1. Introduction
Microalgae are a novel source of different metabolites and products for different industries such as pharmaceutical, animal feed, nutraceuticals, cosmetics, biofuels as well as a large number of products such as polyunsaturated fatty acids, antioxidants, dyes, fertilizers, soil conditioners, bioflocculants, biodegradable polymers, and polysaccharides.
The production of microalgae for obtaining high value products can be a promising business for a country like Colombia, since according to the BACEX Foreign Trade Data Bank of Colombian government, during the last 9 years, different segments of the Colombian Industry have imported USD 68 Million in coloring pigments for food (carotenoids, phycocyanins, chlorophylls, and others). The latter represents an essential segment due to the growing demand by the pharmaceutical, food, and cosmetic industries, where there are no Colombian companies that produce these metabolites.
Among all the products obtainable from microalgae, dyes (especially phycocyanins and carotenoids) are the most required raw materials in the national pharmaceutical and food industry 1,2; which are imported in considerable quantities due to the lack of companies present in the national territory dedicated to the production of this type of raw materials.
Carotenoids are fat-soluble substances with colors ranging from brown, red, orange to yellow. They perform two critical functions in photosynthesis: 1) absorb light in regions of the visible spectrum, in which chlorophyll is not efficiently absorbed; 2) protect photosynthetic systems. The photoprotection mechanisms eliminate the more active states of chlorophyll, as a result of the excessive absorption of light radiation. The latter hinders the formation of reactive oxygen species (ROS), makes carotenoids good antioxidants 3. The main carotenoids of microalgae are β-carotene, lycopene, astaxanthin, zeaxanthin, violaxanthin, and lutein.
Carotenoids are essential for microalgae since they act as a protective barrier against high light radiations and dissipate excess energy in the form of heat 4. According to the literature, strains such as Muriellopsis sp 5, Scenedesmus sp 6, Chlorella zofingensis7, Chlorella protothecoides8, Chlorella sorokiniana9,10, Coccomyxa acidophila11 and Scenedesmus almeriensis6 are potential candidates for obtaining at the industrial level of carotenoids with industrial interest; However, it is necessary to make adjustments to the cultivation conditions (light, concentration of nutrients, carbon source) that allow improving the metabolism of the product of interest without affecting the total cost of the process.
The carotenoid market has grown steadily in recent years, registering values close to USD 1.24 trillion in 2016 and is expected to reach USD 1.53 trillion in 2021. The β-carotene market is approximately 1200 tons per year (261 million dollars in 2010) and is expected to be 334 million dollars in 2018; currently, its sale price ranges from USD 300/kg to USD 3000/kg, depending mainly on the final concentration and the type of product 12,13. On the other hand, natural Astaxanthin has a market of 300 tons per year with an approximate cost of 1.2 million USD and can reach a sale price of up to USD 7000/kg 14.
Nitrogen (both NO3 - and NH4 +) is one of the critical nutrients in the production of biomass and different algal metabolites 15. Several algal biotechnological processes need to exploit this feature to improve the performance of specific metabolites such as carbohydrates 16 lipids 17, hydrocarbons 18 and biomass 19. The objective of this work is the evaluation of the carbon/nitrogen ratio on the production of microalgae-based carotenoids in a strain of Scenedesmus sp.
2. Materials and Methods
2.1. Microorganism
Scenedesmus sp Scene_UFPS01 was obtained from the collection of microorganisms of the Francisco de Paula University Santander (Colombia) and maintained in Bold Basal medium 20. Initially, the strain was cultured in 500 mL glass reactors with 250 mL of sterile basal Bold medium, agitated by the injection of prefiltered air at an approximate flow of 150 mL air/min, constant radiation of 110 μmolm-2s-1 and a light-dark cycle of 12:12 hours.
2.2. Carbon/Nitrogen ratio
The effect of the Carbon/nitrogen ratio on the biomass growth and total carotenoids deposition was tested. First, three different carbon sources were evaluated: sodium acetate, sodium carbonate, and, sodium bicarbonate. The application of a 32 Central non-factorial design (3 levels, two factors) with response surface experimental design was applied for each of the carbon sources. The experimental design was evaluated using STATISTICA 7.0 software 21 (Table 1).
Table 1 Variables and levels for the evaluation of C/N ratio
| Factors | Levels | ||
|---|---|---|---|
| Carbon source (g/L) | 0.3 | 0.4 | 0.5 |
| Sodium nitrate (mL/L) | 6 | 7 | 8 |
The final concentration of available carbon on each of the carbon sources was adjusted according to their chemical formula (Table 2- 4). The nitrogen stock used in the Bold basal media (25 g/L of NaNO3) was used. As a control, Bold Basal medium, without the addition of extra carbon and a NaNO3 concentration of 10 mL/L (0.25 g/L) was employed.
Each of the experiments was carried out using 250 mL of Bold basal medium 20 with the adjusted nitrogen and carbon source and 30 mL of pre-cultured Scenedesmus sp (12% v/v, alga/medium). Each of the experiments was maintained agitated by injecting prefiltered air at an approximate flow of 150 mL air/min, constant radiation of 110 μmol m-2 s-1 and a light:dark cycle of 12:12 hours during 40 days.
Table 2 Experiments for Sodium Acetate/Sodium nitrate ratio
| Experiment | Sodium Acetate (g/250mL) | Sodium nitrate (mL/250mL) |
|---|---|---|
| 8 | 0.26 | 1.40 |
| 5 | 0.26 | 1.75 |
| 3 | 0.34 | 1.50 |
| 1 | 0.17 | 1.50 |
| 2 | 0.17 | 2.00 |
| 9 | 0.26 | 2.10 |
| 6 | 0.14 | 1.75 |
| 7 | 0.38 | 1.75 |
| 4 | 0.34 | 2,00 |
Table 3 Experiments for Sodium bicarbonate/Sodium nitrate ratio.
| Experiment | Sodium bicarbonate (g/250 mL) | Sodium nitrate (mL/250mL) |
|---|---|---|
| 8 | 0.53 | 1.40 |
| 5 | 0.53 | 1.75 |
| 3 | 0.70 | 1.50 |
| 1 | 0.35 | 1.50 |
| 2 | 0.35 | 2.00 |
| 9 | 0.53 | 2.10 |
| 6 | 0.28 | 1.75 |
| 7 | 0.77 | 1.75 |
| 4 | 0.70 | 2.00 |
2.3. Biomass and total carotenoids quantification
Total biomass (in dry weight) was measured according to the method described by 22 GF/C filters were pre-combusted for 1 hour at 100°C and stored in a desiccator for up to 4 hours, their weight (Cell-free) was recorded employing a 6-digit analytical balance and stored in Petri dishes on a silica gel bed until their use. Once the experiments were completed, 80 mL of medium was filtered (by triplicate) and dried at 60 ° C overnight. The next day the samples were taken to desiccator until a constant weight was reached, and the final weight was recorded using a 6-digit analytical balance.
The extraction and quantification of total carotenoids were developed using the method proposed by 23. The filtered biomass obtained from the previous step was suspended in 20 mL of phosphate buffer (8 mM Na2HPO4, 2 mM NaH2PO4, 140 mM NaCl, pH 7.4) and mixed with 10 mg of 0.2 mm glass beads. The mixture was stirred using a vortex at maximum speed for 10 minutes. To separate the carotenoids, 5 mL of chloroform was added and centrifuged at 3400 RPM for 8 minutes. The process was repeated until the pellet was colorless. The chloroform fraction was stirred and concentrated by rotoevaporation. Concentrated carotenoids were suspended again in chloroform and read spectrophotometrically using Eq 1.
3. Results and Discussion
Scenedesmus sp is an alga belonging to the class Chlorophyceae, which is a small and immobile colony-forming cell in which they are aligned in the form of a plate. The cells contain a single nucleus, which consists of a chloroplast in the central part. Figures 1 and 2 show the different cultures carried out with Scene_UFPS01, in the same way, it is possible to recognize the characteristic morphology of the genus.
In recent years, the design of culture media that adjust to the needs of the strain to be cultured has become increasingly frequent, as an alternative to traditional media. Therefore, they are designed using specific concentrations of sodium nitrate, potassium phosphate, sodium acetate, and other critical nutrients for the production of biomass and deposition of metabolites of industrial interest. It has been found that, for media with acetate, there are no significant variables that influence the production of proteins, while, in media with carbonate, sodium nitrate and potassium phosphate significantly influence the production of this metabolite 24.
Under stress conditions, some microalgae positively regulate specific biosynthetic pathways, which leads to the accumulation of specific compounds. For example, the change in the composition of nutrients can induce stress in the physiological activities of algae, which can trigger a marked increase in the production of carotenoids.
For example, physiological stresses induced by nitrogen (N) limitation, high salinity, or extreme light intensity can initiate the synthesis of secondary carotenoids 25. Recent studies indicate possibilities that microalgae can be used as a natural source of bioactive compounds, such as carotenoids, which have high antioxidant properties. These products can have several applications in nutrition and human or animal pharmacology 26.
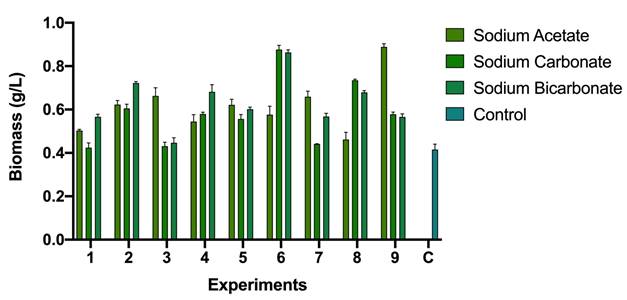
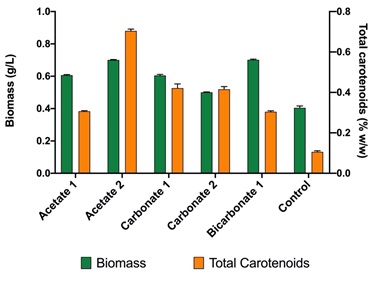
According to the results presented in Figure 2; by the adjustment of the carbon (either carbonate, bicarbonate or acetate) and nitrogen source (sodium nitrate), it is possible to increase the concentration of biomass. In the case studied, values of up to 0.8 g/L of biomass (in dry weight) were obtained, which is double that of the control (0.4 g/L).
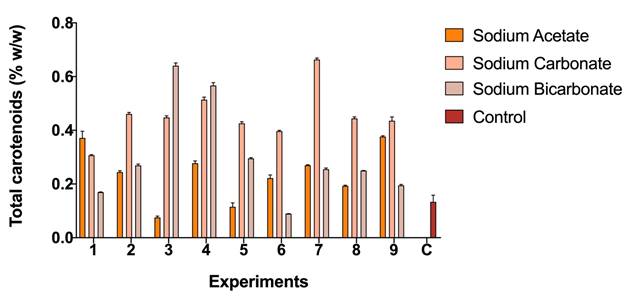
Figure 3 shows the content of carotenoids concerning the biomass obtained for the strain Scene_UFPS01. According to the results, sodium carbonate has the highest values of total carotenoids in all its experiments, with values higher than the control (0.302% w/w) and up to 0.66% P / P. On the other hand, sodium acetate, and sodium bicarbonate obtained values very close to the control. Přibyl, et al. 23 obtained higher concentrations of carotenoids for Dunaliella. salina (8-10% w/w) and Haematococcus pluvialis (6% w/w) under stress due to high deficiency of light, salinity and nutrients, which allows appreciate that the implementation of other stressors may be necessary to encourage the synthesis of total carotenoids.
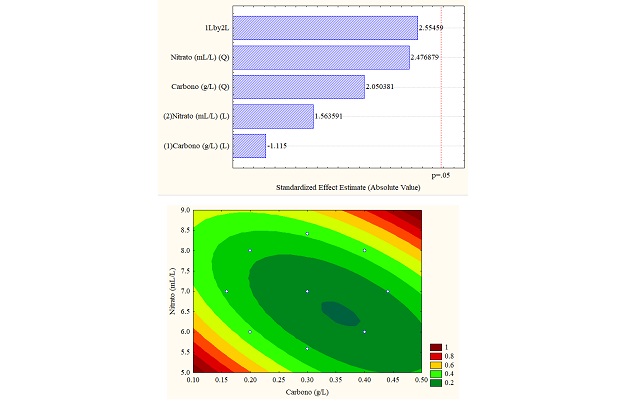
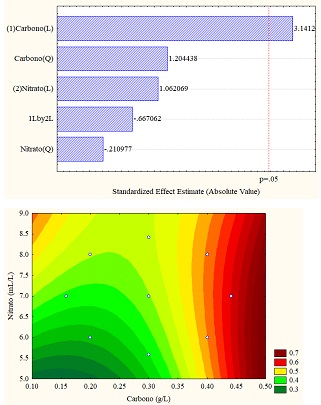
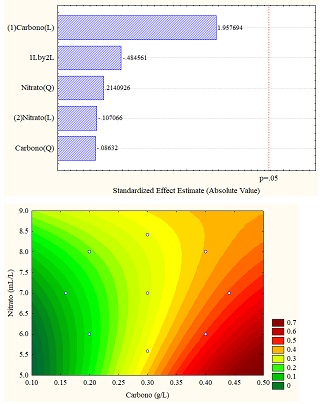
The different results obtained through the application of the design of experiments are better understood through statistical analyzes obtained in the Pareto diagram and response surface graphs, which allow establishing the influence of the variables (carbon vs. sodium nitrate). For the study case, the total carotenoid concentration (in% w/w) was the response variable, which can be seen in Figure 4 (a, b and c). Figure 4b for sodium carbonate shows the significant influence of the carbon concentration in linear function since it exceeds the threshold p = 0.5; however, for the other carbon sources, the variables do not present a significant influence.
Regarding the data obtained from the response surfaces Figure 4 (a, b, and c), it is sodium acetate that reaches values of up to 1% in high concentrations of sodium and carbon nitrate or deficient concentrations of both, exceeding the values obtained in control.
Over the last 20 years, different and unique strains of Scenedesmus sp has been tested for the production of lipids, and carotenoids Table 5 summarize the most relevant studies on this area.
Table 5 Comparison table on the concentration of total carotenoids for different strains.
| Microalgae | Total Carotenoids | Reference |
|---|---|---|
| Scenedesmus oblicuos | 17.4 mg/L | Bishop, 1996 (27) |
| Scenedesmus quadricauda | 1.75 mg/L | Song & Pei, 2018 (28) |
| Scenedesmus bijugus | 2.9 mg/g ó 0.47 mg/L | Minhas et al., 2016 (29) |
| Scenedesmus almeriensis | 3.8 mg/L | Sanchez et al., 2008 (6) |
| Scenedesmus oblicuus | 0.98 mg/g | Qin et al., 2008 (30) |
| Scenedesmus quadricauda | 6.74 mg/g | Kozlova et al., 2017 (31) |
| Scenedesmus bajacalifornicus | 25.07 mg/L | Patil & Kaliwal, 2016 (32) |
| Scenedesmus sp | 20 mg/L | Přibyl et al., 2015 (33) |
| Scenedesmus sp Scene_UFPS01 | 20-65 mg/L | This work |
3.1. Optimization of Carbon/Nitrogen ratio for biomass and total carotenoids production
Taking into account the results obtained, we obtained a series of equations that represent the mathematical models of carotenoid production according to the concentration of the carbon source and the source of nitrogen used. Table 6 presents the equations obtained from the response surface graphs and the operating conditions for each of the cases.
Table 6 Equations of C/N ratio.
| Experiment | Equation % P/ P | Carbon surce (g/L) | Sodium nitrate (mL/L) |
|---|---|---|---|
| Control | - | - | 10 |
| Acetate 1 | Z= 6.0479974522161− 10.003528882418x + 6.3576591263102 𝑥2− 1.292605102042y +0.07680108027039y2 +0.84679798968709xy+0 | 0.1 | 2 |
| Acetate 2 | 0.7 | 9.5 | |
| Carbonate 1 | Z=−0.4214753703782+0.043354041205401x+3.736567958183𝑥2+0.1829099473951y−0.0065452112483499 𝑦2−0.22123319211982xy+0 | 0.6 | 5 |
| Carbonate 2 | 1 | 5 | |
| Bicarbonate 1 | Z=−0.13286361959801+4.7572139901267x−0.73017190853271𝑥2−0.12892867758564y+0.018109867293955𝑦2−0.13818540495544xy +0.0 | 0.6 | 2 |
The results obtained are presented in Figure 5, in which higher concentrations of total carotenoids were achieved than those obtained with the control (0.13% w/w) for acetate 2 and carbonate 1, which is related to the results obtained previously, due to the presence of concentrations less than 1 g/L of carbon and medium-high concentrations of sodium nitrate. Similar behaviors were found by 34 who managed to improve the production of carotenoids with the use of sodium carbonate. On the other hand, 35 did not find essential results in the implementation of diverse carbon sources since the content of carotenoids (Lutein) did not vary significantly.
4. Conclusions
The results of this study indicate that carbon/nitrate ratio It is an exciting process to improve the production of biomass and metabolites of industrial interest such as carotenoids. Concerning to carbon source, results shown that the alga can effectively use the different concentrations of sodium carbonate, sodium bicarbonate and sodium acetate, with an increase on biomass production. Of up to 0.7 g/L (with a control of 0.4 g/L). However, is the specific ratio between the carbon source employed and the concentration of the nitrogen source shows that an outstanding increase on the final biomass and the concentration of total carotenoids that can be produced. Finally, the effect of well-known strategies such as light, salinity and pH, coupled with C/N ratio must be studied to achieve a proper method to stress the cell culture and enhance the synthesis of carotenoids in Scencedesmus sp.