacuum pulses and studies the effect of applying them at different levels.
2. Materials and methods
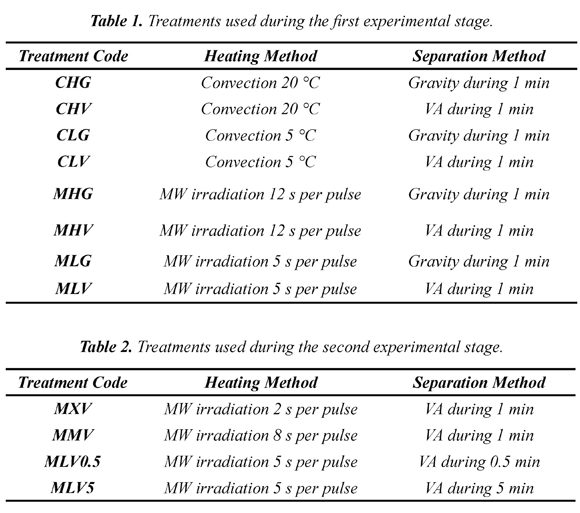
The project was divided into two stages. Table 1 summarizes the treatments applied in the first stage, which focused on comparing the MW heating with convection heating and VA separation with gravitational separation. Two modes of heating and two modes of extraction were analyzed. Each heating mode was studied at two levels and each treatment was done in triplicate.
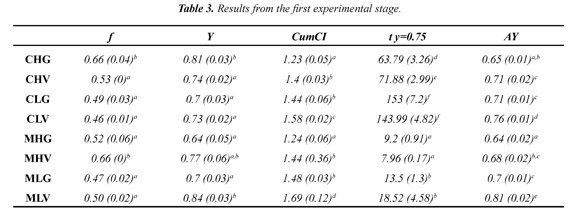
The experiments of the second stage were planned to quantify trends in the separation efficiency and processing time. Therefore, the treatment from the former stage with best results was chosen and two levels of MW and two levels of VA were applied as shown in Table 2.
All sucrose solutions were prepared with a concentration of 10 °Brix. This was measured with a refractometer (Atago PAL-1, Tokyo) and the samples were frozen in cylindrical containers using 0.2 kg per sample. The containers were immersed in an ethylene glycol bath at -8 +/- 1° C for 24 hours. Subsequently, the samples were heated and the liquid fraction separated under different conditions.
MW heating was carried out in a Panasonic equipment (NN-GF560M, China) with a power of 1200 W and pulses from 2 to 12 seconds. Additionally, the convection heating used a thermostatic bath at 5 ° C or at 20 ° C.
The separation of the liquid was made using two methods: gravity and suction. In the first case the samples were allowed to drip without intervention for one minute, whereas in the second case a vacuum pump (model ChemStar 1400N-01, USA) was used to extract the liquid fraction using a gauge pressure of -57 kPa (absolute 20 kPa). After each heating and separation step, extracted liquid was collected in a beaker and its concentration and mass recorded. At the end of the process all collected data (time, mass and concentration) were used to calculate the following parameters:
Thawed fraction (f)
Calculated as the ratio between the thawed mass (mliq) and the initial mass (m0), defined by the equation:
f = mliq / m0 (1)
Solute Yield (Y).
Defined as the mass of solids present in the collected fluid (msliq) divided by the initial mass of the solute in the original solution (ms0), given by the equation:
Y = ms liq / ms 0 (2)
Concentration Factor (CI) and cumulative concentration factor (CumCI).
Defined by the relationship between the concentration of solids in the liquid fraction of the sample obtained in one extraction step (xsliq) and the solids concentration in the initial solution (xs0):
CI = Xs liq / Xs 0 (3)
This parameter is crucial to the selection of the data that is being analyzed ; a CI value lower than 1 shows that sample obtained in that step is less concentrated than the initial sample.
Meanwhile, the cumulative concentration factor is the concentration of solids in the sample collected throughout the process divided by the solids concentration in the initial sample and can be calculated as:
CumCI = Y / f (4)
Ratio of area under the curve as a Y as function of f (AY)
Defined as the ratio of the area under the curve Y(f) of each treatment and the area under the curve Y(f) of the ideal treatment. It is expected that sucrose solutions at 0 °C will have a saturation concentration of 64 % w / w. Therefore the maximum area under the curve will be 0.926 and the minimum area 0.5. This maximum area corresponds to an ideal condition where the extracted samples have the saturation concentration and therefore all the solids are extracted in the first steps of the process.
AY = Area / (ideal Area) (5)
3. Results and discussion
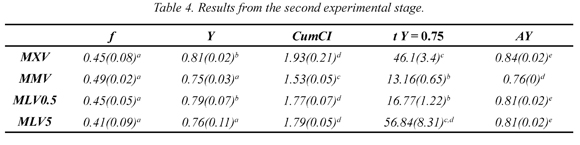
Results from the first experimental stage can be seen in Table 3. Numbers in parentheses represent standard deviations and superscripts represent statistically equal groups of data. The values of f, Y, and CumCI were estimated for the time when CI = 1, which is the practical moment to complete the process, thereafter the yield will increase but obtained samples will have a lower concentration that of the initial solution. Parameters for the exact condition CI = 1 were estimated interpolating between the two steps that showed CI values above and below 1. It is worth noting that almost all of the f values were in a range between 0.46 and 0.52, indicating that melting more than half of the sample is not advisable. Furthermore, the Y values were mainly in a range from 0.64 to 0.77 with an average of 0.71. Only two treatments were separated from this statistical group and reached values greater than 0.8 (treatments MLV and CHG). These can be seen as the best procedure from the standpoint of solute recovery; however, it is not easy to draw conclusions observing this sole indicator. According to Equation 4, CumCI combines the effect of each treatment on Y and f. Thus, from the CumCI point of view, CLV and MLV are the treatments that provide best separation efficiency, concentrating the samples 1.58 and 1.69 times respectively. Both treatments have in common that VA pulses were used in the separation step and low energy levels were used in the heating step. On the other hand, treatments that showed lower concentration levels were CHG and MHG. Both used gravity in the separation stage and applied high energy levels.
Another indicator that can be used to measure the separation efficiency is the area under the curve Y as a function of f as suggested by Moreno et al., (2014). The results in Table 3 show five distinct AY groups and treatments MLV and CLV are the ones with higher AY value. It is worth noting that these treatments have already been highlighted in the analysis of CumCI. Furthermore, CHG and MHG also showed up again as the less suitable procedures according to this analysis. Looking in more detail at the AY values, a positive effect of VA on the separation efficiency is found, all samples using VA show a greater AY values than those obtained using a similar treatment without VA. These results suggest that there is some concentrated fluid trapped between dendrites (van der Sman et al., 2013) that cannot be easily separated by diffusion. In contrast, suction generates a hydrodynamic effect that helps the fluid to move faster through the dendritic network (Petzold et al., 2013), easing the elution process (Nakagawa, et al., 2010).
Furthermore, if AY values from two similar treatments utilizing different heating methods are compared, it is observed that almost all are statistically equal, thus MW heating or convection might not have a differentiating effect on the separation efficiency. However, in these experiments there is an exception that is the pair of treatments CLV, MLV. These treatments show that the use of MW pulses under defined conditions can increase AY as observed and reported earlier in a similar work with different processing conditions (Moreno et al., 2013) and are in contrast with those reported earlier (Aider & Ben Ounis, 2012), where no significant influence of the MW pulse on the efficiency of separation was observed. This contradictory result shown by CLV, MLV suggests that MW pulses below a certain energy level can increase AY if they are combined with the hydrodynamic effect of VA discussed above. The radiated energy level where the positive influence of the MW heating was observed is of 2.4 kJ.
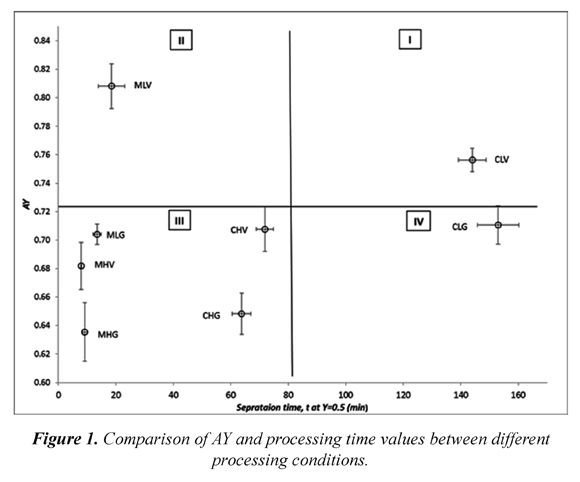
Until now, the indicators analyzed didn't consider the time spent on heating and separation steps, therefore in the following paragraph time consumed to recover 75 % of the initial solid ( t at Y =0.75) is analyzed. Time consumed to achieve CI=1 can also be analyzed, however authors decided to use time at Y=0.75 in order to ease the comparison by increasing the time differences. In Table 3, five statistically distinct groups are observed, but in practical terms there are three groups of processing time: the first with less than 20 minutes, another group with more than 140 minutes and a group in between. It's worth noting that shorter treatments are those undergoing MW heating, the longest are those convection heated at 5 ° C and the intermediate received heat by convection at 20 ° C. These results show the benefits of MW heating since processing times are reduced by up to 11 times. However, this reduction does not necessarily favor the separation efficiency. Thus, Figure 1 was built in order to depict this situation in a simple manner. This figure has been divided into four areas to classify the effect of each treatment.
This division was made using the midpoint in the range of experimental data. It would be desirable that all the treatments were in the quadrant II and none in the quadrant IV; however, experimental results show that studied treatments are mainly in quadrant III. Furthermore, in quadrant II there is only one treatment (MLV), which is the best in terms of separation efficiency and separation time. This results are in agreement with those reported earlier (Moreno et al., 2013), even though their experimental conditions (single MW pulse and continuous vacuum) differ from the ones used in this work, both agree on the synergistic effect created by combining MW and VA. This synergy has been studied in more detail in the second experimental stage and results are described in the following paragraphs. Finally, in quadrant IV there is CLG treatment (convection heated at 5 °C and separated using gravity) that has, the less desirable processing conditions and therefore should be avoided when designing FC block processes.
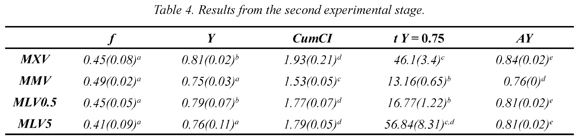
As mentioned in the previous paragraph, it would be useful to experiment near the MLV conditions (MW for 5 s and VA for 1 min) in order to quantify synergy generated by combining MW and VA. Thus, a new series of experiments was developed (Table 2) and their results are shown in Table 4. It is noteworthy that in these results, CumCI values are higher than those previously shown (Table 3). On the other hand AY values are also higher; however, these are statistically equal to the highest values encountered in the first experimental phase.
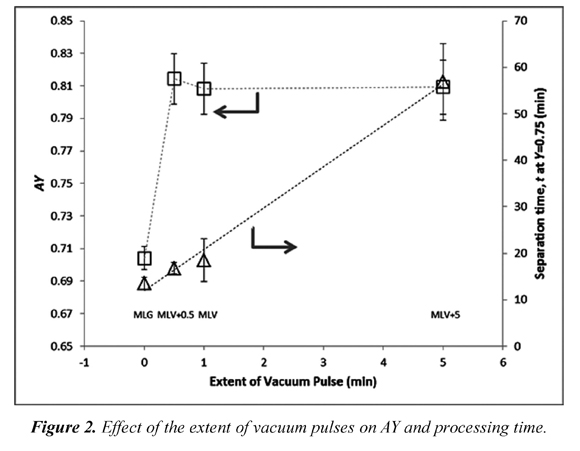
Figure 2 and 3 show some interesting trends. Figure 2 illustrates the effect of the length of each VA pulse (from 0 to 5 minutes) on AY and on separation time. It is clearly seen that there is a positive change in the values of AY when VA is applied, but an increase in the extent of the VA pulse does not generate significant changes in the separation efficiency. Moreover, this same figure shows the effect of VA pulse extension on processing time; as expected, the pulse prolongs the treatment and it does it in a linear way .Thus, one can conclude that the use of VA pulses improves the separation of the solute, but a 30 second pulse is sufficient for an increase in separation efficiency without unnecessarily extending the separation process.
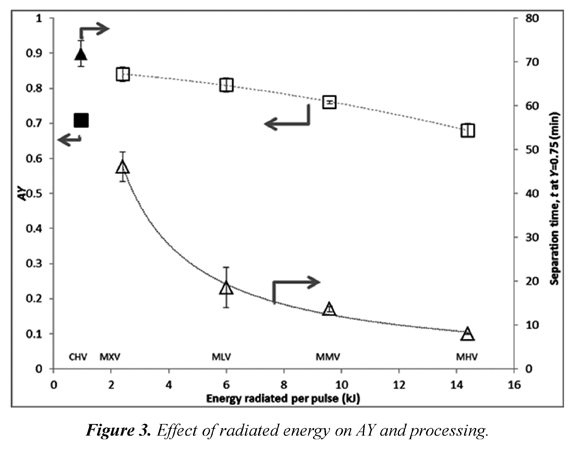
Figure 3 shows the effect of MW on AY and separation time. It is observed that increasing energy in each pulse decreases Thus, by increasing the amount of energy radiated the amount of molten ice per pulse is increased diluting the recovered solids. On the other hand, separation time is increased when MW energy is decreased.
According to the experimental results, a decrease in radiated power from 2.4 kJ to 1.2 kJ has a positive effect on AY increasing it by 1% but also has an effect on the processing time increasing it by about 50%. Separation time using convection heating CHV is lower than that spent using 1.2 kJ but higher than the one spent with MW pulses of 2.4 kJ, additionally in both cases separation efficiency obtained using CHV is lower than both MW treatments. Thus, MW pulses of 2 seconds (2.4 kJ) promote better separation efficiency and separation time compared to convection treatments. This result is explained by the way the energy is delivered to the sample. The affinity of microwaves with liquid water molecules makes this radiated energy to be absorbed in the bound water sites (unfrozen water) which correspond to sites of higher solids concentration (Okawa et al., 2009). In contrast, convection heating is not selective and simply melts the outer layers of the sample regardless of their concentration. Some have tried successfully to increase the separation efficiency (Moreno et al., 2014) by freezing cylinders from the center in radial direction and thawing them in the opposite direction. They achieved CumCI values of 1.64 that are close to those observed with VA and MW treatments MLV but didn't exceed the values obtained with MXV (1.93). Thus, from the standpoint of separation efficiency and separation time it is advisable to use of MW pulses close to 2.4 kJ for 200 g samples (12 kJ /kg) combined with vacuum pulses of 30 seconds, rather than convection heating. However these observations should be taken as a trend and not as an exact value because the minimum vacuum pulse is expected to be dependent on mass transfer variables, thus it will change with sample size and with pressure difference during the pulse. Therefore future work should include these variables in the experimental work.
4. Conclusions
All treatments that used VA as separation method showed a higher separation efficiency in terms of AY and CumCI than those processes that used gravity. Additionally, it has been found that increasing the suction time from 30 seconds to 5 minutes does not significantly change AY but increases the separation time in a quadratic way.
It has been found that increased radiated energy tends to decrease the separation time and also the separation efficiency. However, treatments with low levels of radiated energy (2.4 kJ/pulse) combined with 30 s vacuum pulses offered better separation efficiency and lower separation time than those that used convection heating.
5. Acknowledgements
The Authors would like to thank the financial support of the Engineering School at Universidad Panamericana and also the support received from Mr. Roberto Martínez during the building of the experimental rig.
6. References
Aider, M., & Ben Ounis, W. (2012). Skim milk cryoconcentration as affected by the thawing mode: gravitational vs. microwave-assisted. International Journal of Food Science and Technology, 47 (1), 195-202. [ Links ]
Aider, M., & de Halleux, D. (2009). Cryoconcentration technology in the bio-food industry: Principles and applications. Lwt-Food Science and Technology, 42 (3), 679-685. [ Links ]
Auleda, J. M., Raventos, M., & Hernandez, E. (2011). Calculation method for designing a multi-plate freeze-concentrator for concentration of fruit juices. Journal of Food Engineering, 107 (1), 27-35. [ Links ]
Belen, F., Sanchez, J., Hernandez, E., Auleda, J. M., & Raventos, M. (2012). One option for the management of wastewater from tofu production: Freeze concentration in a falling-film system. Journal of Food Engineering, 110 (3), 364-373. [ Links ]
Escalante-Minakata, P., Ibarra-Junquera, V., Chavez-Rodriguez, A. M., Ornelas-Paz, J. D., Emparan-Legaspi, M. J., Perez-Martinez, J. D., & Villa-Velazquez-Mendoza, C. I. (2013). Evaluation of the Freezing and Thawing Cryoconcentration Process on Bioactive Compounds Present in Banana Juice from Three Different Cultivars. International Journal of Food Engineering, 9 (4), 445-455. [ Links ]
Fernández-Torres, M. J., Randall, D. G., Melamu, R., & von Blottnitz, H. (2012). A comparative life cycle assessment of eutectic freeze crystallisation and evaporative crystallisation for the treatment of saline wastewater. Desalination, 306, 17-23. [ Links ]
Iritani, E., Katagiri, N., Okada, K., Cao, D. Q., & Kawasaki, K. (2013). Improvement of concentration performance in shaking type of freeze concentration. Separation and Purification Technology, 120, 445-451. [ Links ]
Kuo, C.-H., & Lee, C.-L. (2010). Treatment of oil/water emulsions using seawater-assisted microwave irradiation. Separation and Purification Technology, 74 (3), 288-293. [ Links ]
Miyawaki, O., Kato, S., & Watabe, K. (2012). Yield improvement in progressive freeze-concentration by partial melting of ice. Journal of Food Engineering, 108 (3), 377-382. [ Links ]
Miyawaki, O., Liu, L., Shirai, Y., Sakashita, S., & Kagitani, K. (2005). Tubular ice system for scale-up of progressive freeze-concentration. Journal of Food Engineering, 69 (1), 107-113. [ Links ]
Moreno, F. L., Raventos, M., Hernandez, E., & Ruiz, Y. (2014). Block freeze-concentration of coffee extract: Effect of freezing and thawing stages on solute recovery and bioactive compounds. Journal of Food Engineering, 120, 158-166. [ Links ]
Moreno, F. L., Robles, C. M., Sarmiento, Z., Ruiz, Y., & Pardo, J. M. (2013). Effect of separation and thawing mode on block freeze-concentration of coffee brews. Food and Bioproducts Processing, 91 (4), 396-402. [ Links ]
Nakagawa, K., Maebashi, S., & Maeda, K. (2010). Freeze-thawing as a path to concentrate aqueous solution. Separation and Purification Technology, 73 (3), 403-408. [ Links ]
Nakagawa, K., Nagahama, H., Maebashi, S., & Maeda, K. (2010). Usefulness of solute elution from frozen matrix for freeze-concentration technique. Chemical Engineering Research & Design, 88 (5-6), 718-724. [ Links ]
Okawa, S., Ito, T., & Saito, A. (2009). Effect of crystal orientation on freeze concentration of solutions. International Journal of Refrigeration-Revue Internationale Du Froid, 32 (2), 246-252. [ Links ]
Petzold, G., & Aguilera, J. M. (2013). Centrifugal freeze concentration. Innovative Food Science & Emerging Technologies, 20, 253-258. [ Links ]
Petzold, G., Niranjan, K., & Aguilera, J. M. (2013). Vacuum-assisted freeze concentration of sucrose solutions. Journal of Food Engineering, 115 (3), 357-361. [ Links ]
Rich, A., Mandri, Y., Mangin, D., Rivoire, A., Abderafi, S., Bebon, C., Semlali, N., et al. (2012). Sea water desalination by dynamic layer melt crystallization: Parametric study of the freezing and sweating steps. Journal of Crystal Growth, 342 (1), 110-116. [ Links ]
Sanchez, J., Ruiz, Y., Auleda, J. M., Hernandez, E., & Raventos, M. (2009). Review. Freeze Concentration in the Fruit Juices Industry. Food Science and Technology International, 15 (4), 303-315. [ Links ]
Van der Sman, R. G. M., Voda, A., van Dalen, G., & Duijster, A. (2013). Ice crystal interspacing in frozen foods. Journal of Food Engineering, 116 (2), 622-626. [ Links ]
Virgen-Ortiz, J. J., Ibarra-Junquera, V., Osuna-Castro, J. A., Escalante-Minakata, P., Mancilla-Margalli, N. A., & Ornelas-Paz, J. D. (2012). Method to concentrate protein solutions based on dialysis-freezing-centrifugation: Enzyme applications. Analytical Biochemistry, 426 (1), 4-12. [ Links ]

Revista Ingeniería y Competitividad por Universidad del Valle se encuentra bajo una licencia Creative Commons Reconocimiento - Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.