1. Introduction
The textile industry is characterized by being highly polluting by using a large amount of energy, chemicals, and water during the production process. Textile manufacturing contains different processes, the most common of which are rinsing, washing, bleaching, and dyeing. The largest amount of contaminating dye is discharged from the dyeing stage, and also in the rinsing stage. Industrial wastewater from textile industries contains different types of dyes and other organic and inorganic pollutants. Especially in fiber processing, a high degree of water contamination occurs once it is used, and in most cases, it ends up in bodies of water without pre-discharge treatment 1,2.
Discharges of textile wastewater with recalcitrant substances not only represent a problem in wastewater treatment plants (WWTP), but also seriously affect the biological processes of the organisms present in the bodies of water, increasing the chemical demand for oxygen (COD) and biochemical oxygen demand (BOD). Harmful effects on human health are also the result of this increase. Several studies have shown the mutagenicity of some dyes and their potential carcinogenic effect 3,4.
The textile industry represents about half (54%) of the colored effluents in the world, followed by the dyeing industry (21%), the pulp and paper industry (10%), the tannery and painting industry (8%) and the dye manufacturing industry (7%) 2.
Commonly used treatments for wastewater from the textile industry are flocculation and adsorption, in combination with biological and oxidative treatments. These processes generate large volumes of sludge, in which contaminants are retained because they are simply transferred from one phase to another 5.
When these contaminants are not completely eliminated, it is not convenient to recirculate the treated water and incorporate it back into the production process, because these waters contain not only part of the main waste from this process, but also various by-products of chemical oxidation or implemented biodegradation, which affects the production conditions and, therefore, the quality of the products 5,6.
Various research publications have reported different methods of dye removal in wastewater, such as adsorption, advanced oxidation, biological treatment, electrochemical treatment, and membrane filtration. The best method cannot be stated as each method has its advantages and disadvantages with respect to cost, efficiency, feasibility, and environmental impact. An ideal technology for dye removal is one that is capable of removing it in short periods of time and without causing secondary contamination 2,5,6.
As alternatives to the treatment of these waters, there are the Advanced Oxidation Processes (AOPs), which have demonstrated a remarkable feature in the treatment of residual waters, which contain toxins, organic and inorganic contaminants, pesticides, and other harmful contaminants such as nanomaterials, which they are refractory to other decontamination methods 5. AOPs are treatments based on the in-situ generation of reactive oxidizing species (ROS) such as hydroxyl radicals (•OH), or the superoxide radical, •O2 - with a high oxidizing capacity. These ROS can attack organic pollutants in water by breaking them down, modifying them to more biodegradable constituents, and eventually mineralizing them to CO2, H2O, and inorganic ions 6,7.
AOPs also allow dye removal, a parameter that is difficult to reduce with conventional treatments. AOPs can be applied both individually and in combination with other technologies. As a pretreatment to any biological process, AOPs can transform compounds that are toxic to microorganisms into biodegradable compounds. As a post treatment, it leads to a final oxidation of an effluent before it is discharged into the sewer system; or it is applied when the effluent does not meet the standards at the exit of the biological treatment 7-9.
UV-based AOPs have been investigated to produce ROS. Dye discoloration by UV catalysis in the presence of H2O2 has been applied as a simple and cost-effective procedure in textile wastewater treatments 10,11. The UV/H2O2 reaction mechanism for dye discoloration has been explained from the formation of free radicals as active species. Hydrogen peroxide can absorb UV radiation in a specific wavelength range, causing the following chain reactions (Eq. 1-6), which show how the efficiency of the production of •OH radicals can be affected by their recombination.
This study assessed the effect of the power of UV irradiation by using interchangeable 15 W lamps, and the effect of the concentration of H2O2 on the treatment of real textile wastewater. An economic analysis was performed to demonstrate economic viability in a local textile industry.
2. Methodology
Tests were carried out in a lighting system equipped with 5 UV-C T8 lamps (Lumek®) of 15 watts of power each with a germicidal mercurial light of 254 nm.
5L of water was taken from a local textile company and the parameters pH (pH meter 3110 SET 2), Turbidity (Turbid meter Scientific inc Micro TPI), Conductivity (conductivity meter HANNA Instruments), Nitrogen (Total nitrogen by Nano color PF-12 Plus), BOD5 (Respirometer method by Oxitop WTW OC 110), COD (Standard Methods 5220 D with Thermoreactor WTW TR 420 and spectrophotometer Jenway 7205), TOC (Total carbon by Nano color PF-12 Plus), Alkalinity (Standard Method 2320 B with pH meter 3110 SET 2), Suspended solids ( Standard Methods 2540 D with muffle Terrigeno), settleable solids in 30 minutes (Standard Methods 2540 F), Total solids (Standard Methods 2540B), Dissolved solids (Standard Methods 2540 C with muffle Terrigeno), Biodegradability, color (Spectrophotometer method with Jenway 7205) and Spectrophotometric scan (Spectrophotometer method with UV-1100 Jenway 7205) were measured.
Also, HCl (36%, JTBaker) was used to alkalinity test, and K2Cr2O7 (100%, R. A. Químicos), Ag2SO4 (p.a. Mol Labs) and HgSO4 (p.a. Honeywell) were used to COD analysis. The H2O2 (30% w/w, Sigma Aldrich) and Na2S2O5 (97%, Panreac) were used in the degradation tests.
The evaluation of the degradation of industrial textile wastewater was carried out with a 32 factorial experimental design. The effect of the addition of hydrogen peroxide was analyzed by varying its concentration in 0.05, 0.10 and 0.15 M, and the intensity of the radiation in 15, 45 and 75 W, corresponding to 1, 3 and 5 lamps. Photolysis and oxidation with hydrogen peroxide were evaluated individually as control tests with the mean value of each factor.
For the textile wastewater degradation experiments, the number of lamps corresponding to the radiation power indicated were turned on for 10 minutes to achieve thermal equilibrium. Then 100 mL of the residual water was taken, placed in a reservoir and then a control sample was taken to analyze the initial absorbance. The required amount of hydrogen peroxide was added as indicated. The lamps were turned on to initiate the reaction and samples were taken at different intervals for one hour to evaluate the oxidation process. Samples were filtered prior to absorbance reading to avoid turbidity interference in the analysis.
The experimental design was applied again to the wastewater, but with the application of a pre-treatment, which consisted of a filtration process, followed by a 1:10 dilution and a pH adjustment close to neutral. All experiments were performed in duplicate. Finally, a study of energy-economic costs was developed to determine the best treatment applied to this type of wastewater.
3. Results
3.1. Characterization
Table 1 shows the results of the characterization of wastewater from a local textile industry. The results obtained show a slightly basic pH value, both due to the chemical nature of the dyes and the use of detergents in the fabric finishing process. The pH also reveals a high alkalinity of the industrial wastewater in the study. The alkalinity measurement was 1200 mg CaCO3×L-1, which showed a high content of weak bases. In addition, a high turbidity value is reached due to the high presence of suspended solids originated from the fibers of the textiles that are released in the process.
Table 1 Physico-chemical analyzes to the textile wastewater before treating
| Parameter | Value |
|---|---|
| pH | 7.58 |
| Turbidity (NTU) | 97.71 |
| Conductivity (µS×cm-1) | 3,810 |
| Total nitrogen (mg N×L-1) | 19.6 |
| BOD5 (mg O2×L-1) | 544.4 |
| COD (mg O2×L-1) | 1,148.33 |
| Alkalinity (mg CaCO3×L-1) | 1,200 |
| Suspended solids (mg L-1) | 672 |
| Settled solids (30 min) (ml×L-1) | 2 |
| Total solids (mg×L-1) | 10,962 |
| Dissolved solids (mg×L-1) | 10,186 |
| Biodegradability | 0.47 |
| Color (wavelength, nm) | Red (610), Green (546), Blue (436) |
| Spectrophotometric scan (nm) | 200-800 |
Source: own elaboration
A COD value of 1,148.33 mg O2×L-1 (Table 1) was obtained, which exceeds the maximum permissible limit in non-domestic wastewater discharges from textile manufacturing activities in Colombia, established in article 13 of resolution 0631/2015 12, which is 400 mg O2×L-1. However, according to the literature 8, this value is relatively low considering the levels reported by the dyeing industries and makes the use of AOPs for its treatment feasible. The content of total suspended solids and settleable solids, as well as the BOD5 also exceeded the maximum permissible limits established in such resolution 12.
The results of the biodegradability relationship based on the criteria established in the literature 13 indicate that this water should be classified as poorly biodegradable (BOD5/COD between 0.3 and 0.7). Based on these results, it would not be feasible or advisable to apply a biological treatment system. Additionally, a discharge of these waters to the environment would greatly affect their characteristics, as well as bring unknown effect.
3.2. Spectrophotometric scan
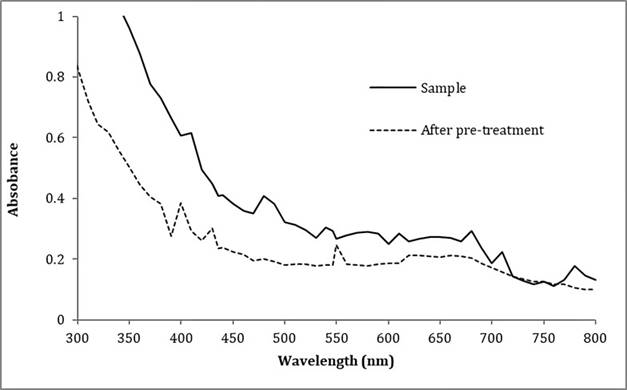
A spectrophotometric scan of the wastewater sample was carried out in order to determine the appropriate wavelength to measure the degradation of the pollutants. Figure 1 shows that the 480 nm and 540 nm wavelengths have small absorption maximum peaks, at other wavelengths such as 610, 680, 710 and 780 nm the absorbance is significantly reduced after the filtration and dilution process of the samples. The predominant color in the water to be treated is between blue and green, however, the sample shows a great absorption in the near UV region, typical of the electronic transitions in the outer layers of the molecules.
3.3. UV/H2O2 degradation tests
3.3.1 UV/H2O2 degradation to untreated wastewater
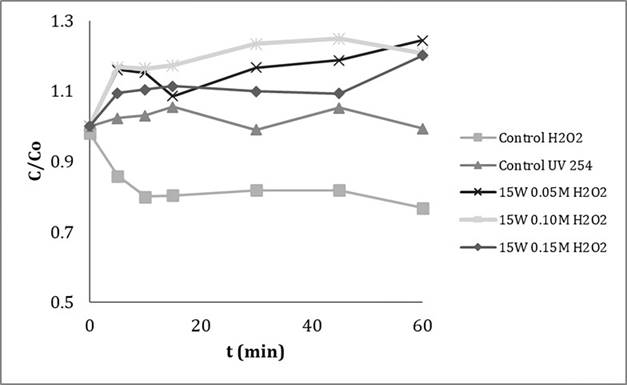
The degradation presented by the action of H2O2 occurs due to its great oxidizing power against organic matter, however a high concentration would be required to efficiently reduce the pollutants present in the wastewater (Figure 2). On the other hand, no degradation is evidenced after exposure of the sample to radiation of 254 nm, which may be due to a low photosensitivity of the species present in the water, and the turbidity of the solution due to the color and presence of solids in suspension; in this way, there is no effective penetration of the radiation and therefore a photodegradation process does not take place.
Source: own elaboration
Figure 2 Textile wastewater degradation without pretreatment. Measurement at 480 nm.
The tests with the UV/H2O2 showed negative degradation percentages. The experiments with an applied power of 15 Watts, report values of -24.4%, -20.9% and -20.1% respectively (Figure 2). This behavior is since the generation of highly oxidizing and non-selective hydroxyl radicals are also capable of destroying the suspended solids of the textile fibers, causing the formation of by-products that contribute to the absorbance of the original solution at the measurement´s wavelength, 480 nm.
A similar case could be evidenced in the work of Bahnemann 9, where in the degradation of 4-chlorophenol, the first by-products obtained were hydroquinone and benzoquinone, which present a greater absorption of radiation than 4-chlorophenol itself at certain lengths.
A Total Organic Carbon (TOC) measurement was carried out for the experiment with radiation of 15W and a concentration of 0.10 M H2O2 to corroborate the hypothesis made with the absorbance analysis, and it was possible to verify that the content of organic compounds increases after exposure to the treatment (Table 2). On the other hand, an analysis of the total solids (TS) of the sample also showed that the UV/H2O2 process is degrading not only the molecules of dissolved compounds but also the solid material from the textile fibers (Table 2). Considering that with a low level of radiation the suspended solids are degraded, it is decided to continue with the procedure applying a pretreatment that consists of filtration and neutralization.
3.3.2. UV/H2O2 degradation to pretreatment wastewater
A sequence of pretreatments prior to the AOP process was carried out, which were vacuum filtration and neutralization, in addition to a 1:10 dilution in distilled water; in order to have a suitable solution to observe the effects of the radiation intensity of the UV/H2O2 process on the treatability of textile waters. The degradation experiments specified in Table 3 were performed and a spectrophotometric method was used at wavelengths of 480 and 540 nm to determine the absorbance reduction, obtaining the reported degradation rate.
Treatment with hydrogen peroxide in the absence of radiation (C1) produces a reduction in absorption due to the oxidizing characteristic of this substance, however, the need for a higher concentration to achieve a satisfactory reduction is reiterated.
Table 3 Experimental design for wastewater subjected to pretreatment.
| Test | Radiation Power (W) | H2O2 (M) | Absorbance reduction (%) |
|---|---|---|---|
| C1 | 0 | 0.10 | 2.8 ± 7.3 |
| C2 | 45 | 0 | 1.3 ± 3.1 |
| 1 | 15 | 0.05 | 3.7 ± 1.6 |
| 2 | 15 | 0.10 | 15.6 ± 6.8 |
| 3 | 15 | 0.15 | 13.2 ± 1.0 |
| 4 | 45 | 0.05 | 56.6 ± 2.6 |
| 5 | 45 | 0.10 | 62.9 ± 4.2 |
| 6 | 45 | 0.15 | 61.2 ± 4.1 |
| 7 | 75 | 0.05 | 68.9 ± 1.3 |
| 8 | 75 | 0.10 | 70.0 ± 4.0 |
| 9 | 75 | 0.15 | 67.6 ± 2.7 |
Source: own elaboration
It is evident that the use of radiation (C2) does not have a significant effect on the removal of pollutants present in pretreated textile wastewater (Figure 3). It should be noted that despite having removed the solids by filtration and diluted this residual water, it still has a high level of color that does not allow radiation to penetrate and can act on a large number of molecules.
The tests carried out with UV/H2O2 technology show the effectiveness of the generation of oxidant species when a radiation of 45 and 75 W of power is applied, evidenced by the similarity in the percentages of reduction of the absorption of the samples. A slight dependence on the H2O2 concentration is also observed, since it presents a greater percentage of reduction of the absorption in the intermediate concentration level, in the 3 power levels evaluated. However, a statistical analysis using ANOVA shows that the H2O2 concentration in the evaluated range is not statistically significant, while the value of the radiation power is.
3.4. Cost analysis
Although the highest absorption reduction was obtained with the highest power level, the result with the 45 W power level is similar. Taking in account that the higher cost of the technology is due to the lighting of the system, an analysis of energy consumption of all the tests is carried out to verify if, in this case, a higher expenditure for radiation consumption is justified (Eq. 7).
Where P is Power in kW, t is reaction time (h), V is treated volume (L) of the original wastewater and d is percentage of absorption reduction.
Table 4 shows the results obtained from the analysis of the energy consumption of the treatments applied to wastewater from the textile industry with pretreatment.
Table 4 Energy consumption analysis
| Test | kWh×L-1 |
|---|---|
| C2 | .2.5983 |
| 1 | 0.305 |
| 2 | 0.072 |
| 3 | 0.085 |
| 4 | 0.060 |
| 5 | 0.048 |
| 6 | 0.055 |
| 7 | 0.082 |
| 8 | 0.080 |
| 9 | 0.083 |
Source: own elaboration
Experiment 5, that is, with a radiation power of 45 W and 0.10 M H2O2, shows the lowest energy consumption for each liter of solution treated. This shows that the application of a higher radiation power, in the evaluated range, does not represent a substantial benefit to improve the degradation process. It should be noted that lighting in this technology cannot be replaced by solar radiation, since UVC radiation is fully absorbed by the atmosphere.
4. Conclusions
AOPs are efficient alternatives for the treatment of wastewater from the textile industry, since the generation of hydroxyl radicals, as it is not selective, reduces the amount of all the components present in this type of complex waters.
The UV/H2O2 combination was found to attack both dissolved and suspended solids, but to improve the efficiency of the process, a primary treatment process is required to remove solid materials. In this way, radiation will also have a greater effect and recalcitrant pollutants that cannot be treated with conventional technologies can be destroyed more efficiently.
The results showed that the treatment with 0.10 M H2O2 and 45 W was effective and energetically viable, for the real water evaluated, achieving an absorbance removal of 62.9% with an energy consumption of 0.048 kWh L-1 for a total nominal degradation.