1. Introduction
The department of Norte de Santander is renowned for its agricultural production, mainly coffee, rice, and cocoa. Based on the primary information collected through a survey applied to coffee growers and rice farmers in the study area, it was determined that Chlorpyrifos is one of the most widely used agrochemicals in this type of crop. This type of substance is usually spread over the crop employing fumigation equipment, which, when cleaned, causes liquid waste that subsequently reaches water sources, which alter its composition and cause contamination.
Due to the presence of pesticides in water sources, different investigations have been carried out for the removal of contaminants. For this reason, research such as that carried out by Thind, P., Kumari, D. and John, S 1 on ultraviolet photocatalysis using Chlorpyrifos TiO2 / H2O2, developed at the University of Technology, Chandigarh India; determined the optimal conditions for this type of compounds. According to the results, it was possible to obtain a degradation of 68.29% of the COD. On the other hand, Berberidou, C., Kitsiou, V., Lambropoulou, D., Antoniadis, A., Ntonou, E., Zalidis, G. and Poulios, I 2, evaluated an alternative method for water treatment residuals with pesticide content at the Aristotle University of Thessaloniki, Thessaloniki, Greece, in which solar photocatalytic oxidation and constructed wetlands were used, thereby obtaining 87% COD removal.
The aim of this study is to evaluate the efficiency of a two-step system of heterogeneous photocatalysis coupled with aerobic biological process, as a sustainable process for the removal of Chlorpyrifos on agricultural wastewater; and thereby minimize the impact of pesticides on the local agricultural community of Norte de Santander.
2. Methodology
2.1. Wastewater characterization
Samples of the fumigation equipment cleaning were taken after applying the pesticide in a coffee crop in the village of El Alto, in the municipality of Bucarasica and a rice crop in the village of El Encanto (Cúcuta, Norte de Santander). Subsequently, its physicochemical characterization was carried out, and from this characterization, synthetic samples similar to the original ones with which the research tests were performed were prepared. The parameters analyzed were: Chemical Oxygen Demand - COD, pH, Temperature, and Dissolved Organic Carbon - COD 3.
2.2. Sludge characterization
Aerobic sludge from a domestic wastewater treatment plant was used as inoculum. The sample was analyzed to determine its quality and potential for use in the treatment of wastewater. The parameters analyzed were Total Suspended Solids (SST), Volatile Suspended Solids (SSV), Hydrogen Potential (pH), Sludge Volume Index (IVL), and Oxygen Consumption Rate 3.
2.3. Breathing inhibition test
The toxic effect of the pollutant load of the wastewater on the microorganisms present in the inoculum sludge was determined through the method of Inhibition of Respiration for Activated Sludge - OECD 209 4) was used. Chlorpyrifos concentrations equivalent to 0, 200, 400, 600 and 800 mgO2.L-1 of COD were evaluated against a sludge volume equivalent to 1200 mg.L-1 of SSV in a 300 mL container. The percentage inhibition of Chlorpyrifos on microorganisms was calculated using Eq. 1.
2.4. Biodegradability test
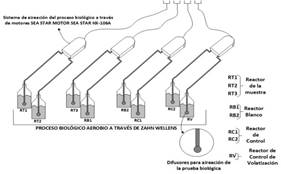
Biodegradability of the samples was determined from the Zahn-Wellens EMPA - OECD 302B method 5. For the samples, eight 1L amber glass flasks were prepared as presented in Figure 1. 3 with samples were used for monitoring the biodegradability of Chlorpyrifos (RT) (Table 1). two flasks without pesticide samples (RB). Two flasks as control reactors (RC) and one flask as volatilization control vessel (RV). Each of the tests had a duration of 28 days.
Table 1 Sample preparation
| Sample reactors | ||||
|---|---|---|---|---|
| RT | RB | RC | RV | |
| Zahn wellens media (mL) | 741 | 741 | 741 | 741 |
| Sludge (mg.L-1 of SSV) | 1200 | 1200 | 1200 | 0 |
| Chlorpyrifos (mgO2.L-1 of COD) | 400 | 0 | Ethylene Glycol as reference compound | 400 |
The percentage of biodegradation of Chlorpyrifos was evaluated in terms of COD and COD. The first sample was taken 3.5 hours after starting the test, and subsequently every 4 days until the end of the test. The percentage of biodegradation was determined by Eq. 2.
2.5. Evaluation of photodegradation of wastewater containing Chlorpyrifos
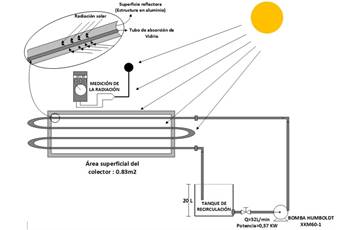
The photocatalysis process was carried out in a Laboratory Scale-CPC Composite Parabolic Collector (Figure 2), using titanium dioxide - TiO2 Degussa P25 as a catalyst, sunlight as an energy source and a hydrogen peroxide concentration of 100 ppm 1. The CPC was located in the North-South direction, taking into account the path of solar irradiation in Cúcuta 6, looking for a plane perpendicular to the sun's rays and thus having greater efficiency in the collection of solar radiation.
The UV radiation was measured by adapting an SP110 Pyranometer solar radiation sensor with a UNI-T UT71C multimeter. The samples were taken at accumulated energies of 0, 20, 40, and 60 KJ.L-1, taking into account the levels used by Becerra 7, to perform monitoring of degradation by COD. Accumulated energy measurements were calculated, taking into account Eq. 3 8.
The experimental design consisted of a non-factorial design 32 (three levels, two factors). The variables evaluated were the pH and catalyst concentration according to a study reported by Malato, et al. S.f. 9; Also, the percentage (%) of degradation expressed in terms of COD was used as the response variable. The evacuated pH was established, taking into account what was reported by Thind et al. 1; also, the catalyst concentrations were fixed according to those used by García 10. The levels used in pesticide degradation are shown in Table 2, and its resolution in Table 3.
Table 2 Variables of the experimental design
| Factors | Low | Medium | High |
|---|---|---|---|
| TiO2 (mg.L-1) | 100.0 | 350.0 | 600.0 |
| pH | 3.0 | 6.0 | 9.0 |
Table 3 Full design for the variables selected
| Experiment | Block | TiO 2 (mg.L -1 ) | pH |
|---|---|---|---|
| 1 | 1 | 100 | 9 |
| 2 | 1 | 350 | 6 |
| 3 | 1 | 600 | 3 |
| 4 | 1 | 600 | 9 |
| 5 | 1 | 600 | 6 |
| 6 | 1 | 100 | 3 |
| 7 | 1 | 100 | 6 |
| 8 | 1 | 350 | 6 |
| 9 | 1 | 350 | 9 |
| 10 | 1 | 350 | 3 |
Finally, the degradation percentage of the sample studied was applied in Eq. 4 1.
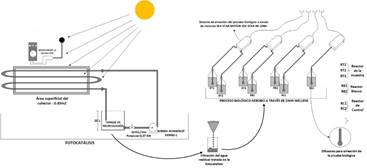
2.6. Coupling of photocatalytic and biological processes
From the optimal operating conditions of the process by photocatalysis obtained from the design of experiments, results obtained from the design of experiments, the wastewater was treated using accumulated energy of 60 KJ.L-1, using 100 mg.L-1 peroxide of hydrogen and 400 mgO2.L-1 of COD of wastewater.
Once the process was finished, the photo treated water was filtered, the pH was neutralized, and its biodegradability 5) was determined, as seen in Figure 3.
3. Results and discussion
3.1. Wastewater characterization
According to the results obtained (Table 4), the values of COD from the coffee crops double the permissible limit value in point discharges of non-domestic wastewater (150 mgO2.L-1), established in resolution 0631 of 2015 11. On the other hand, the samples show COD concentrations of 73 and 46.3 mg.L-1, respectively. These results are within the COD range of rivers contaminated with pesticides in Colombia 12. This type of analysis is essential since organic carbon is considered as the most common cause of water quality damage 13.
Table 4 Characterization of wastewater from coffee and rice crops
| Parameters | Coffee crops | Rice crops |
|---|---|---|
| COD (mg 02.L-1) | 340.54 | 129.73 |
| pH | 8.32 | 7.90 |
| Dissolved Organic Carbon (mg.L-1) | 73.00 | 46.37 |
| Temperature (ºC) | 23.40 | 26.50 |
When performing the pH and temperature analyzes at the sampling sites, a pH of 8.32 and 7.9 and a temperature of 23.4 and 26.5° C were found for the water samples of the coffee and rice crop respectively. From these results, it can be inferred that the temperature allows the implementation of possible aerobic biological treatments since these processes are active up to temperatures close to 50º C 14. Regarding the pH, it is possible to determine that it complies with current regulations since it is within the range of 6 to 9, this water being suitable for the development of biological processes14. Finally, taking into account the results obtained for COD and COD concentrations, it is observed that the discharge of these waters can induce the contamination of water sources and the reduction of the conditions for aquatic life of both animal and plant species 14.
3.2 Sludge characterization
Table 5 shows that aerobic sludge possesses optimal characteristics for the development of biodegradability tests; since its pH (7.9) can allow the optimal reproduction of microorganisms and increase the sedimentation of organic load 15. On the other hand, the IVL was determined for the pure sludge and a 1: 4 dilution, whose result was 66.15 ml.g-1, a value that is below 80 ml.g-1 indicating that the sludge has proper compaction and sedimentation characteristics of flocculent biomass 16. On the other hand, the IVL value was determined for the pure sludge and a 1: 4 dilution, whose result was 66.15 ml.g-1, a value that is below 80 ml.g-1 indicating that the sludge has proper compaction and sedimentation characteristics of flocculent biomass 16. Finally, the Oxygen Consumption Rate was determined, obtaining a value of 17.97 mg O2 g-1 SSV.h, which is in the range of 12 to 20 mg O2 g-1 SSV.h, indicating that the mud has a normal TCO. This represents good sedimentation, low probability of toxic presence, high biological activity, and timely microbial growth 7.
3.3. Breathing inhibition test
Table 6 shows that the inhibition percentages behave proportionally to the concentration of agricultural wastewater; the higher the concentration, the greater the inhibition. From this information, a curve was constructed with the percentages of inhibition based on COD concentrations. The latter allowed us to determine the effective concentration that causes a 50% reduction (EC50). According to the results obtained, the interpolation of the graph was 550.47 mg.L-1. The latter corresponds to the limit value of concentration possible in biodegradability tests. To develop the tests under aerobic biological processes, a base concentration of 400 mg.L-1 was used, which corresponds to an inhibition percentage of 46%, below the EC50.
3.4. Biodegradability test
The results of the biodegradation in terms of COD and COD can be found in Table 7. From the follow-up to the different tests performed, it was found that the pH was within the optimum range of 6-8.5, which is stipulated in the OECD 302B5, in which microorganisms can reproduce and develop properly17. Likewise, the average temperature of the experiments remained at 26ºC, being optimal for the development of the bacterial activity, since it is below the maximum limit of 50ºC13. Therefore, these parameters were not a limiting factor in the activity of degradation by microorganisms.
Table 7 COD and DOC biodegradation in samples.
| Time (days) | COD Biodegradation (%) | DOC Biodegradation (%) | ||||
|---|---|---|---|---|---|---|
| RT* | RC** | RV*** | RT* | RC** | RV*** | |
| 0,15 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 58 | 100 | 33 | 40 | 98 | 5 |
| 8 | 67 | 78 | 50 | 23 | 86 | 23 |
| 12 | 76 | 89 | 67 | 64 | 100 | 53 |
| 16 | 82 | 96 | 67 | 66 | 94 | 60 |
| 20 | 82 | 93 | 67 | 62 | 100 | 80 |
| 24 | 64 | 93 | 83 | 74 | 98 | 89 |
| 28 | 64 | 100 | 83 | 100 | 84 | 91 |
*reactors with the sample
**Control reactors with Ethylene Glycol
***Volatilization control reactors
According to the results, the sludge employed as inoculum was suitable for the biodegradation process; since the ethylene glycol (control reactor, or RC) was degraded entirely before the 14th day of the test. The above is following the provisions for the Zahn Wellens test, in which it is required that the percentage of degradation after 14 days must be higher than 70%.
On the other hand, the reactors containing the test substance (RT) presented 64% COD removal and 100% COD at 28 days after the test. Finally, in the volatilization reactors (RV), 83% of the COD and 91% of the COD were removed, so it can be inferred that the pesticide was transported to the atmosphere and did not biodegrade. During the activated sludge process, there is a risk of volatilization of certain compounds, mainly due to the diffusion of the air or the turbulence that it generates when entering the reactors18. A similar case occurred in the study of Barba & Becerra19, in which pesticide 2.4 D was removed due to physical volatilization phenomena and not biochemical processes. A result that is important to highlight that the organic matter contained in the sample reactor (RT) was not volatilized; This is due to a fast change in the concentration of COD (from 400 to 260 mgO2.L-1 in 3.5 hours).
The Adsorption of the pesticide in the aerobic sludge could occur because the organic matter adheres to the floc and the deposited residue oxidizes as the aeration enters (18. However, the degradation was not active when there was evidence of a possible accumulation of non-biodegradable and toxic compounds that inhibited the microorganisms, causing their death and immobilization, thus preventing degradation of the contaminating organic load of agricultural wastewater containing Chlorpyrifos (20. This is corroborated by the reduction of volatile suspended solids. The latter is consistent with the studies carried out by Marcelino et al., 21 and Becerra 7, in which they found that the test substances present in wastewater are absorbed in the aerobic sludge, rather than mineralized. Research carried out in Europe by Greenpeace 22, has shown that the volatilization phenomena presented by aerobic biological treatments affect, both directly and indirectly, the quality of natural resources and biodiversity. Studies have determined at least 53 pesticides in samples of captured pollen and approximately 17 in samples of honeycomb pollen; Chlorpyrifos is one of the 7 agrochemicals detected that harm bee.
Finally, it can be concluded that the biological process studied was not efficient for the degradation of the contaminating organic load of agricultural wastewater containing Chlorpyrifos, since volatilization phenomena caused the removal of the pesticide. Therefore, it is necessary to couple it with an advanced oxidation process, to treat effluents with toxic substances that inhibit the microorganisms of biological processes.
3.5. Evaluation of photodegradation of wastewater containing Chlorpyrifos
Table 8 shows the results of the analysis of variance (ANOVA), where it was found that the model is statistically significant, since the maximum error accepted by the test is 5% and in this case 3 of the 5 effects present P values below 0.05, representing a 95% level of confidence in the results.
Tabla 8 ANOVA table
| Source | Sum of squares | Liberty degrees | Medium Square | F-value | P-Value |
|---|---|---|---|---|---|
| A:Concentración de TiO2 | 223.626 | 1 | 223.626 | 10.67 | 0.0309 |
| B:pH | 739.038 | 1 | 739.038 | 35.26 | 0.0040 |
| AA | 105.885 | 1 | 105.885 | 5.05 | 0.0879 |
| AB | 98.8036 | 1 | 98.8036 | 4.71 | 0.0957 |
| BB | 183.018 | 1 | 183.018 | 8.73 | 0.0418 |
| Total error | 83.8398 | 4 | 20.9599 | ||
| Total (corr.) | 1490.19 | 9 | |||
| Model correlation R2= 94.37% | |||||
| R2 (adjusted from the liberty degrees) = 87.34% | |||||
On the other hand, it can be affirmed that the quadratic model is adequate to describe the relationship that exists between the variables that affect the percentage of photocatalytic degradation since at least 3 degrees of freedom must be available and, in this model, there are 4 23. Likewise, it presented an R2 correlation of 0.9437, being close to 1, reducing the probable causes of error by 5.63%. Taking into account the results, it was shown that the pH has a more significant effect than the catalyst concentration on the percentage of degradation.
Taking into account the above, the degradation will be more efficient in acid medium, as cited in Blanco et al. 9, In which it is explained that the photocatalytic process is more efficient in a range of 3 to 5 pH units. Likewise, the TiO2 catalyst acts better in smaller quantities; this happens due at higher concentrations the residual water can be supersaturated with said catalyst and prevent the entry of the sun's rays and consequently decrease the degradation of the pesticide 1.
3.5.1 Regression coefficients and surface
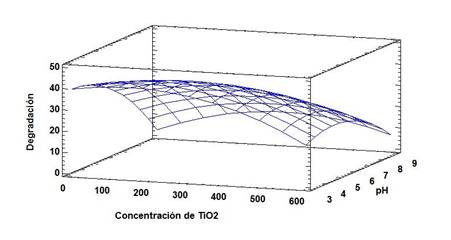
From the results, it was possible to construct a quadratic equation (Eq 5) that explains the behavior of the variables and maximizes the degradation of chlorpyrifos in the evaluated sample (Figure 4).
In Figure 4, the region between the concentrations of 100-300 mg.L-1 of TiO2 and pH of 3-5 represents the combination of optimal pH and concentration, where the highest percentage of degradation is generated. Finally, the photodegradation of the contaminant load of the wastewater containing chlorpyrifos had a degradation percentage of 44.18%, similar to that achieved by Thind et al.,1 of 68.29%, and that obtained by Duarte of 56% for the degradation of thiodan 3524. It is important to highlight that despite not achieving total mineralization, it is considered that heterogeneous photocatalysis is an interesting alternative to treat wastewater containing pesticides, since it allows to obtain effluents with a low concentration of organic compounds.
3.6 Coupling of photocatalytic and biological processes
For the development of a coupling process between a photocatalytic and biological method, the operational conditions obtained in the previous phases were used. The percentage of degradation of chlorpyrifos was monitored employing COD and COD analysis at 60 kJ.L-1, obtaining the results of Table 9. Results shown a COD degradation of up to 47%. This value is within the predictions of the developed model (30.13 to 52.84%).
Table 9 Degradation of the sample after the photocatalytic process
| Photocatalysis | COD (mgO 2 /L) | COD (ppm) |
|---|---|---|
| Inicial value | 551.35 | 69.62 |
| Final value | 194.59 | 36.77 |
| Degradation (%) | 64.71 | 47.19 |
The effluent obtained from the photocatalytic process was filtered, and a volume of nutrients and a volume equivalent to 1,200 mg.L-1 of SSV was added, as shown in Table 10. The biological process lasted 28 days.
Table 10 Characteristics of the sludge employed
| Sludge | |
|---|---|
| TSS (mg.L-1) | 35.986 |
| VSS (mg.L-1) | 12.246 |
| IVL (mL.g-1) | 76.70 |
It was observed that the sludge retained its quality since its SSV content was high and the IVL was below 80 mL.g-1, presenting proper compaction and sedimentation characteristics. The biodegradability test was monitored using the parameters of pH, temperature, SSV, COD, and COD.
The biological test presented an average value of approximately 27ºC and pH 6-8.5, is optimal for the development of the bacterial activity 15,17,25; Also, the reference compound exhibited a degradation higher than 70% in less than 14 days, as cited in NTC 4255.
Finally, the efficiency of the entire process was evaluated in terms of COD and COD, obtaining oxidation of organic matter of 88.24% and mineralization of 67.78% (Table 11). Also, the effluent studied showed low COD concentrations, which is below the maximum discharge limit for non-domestic wastewater, cited in resolution 0631 of 201511. These results allow us to deduce that the coupled process is effective in reducing pesticide levels.
Table 11 Result of the coupled treatment
| Coupled treatment | COD (mgO 2 /L) | COD (ppm) |
|---|---|---|
| Inicial value | 551.35 | 69.62 |
| Final value | 64.86 | 22.43 |
| Degradation (%) | 88.24 | 67.78 |
Taking into account the previous results it is possible to conclude that the wastewater subjected to coupling process was not wholly mineralized, and only a fraction of this waste was transformed into carbon dioxide, and water and the other part was transformed into intermediates 26. Also, it was determined that the degree of oxidation of the pollutant studied was 88.24%.
These results are comparable with those reported by other works using techniques such as photo-Fenton / biological, with values of 94% mineralization (35.5% by the advanced oxidation system and the rest to the biological treatment) 26. Finally, it can be concluded that the coupling of different methods is efficient to degrade the polluting organic load of chlorpyrifos since the application of a single method proved to be insufficient to reduce the contamination of pesticides.
4. Conclusions
The biological process proposed was not efficient in reducing the contaminating organic load of the wastewater containing the active ingredient chlorpyrifos, because in this process its toxic compounds inhibited the microorganisms, which generated adverse effects on the biodegradation. Also, it was affected by the phenomenon of volatilization, which can release toxic compounds harmful to the environment, which could cause adverse effects on ecosystems susceptible to such pollutants.
The heterogeneous photocatalytic process using a CPC showed maximum mineralization of 44.18%. This process is a viable option as a pre-biological treatment for the degradation of several toxic compounds. Finally, by optimizing the concentration of TiO2 and pH (159.19 ppm and 3.47 respectively), its efficiency could be significantly improved.
By developing the process of treating this type of water using a coupling between the two proposed methods, degradation values of up to 88.24% and mineralization of 67.78% could be obtained. This allows to obtain fewer polluting effluents that can be reused; which can increase the availability of water, and thus reducing the impact of ecosystems by the accumulation of toxic elements.