1. Introduction
Colombia has one of the largest coal reserves in Latin America, today and is one of the largest exporters of thermal coal in the world. Colombian coal is recognized worldwide for its excellent thermal properties, low ash content and high volatile matter content. In spite of Colombia possessing high heat value coal reserves, there are deposits of low range coal, with high mineral matter content, which make it less attractive for energetic uses. Nevertheless, it is possible to enhance the properties of this low rank coal through coal cleaning processes (reduction of the mineral matter content) and improve its thermal properties.
Over the past decades, there has been a considerable amount of research in opening new markets for coal, one of these markets, has attracted a considerable amount of interest in the use of coal as substitute for oil and gas. Researches has shown that the coal used for this purpose must be cleaned until superclean levels, which is defined as coal containing less than 2% ash 1. To achieve this level of cleaning, the coal must be micronized to produce a median size of approximately 5 micrometers to liberate the ash components 1,2.
The most commonly used physical method to clean fine coal is froth flotation, however, due to problems related to ultrafine particles treatment, froth flotation efficiency decreases as particles reach colloidal range. Selective coagulation processes can be used to enhance the performance of froth flotation or as an alternative method for cleaning ultrafine coal 2,3. In these processes, coal particles are selectively agglomerated by various means, while the associated mineral matter is dispersed by pH control and/or dispersants, separation of the mineral matter is achieved through differential sedimentation, screening, filtration or flotation. In order to reduce the commercial costs of coal cleaned using flocculants and organic liquids, a selective hydrophobic coagulation process can be used instead, only by controlling the pH of the slurry 2-4.
The objective of this work was to evaluate the effects of pH control and agitation time of the slurry in the ash rejection and combustible recovery of the coal sample after the selective hydrophobic coagulation process was performed. This is done through a 22 central composite experimental design, the pH was choose as the independent variable due the strong influence it has on the coal surface properties and the agitation time was chosen due the effect it mass recovery.
2. Experimental
2.1 Equipment
The coagulation tests were carried out in a cylindrical acrylic agitation tank (23 cm high x 19 cm diameter), provided with 4 baffles (1.8x19 cm), equally spaced along the inside of the wall, made of acrylic as well (designed and built by the authors). In the centre of the tank, an acrylic impeller was placed at 6cm from the flat bottom of the tank 5,6. This tank-impeller design was chosen to guarantee the correct distribution of the pulp and to maximize the interaction of the sample with the pH modifiers during the tests 7. A laboratory type oven and a vacuum filtration system were used to dry and filter the processed sample.
2.2 Materials
To perform the selective hydrophobic coagulation tests, a Colombian coal sample from the Guajira, Colombia was used. This coal sample was provided by Panama Coal Company. In order to ensure the floatability of the coal, the samples were ground and sieved down to < 38 μm size and stored in an air tight container to prevent the coal from oxidizing before performing the coagulation tests. Sodium hydroxide and hydrochloric acid were used as pH modifiers, concentrations above 10M were chosen to provide maximum control in pH change without modifying the volume of the slurry in a significant way. Tap water was used as medium for the coagulation tests.
2.3 Experimental procedure
A 50 g coal sample (-400 mesh) was diluted in 2 liters of tap water, until a solid concentration of approximately 0.4% by weight 4,7. The pH of the slurry was adjusted to the desired values adding hydrochloric acid or sodium hydroxide according to experimental design. The slurry was then agitated using a 6cm acrylic impeller. The agitation time (Agt) and the pH were varied according to the experimental design to evaluate the effect of variables during the selective hydrophobic coagulation (SHC) process. The slurry was allowed to stand for 3 to 4 minutes, before being collected, for coagula growth and sedimentation 7. The coagula were separated from the supernatant with a device, and then it was filtered using a vacuum pump and a buchner funnel equipped with a Whatman® type 40 filter. A single cleaning stage was used in the SHC Batch tests. The pH of the slurry and the agitation time were chosen as entry variables for the selective hydrophobic coagulation (SHC) tests.
2.4 Experimental Design
For the batch SHC tests, a 22 factorial central composite design was chosen. A center point, with 4 repetitions, were added to the design to evaluate the quadratic effect between the entry variables and a set of 4 axial points were introduced to expand the model and find a better fit, by increasing the number of degrees of freedom 8. Table 1 shows the values of the variables evaluated in the batch SHC experiments; A is the agitation time (Agt) and B is the pH. Parameters such as agitation velocity, temperature, solids concentration in slurry were kept constant to minimize their interaction with the evaluated variables and eliminate their incidence in the results. Figure 1 shows the schematic of the experimental design.
Table 1 Data for the 22 factorial central composite experimental design
| Variable | Level | |
|---|---|---|
| A | Agitation time | High level (5 min) |
| Low level (3 min) | ||
| Mid level (4 min) | ||
| Low level (2 min) | ||
| High level (6 min) | ||
| B | pH | Low level (4) |
| High level (10) | ||
| Mid level (7.23) | ||
| Low level (1) | ||
| High level (13) |
The criteria followed to choose the pH as the independent variable was the strong influence it has on the coal surface properties, such as the surface charge 9. On the other hand, the agitation time was chosen due the effect it may have in response variables such as mass recovery. The experimental error is based on repeated experiments at the central points of experimental design and was ±5%.
3. Results and discussion
3.1 Coal
Ash content analysis was performed in the coal raw and processed samples, the results are shown in Table 2. The results shown in Table 2 suggest that the coal sample is a high rank bituminous coal, given that the calorific value (13,759 Btu/lb) exceeds 11,500 Btu/lb 10 which could be due to the low ash content in the sample (3.97%). It also noticeable the low content of sulphur in dry basis (0.65%).
Table 2 Proximate analysis
| Analysis | As received | Dry basis* |
|---|---|---|
| (%) Ash | 3.72 | 3.97 |
| (%) Volatile | 38.31 | 40.89 |
| (%) Fixed carbon | 51.65 | 55.14 |
| (%) Sulphur | 0.61 | 0.65 |
| Heat Value (Btu/lb) | 12889 | 13759 |
| (%) Nitrogen | - | 1.61595 |
| (%) Carbon | - | 76.9215 |
| (%) Hydrogen | - | 5.06615 |
| (%) Oxygen | - | 15.7464 |
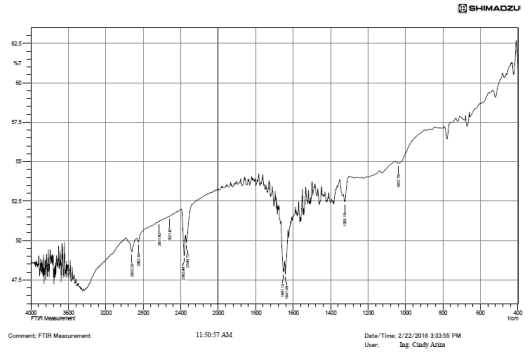
Using Fourier Transform Infrared Spectroscopy (FTIR), as can be seen in the Figure 2, it was possible to qualitatively identify the functional chemical groups present in the raw coal sample. The spectrum shows a variety of vibrations corresponding to groups such as: Minerals (460 - 540 cm-1); C=O, Aromatic-O-Aromatic (1,100 - 1,380 cm-1); Nitrite groups bonded to alkenes and aromatic structures (1,330 - 1,530 cm-1); C=C (1,600 - 1,650 cm-1); short aliphatic chains (2,850 cm-1) and long aliphatic chains (2,920 and 3,920 cm-1); CH2-CH3 (2850 cm-1) and -NH-NH2-OH- groups (3,400 - 3,700 cm-1). It is worth noting results represent characteristic groups, and no specific structure can be identified through this analysis due to the complex structure of the coal. The group C=C, for instance, can be attached to complex and simple aromatic structures as well. Characteristic wavelengths were taken from other papers based on bituminous coal research 11-14.
3.2 Batch selective Hydrophobic Coagulation Tests Results
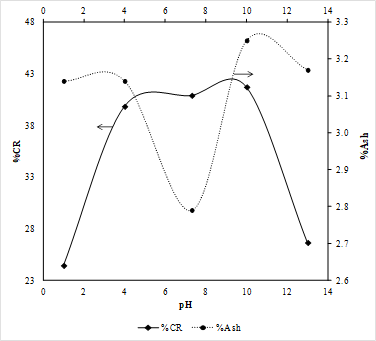
Figure 3 shows the results for the selective hydrophobic coagulation test performed in the coal sample. The highest value for the combustible recovery (CR) is 41.71%, at alkali pH (pH 10), however, a sharp drop in the combustible recovery is found when increasing the pH; the same behavior is present at pH values lower than 4. For acidic condition (pH 1 and 4), mineral matter present in the coal sample were coagulated, while organic matter in coal became dispersed, the same behavior for alkali conditions (pH 10 and 13) was observed; indicating that at extreme acid and alkaline conditions, the concentration of mineral matter in the sample is high, with low combustible recovery. On the other hand, pH range of 4 to 10, it was found that, for this coal sample, combustible recovery reaches its highest values while the ash content decreases significantly; which indicated that pH range of 4 to 10 the organic matter coagulates while the mineral matter present in the coal sample became dispersed 3,4. This may be because that the selective hydrophobic coagulation is more efficient at recovering organic matter from the coal sample to neutral conditions. This behavior may be due to the fact that, for this coal sample, the point of zero charge (pHzpc) was found around pH 6.2, which is close to neutral conditions, favoring the combustible recovery at these conditions 16.
The favorable effect of pH increase on the selectivity (coagulation of coal and dispersion of ash), may be attributed to the chemical effects of hydroxyl ions on the hydrophobicity of coal and the mineral matter present in the coal. In other words, pH range of 4 to 10 induce a significant decrease in the hydrophobicity of the mineral matter in coal, which is evident in the low ash content in the recovered sample, indicating it gets dispersed; while the hydrophobicity of the organic matter high, so the coal particles coagulate and float. At extremely high and low pH conditions (high concentrations of hydrogen ions, and hydroxyl ions respectively) the hydrophobicity of the mineral matter, represented in this case with the ash content is high, resulting in its coagulation with the organic matter present in the coal sample as shown in Figure 3.
3.3 Effect of pH and agitation Time on Combustible Recovery
The combustible recovery was determined using Equation 1 (Piñeres & Barrza, 2009), the effect of pH on CR are shown in Figure 4.
Where Mclean: mass of clean coal, Mfeed: mass of feed, Aclean: ash content of clean coal, A feed: ash content of feed coal. The highest combustible recovery value was found to be 41.8%, at pH 10, it is also noticeable in Figure 4 that at extreme pH conditions, acidic or alkaline, combustible recovery presents its lowest values. The lowest value of combustible recovery was found at pH 13, suggesting that high concentrations of OH+ affect in a significant way the hydrophobicity of the sample inducing the coal to disperse rather than coagulate.
In coagulation and flotation the pH is of outmost importance, OH- and H+ ions affect the surface potential of the coal, inducing a change of polarity degree and structure of the double electrical layer between water and coal 15. For the coal sample processed during the coagulation tests the highest values of combustible recovery were found pH range of 4 to 10, while the lowest are found at acidic condition (pH 1) and alkali conditions (pH 13). These findings suggest that the coal sample contains minerals in its surface which present an increase in hydrophobicity when interacting with H+ and OH- ions.
On the other hand, for pH range of 4 to 10, the electrical interactions of the H+ and OH- ions, the coal organic surface and the mineral matter inorganic properties balance so that the mineral matter gets either dispersed (alkaline conditions) or precipitated (acidic conditions) due to their hydrophilicity, while the coal coagulates; under these pH conditions the double layer of the coal particles change in such a way collide them to they are adhered hit each other and float, which ultimately lead to high mass recovery, translated then into high combustible recovery. It was also observed during the coagulation tests that the coagula presented fine particle and concentrated around the agitator in acidic conditions, and the portion of the sample that went to the bottom of the tank presented the larges particles and dense, with little presence of particles suspended in the supernatant; on the other hand, for alkaline pH conditions, the coagula was formed toward the walls of the tank, and were denser than the ones formed in acidic conditions, and presented more suspended matter and little tailing.
These finding show how the H+ and OH- ions interact changing the coal surface properties, modifying the electrical double layer and making the particles become more attached to each other and either float or tailings depending on the ion present. It is also noticed the little effect that the agitation time has on the combustible recovery, nevertheless, is it possible to point out that the higher the time, the higher the combustible recovery is, except in extremely alkaline conditions, where the coagula is thickest and more attached to the walls of the recipient, and the long agitation period may to affect the structure of the coagula, breaking the biggest ones and dispersing them into the liquid. The behavior of the combustible recovery (CR) at extreme pH values may be due to the formation of complex Aluminum structures in acidic conditions and the oxidation of the pyrite at extremely alkaline conditions 15.
3.4 Effect of pH and Agitation Time on ash Rejection
The ash rejection, AR (w/w %) was calculated using the following equation
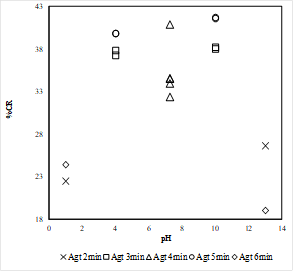
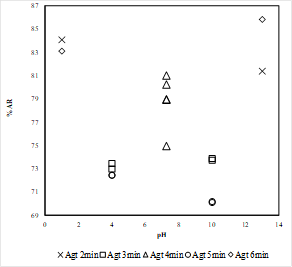
Where Mclean: mass of clean coal, Mfeed: mass of feed, Aclean: ash content of clean coal, Afeed: ash content of feed coal. The results for ash rejection (AR) in the coal sample, for all operating conditions are shown in Figure 5. In general, through the batch selective hydrophobic coagulation tests, ash rejection above 70% was obtained. The highest ash rejection value was found at alkaline conditions, pH 13. However, similar behavior is shown in acidic conditions, pH 1, which suggests that the superficial effect caused by the interactions between the coal and the hydrogen/hydroxyl ions are similar under the conditions given in the coagulation tests.
The highest ash rejection value was found at an agitation time of 6 minutes, which also corresponds to the highest pH value, as shown in Figure 5. Analyzing the Figure 5, it is also possible to observe that the ash rejection values are not significantly affected by the agitation time, especially in acidic conditions, in which the results are almost identical for agitation time (Agt) of 2 and 6 minutes. On the other hand, the differences in ash rejection values become more evident while the pH increases, showing the general tendency of having better ash rejection results at higher agitation time.
3.5 Statistical analysis
Experiments in the hydrophobic selective coagulation process were carried out according to the experimental design. Seventeen experimental run were conducted for coal sample. The data of combustible recovery and ash rejection were analyzed statistically using Design expert software. The results of the significance of the variables and their interactions are presented in Table 3.
Table 3 Significance of the variables and their interactions. Combustible recovery and ash rejection.
| Source | df | %CR | %AR | F critical |
|---|---|---|---|---|
| F calculated | F calculated | |||
| Model | 5 | 29.332 | 18.372 | 6.632 |
| A | 1 | 0.740 | 0.043 | 11.259 |
| B | 1 | 29.350 | 57.769 | 11.259 |
| AB | 1 | 8.349 | 0.883 | 11.259 |
| A2 | 1 | 29.607 | 57.731 | 11.259 |
| B2 | 1 | 29.812 | 57.889 | 11.259 |
| Residual | 11 | - | - | - |
| Lack of Fit | 3 | 3.999 | 2.639 | 7.591 |
| Pure Error | 8 | - | - | - |
| Total | 16 | - | - | - |
A = Agitation Time
B = pH
Table 3. Significance of the variables and their interactions. Combustible recovery (% CR) and ash rejection (% AR), A (agitation time), B (pH). Coefficient correlations are: R2=0.93 for %CR and R2=0.90 for %AR
The results of the significance of the variables and their interactions are presented in Table 3. Table 3 show that the model has a significant (95% confidence level) effect Fcalculated>Fcritic), while for the lack of fit shows no significant effect (Fcalculated<Fcritic), indicating that the regression models were found to be highly adequate for accurate prediction of the response variables. It is possible to see in Table 3 that the agitation time (A) has little significant effect on the %CR compared to the pH, agitation time (A) (Fcalculated<Fcritical), not present significant effect, this confirms the hypothesis made in the discussion of the effect of agitation time and pH on combustible recovery material (Figure 4). The pH has a significant negative effect (Fcalculated>Fcritical) on the %CR, which in this case means that the interactions of H+ and OH- ions with the coal surfaces and mineral matter play a significant role in the cleaning of coal using selective hydrophobic coagulation process, it is also possible to notice that the interaction AB is not significant, while the quadratic effects of agitation time and pH are significant in a negative and positive way respectively. The above indicates that the CR decreases by the inverse of the square root when pH increases, the curvature effects suggest that in some point of the surface of response there is a maximum value of CR in which the pH is high, shown in Figure 3.
The effect of pH on the coal sample depends mainly on the coal surface properties and the change of hydrophobicity/hydrophilicity of the mineral matter present, the presence of H+ and OH- ions maintain the high natural hydrophobicity of the coal sample, as shown in the electrokinetic study of the coal sample, the hydrophobicity of this sample coal is high, with a contact angle of 63° in water and almost 0° for n-hexane 16. All of the above mentioned indicates that by inducing changes on the pH of the coal slurry in a pH range of 4 to 10, maintaining good agitation (constant time of agitation ) there will be a rather high combustible recovery. The analysis for ash rejection shows that the agitation time has no significant effect (Fcalculated<Fcritic), while the pH value does have a significant effect over the %AR. Curvature effects were found for both pH value and agitation time.
Table 3 show that the pH has a significant negative effect on %AR, this may be due to the presence of minerals such as clays in the sample and the oxidation of pyrite. The obtained regression suggests that %AR decreases with a pH increase and the curvature effects indicate that, in a given point of the response surface, there is a maximum value for %AR at pH value, as shown in Figure 5 (constant time of agitation). The positive and negative curvature effects in the response surface also show that there is a maximum and a minimum value of %AR and it may depend on the coal surface properties interacting with the ions present at a given pH value.
5. Conclusions
Through batch tests of selective hydrophobic coagulation performed in a coagulation cell at constant agitation velocity, with a single cleaning stage, it was possible to obtained to 40% of ash removal, this value was reached at pH value 7.3 and 4 minutes of agitation time. It was also possible to conclude that the selective hydrophobic coagulation process for the studied sample is more selective and efficient in a pH range of 4 to 10, due to the fact that the highest values of combustible recovery and lowest ash content were obtained in this interval of pH.
The highest values of ash rejection were found in a pH of 1 and 13, corresponding to 86% and 84% respectively. In general, through selective hydrophobic coagulation of the coal sample, values above 70% of ash rejection were reached in all of the cases.
Statistical analysis shown it was possible to conclude that the pH has a significant effect in combustible recovery and ash rejection, while agitation time is considered not significant.