Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín Científico. Centro de Museos. Museo de Historia Natural
Print version ISSN 0123-3068
Bol. Cient. Mus. Hist. Nat. Univ. Caldas vol.17 no.2 Manizales July/Dec. 2013
POPULATION ECOLOGY AND MORPHOMETRIC VARIATION OF THE CHOCOAN RIVER TURTLE (Rhinoclemmys nasuta) FROM TWO LOCALITIES ON THE COLOMBIAN PACIFIC COAST*
ECOLOGÍA POBLACIONAL Y VARIACIÓN MORFOMÉTRICA DE LA TORTUGA DE RÍO CHOCOANA (Rhinoclemmys nasuta) EN DOS LOCALIDADES DE LA COSTA PACÍFICA DE COLOMBIA
Mario Fernando Garcés-Restrepo1, Alan Giraldo1 & John L. Carr1,2
* FR: 21-VIII-2012. FA: 4-V-2013.
1 Universidad del Valle, Facultad de Ciencias Naturales y Exactas, Departamento de Biología, Sección de Zoología, Grupo de Investigación en Ecología Animal. Calle 13 No. 100-00, Cali, Colombia. E-mail: mariofgarces@gmail.com; ecologia@univalle.edu.co
2 University of Louisiana at Monroe, Department of Biology and Museum of Natural History, Monroe, Louisiana 71209-0520 USA.
Abstract
The Chocoan River Turtle, Rhinoclemmys nasuta (Geoemydidae), is a species of great importance due to its limited geographical distribution and threat status. In Colombia it is considered in the category data deficient (DD) and globally as a near-threatened species (NT). In this study we assessed the population density, variation in the demographic structure and population size, and morphometric variation in two localities. One island population has no human disturbance and the other, mainland locality is human-influenced. Population density was 6.3 times greater in the insular locality, which corresponds with the absence of some predators and human disturbance at this location. Additionally, there was no significant difference between localities in demographic structure and size classes, which may reflect that there is no removal of individuals for consumption or use as pets in the mainland population. On the other hand, body size was smaller on the island, a phenomenon that may be explained by a tendency of species to dwarfism in insular environments, or an effect of increased intraspecific competition. To clarify whether differences in population density and body size are attributable to island effects or to the difference in the degree of human disturbance between the two populations it will be necessary to sample at other locations on the mainland with different degrees of human disturbance. However, it is important to stress the importance of Isla Palma as a site for regional conservation of R. nasuta.
Key words: human disturbance, geographic isolation, population density, habitat degradation, morphometric variation, island effect.
Resumen
La Tortuga de río chocoana, Rhinoclemmys nasuta (Geoemydidae), es una especie de gran importancia para la conservación debido a su distribución geográfica limitada y a su estatus de amenaza, siendo considerada en la categoría de datos deficientes (DD) a nivel de Colombia y casi amenazada (NT) mundialmente. En este estudio se evaluó la densidad poblacional, la variación poblacional en estructura y tamaño, así como la variación morfométrica en dos localidades. La primera localidad corresponde a una isla continental (Isla Palma) sin perturbación humana, y la otra localidad se encuentra en el continente y ha sido afectada por la perturbación humana. Como resultados se obtuvo una densidad poblacional 6,3 veces mayor en la localidad insular, debido a la ausencia de algunos predadores y de perturbación humana. No se encontraron diferencias significativas en la estructura de la poblacion a nivel de proporción de sexos o clases de tamaños, lo cual puede estar reflejando que no existe una remoción de individuos para el consumo o tráfico de mascotas en la localidad continental. Por otra parte, se evidenció una disminución del tamaño corporal en la isla, lo cual puede ser explicado por una tendencia al enanismo en islas de especies no territoriales o a un efecto de aumento de competencia intraespecífica. Para esclarecer si los cambios en densidad poblacional y tamaño corporal son generados por efecto insular o diferencias en grado de perturbación humana, es necesario realizar muestreos en otras localidades continentales con diferentes grados de perturbación humana. Sin embargo, cabe recalcar la importancia de la localidad de Isla Palma a nivel regional para la conservación de R. nasuta.
Palabras clave: perturbación humana, aislamiento geográfico, densidad poblacional, degradación del hábitat, variación morfométrica, efecto isla.
INTRODUCTION
On a global basis, many turtle species show declines in their populations due to a variety of causes that affect population viability, including habitat loss and degradation, introduction of invasive species, environmental pollution, disease and human exploitation (MITTERMEIER et al., 1992; ARESCO & DOBIE, 2000; CONVERSE et al., 2005). Factors inherent to their biology, such as the slow recovery of populations from disturbance and delayed sexual maturity, add to the problems faced by turtle populations (CONGDON et al., 1994; HEPPELL, 1998). The greatest threats that befall particular groups such as freshwater turtles are considered to be human disturbances such as habitat loss and fragmentation (MITCHELL & KLEMENS, 2000; CONVERSE et al., 2005).
Colombia is no exception to these conservation problems for turtle populations. Of the 33 taxa of turtles covered in the Colombian Red Data book (CASTAÑO, 2002; RUEDA-ALMONACID et al., 2007), six species are classified critically endangered (CR), six more are classified endangered (EN), six are considered vulnerable (VU), five near threatened (NT), four others are considered data deficient (DD) and the species Batrachemys raniceps. is possibly extinct in the country. Despite the high diversity of turtles recorded in Colombia, systematic investigation of these species has been minimal. This situation highlights the need for conservation assessments and strategies for this group of organisms, which must start with knowledge of the basic biology of the species (MITTERMEIER et al., 1992).
Among regions within Colombia with the least information about the biology and conservation of turtles is the Chocoan biogeographic region. The only comprehensive studies on this group were published 50 years ago (MEDEM, 1962). Several threats to turtles in this region are present; increased human settlement in the Chocoan region has lead to an increase in the conversion of natural habitats, as well as intensification in the use of turtles as an alternative source of protein (CORREDOR-LONDOÑO et al., 2006; CORREDOR-LONDOÑO et al., 2007; GIRALDO et al., 2012). Additionally, illegal traffic in pet turtles is high in this area; according to seizure data from the regional authorities, turtles are the most traded wildlife (CORREDOR- LONDOÑO et al., 2007).
Among the freshwater turtles recorded in this area is the Chocoan River Turtle, Rhinoclemmys nasuta (Geoemydidae), a species of great importance due to its limited geographical distribution in Colombia and Ecuador (CARR & GIRALDO, 2009). Furthermore, the fact it has been categorized as a near-threatened species globally (NT) by IUCN (2010) and data deficient (DD) in Colombia (CASTAÑO, 2002) make it an important species to study. This turtle is a small to medium-sized species (maximum carapace length of 229 mm) found in freshwater habitats (RUEDA-ALMONACID et al., 2007; CARR & GIRALDO, 2009). We began studying an island population of R. nasuta in 2005 on Isla Palma, Bahía Málaga, in the Pacific coastal region of the Department of Valle del Cauca in Colombia (CARR & GIRALDO, 2009; CARR et al., 2010; GIRALDO et al., 2012). However, the population status, population structure and morphometrics of continental populations are unknown.
Islands populations of species frequently exhibit distinctive biological characteristics when compared to mainland populations. For instance, islands often have greater population densities due to the reduction of competitors and predators (MACARTHUR et al., 1972). Isla Palma is protected from human disturbance within a military use area (GIRALDO et al., 2012), so it is quite possible that population parameters present in the island population are not typical of what may be found in continental locations. In addition, the two populations of R. nasuta are reproductively isolated because the species is unable to cross the saltwater barrier. We wanted to assess any difference in external morphology that might be due to this geographic isolation, such as a difference in body size, which is a phenomenon found in many island populations in response to the historical, physical and biological characteristics of the islands (CASE, 1978; BOBACK, 2003; LOMOLINO, 2005). This study aims to determine the population status in a continental site and see if there are differences from the insular site based on the arguments put forward above.
MATERIALS AND METHODS
Isla Palma and Playa Chucheros belong to the Chocó biogeographic region, specifically to the Pacific coastal subregion; the vegetation type is classified as lowland, very moist tropical forest. The sites have a high rainfall of 7200 to 8500 mm annually, relative humidity of 90%, and an average temperature of 23.5 to 25.7º C (RANGEL-CH. & ARELLANO-P., 2004). These two sites are located in Bahía Málaga, in the central Pacific region of Colombia in the Department of Valle del Cauca (Figure 1). Playa Chucheros is located at the southern entrance of Bahía Málaga and Isla Palma in the North Bay outlet. Isla Palma has an area of 138 ha and is surrounded by uniformly distributed hard-rock cliffs and sandy beaches exposed only at low tide, with an elevation that varies from 6-15 m. Due to high rainfall and a rugged topography, there are numerous freshwater streams draining to the sea (GIRALDO et al., 2012). The island of Isla Palma is used for a lighthouse by the Maritime Directorate General of Colombia (DIMAR), which gives it protection because hunting and logging are not allowed, and no one lives there permanently. On the nearby coast of the continent, we identified a population of R. nasuta at the locality Playa Chucheros. This locality has a backdrop of cliffs up to 18 meters in all directions, which are separated from the sea by a wide beach (CANTERA et al., 1998). There is a population of more than 50 people who live off the exploitation of natural resources and tourism. The species is occasionally captured for food and there is a growing problem of loss of natural habitat due to timber harvesting for the expansion of hotel infrastructure and houses for the inhabitants.
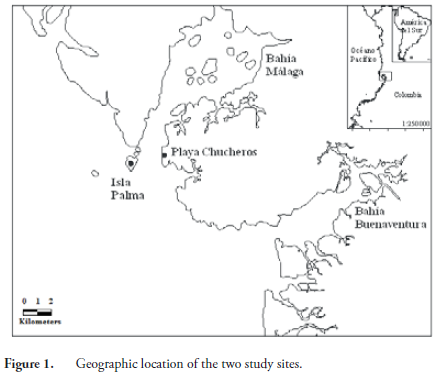
For this study, we sampled each locality three times in 2007 at one month intervals: a capture event and two recapture events. Sampling was carried out in stream sections of variable length starting at the mouth. On Isla Palma we sampled five streams, with a total area studied of 0.40 ha. At Playa Chucheros, we sampled three streams with a total area of 0.18 ha. Specimens were collected between 2000 and 2400 hours by direct visual encounter; the animals collected were marked directly at the site of capture and transported to the camp in cloth bags, where we proceeded to take measurements and record data for each individual. We used the marking methodology proposed by CAGLE (1939) with some modifications (GIRALDO et al., 2012).
We made 32 linear body measurements using a vernier caliper (0.01 mm), three curved measurements using a tape measure (1 mm), and weight (W) was recorded with a Pesola scale (10 g) for each individual (MEDEM, 1976; MERCHÁN, 2003; MERCHÁN et al., 2005). The measurements included: straight median carapace length (LSMS); median curved carapace length (MCCL); maximum straight carapace length (MSCL); straight carapace width between the marginal 5-6 seams (SCW5-6); curved carapace width between the marginal 5-6 seams (CCW5-6); maximum straight carapace width (MSCW); length of vertebral scutes: (LV1), (LV2), (LV3), (LV4), (LV5); length of costal scutes: (LCo1), (LCo2), (LCo3), (LCo4); median straight plastron length (MSPL); curved plastron length (LCP); maximum straight plastron length (MaSPL); plastral seam lengths: gular (GSL), humeral (HSL), pectoral (PSL), abdominal (ASL), femoral (FSL), anal (AnSL); plastral seam widths: gular (GW), humeral (HW), pectoral (PW), abdominal (AW), femoral (FW); bridge length (LB); shell height (SH); pre-cloacal tail length (TLPreCl); post-cloacal tail length (TLPosCl); and head width (HeW).
Population size was calculated using the program MARK, version 6.0 (WHITE & BURNHAM, 1999). We used a closed population model because the periods of capture were separated by only one month, and we used the AIC criterion to choose the best closed population model. Population density was calculated using the estimated number of turtles and the sampled area (WILLIAMS & PARKER, 1987; MILAM & MELVIN, 2001). We performed contingency table analyses to test whether there were differences in the proportional composition of demographic classes (males, females, juveniles) between localities (ZAR, 1999). Each individual's sex was determined based on the presence or absence of secondary sexual characteristics, i.e., pre-cloacal tail length, plastral concavity, body size (MEDEM, 1969; CARR & GIRALDO, 2009), or by primary sexual characteristics (palpation of eggs or eversion of the penis). Juveniles were those individuals that could not be assigned a gender on the basis of secondary sexual characteristics and were generally smaller than 140 mm LSMS. To further characterize population structure, turtles were assigned to four size demographic classes according to carapace length based on 20 mm intervals (LESCANO et al., 2008), then we used contingency table analysis to evaluate differences between the proportional distribution of size classes in the two populations (ZAR, 1999). When preliminary comparisons via ANOVA yielded significant differences in all morphometric variables between males, females and juveniles, geographic variation was assessed with Mann-Whitney tests separately for males, females and juveniles. Similarly, we performed a principal components analysis to graphically detect the existence of groups. Principal components analysis was performed using the statistical module of the computer program STATISTICA 7.0 (STATSOFT, 2004). For morphometric analyses, we used additional data on four juveniles, eleven females and five males obtained during occasional visits to Playa Chucheros.
RESULTS
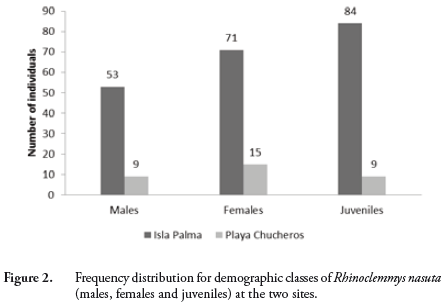
During the three visits to Isla Palma, we captured a total of 208 individuals of R. nasuta; 21 turtles were recaptured on one occasion and two were recaptured twice. We calculated an estimated population size of 624 ind (95% CI = 577.8–670.2; AIC = -1194.37; Model = p(t)c(.)N(.)) and a density of 1560 ind/ha. At Playa Chucheros, we captured 33 individuals for an estimated population size of 99 individuals (95% CI = 86.7–111.3; AIC = -91.25; Model = p(t)c(.)N(.)) and a density of 247.5 ind/ha. The estimated population density of the Isla Palma population is 6.3 times greater than that found at Playa Chucheros. The demographic classes we captured were 53 (26%) males, 71 (34%) females and 84 (40%) juveniles in the Isla Palma population and 9 (27%) males, 15 (46%) females and 9 (27%) juveniles at Playa Chucheros (Figure 2). The contingency table analysis indicated that the proportion of population classes is not significantly different between the two locations (p = 0.326). In terms of size-class composition for each of the demographic classes, there were no significant differences between the localities (males p = 0.096; females p = 0.106; juveniles p = 0.962) (Figure 3).

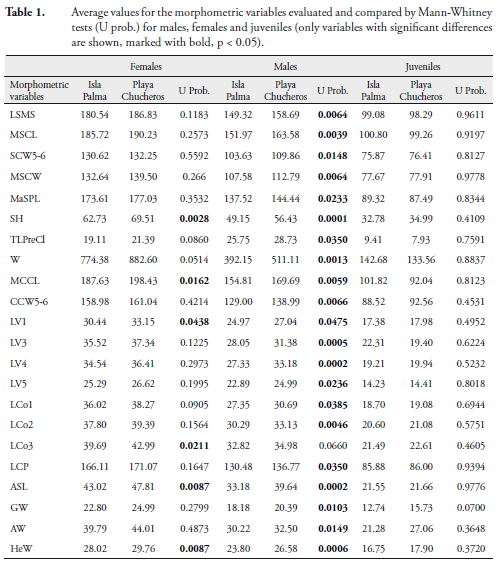
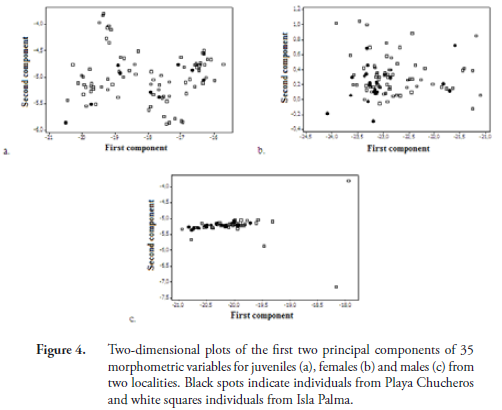
In terms of morphometric variation, we found that juveniles in the two populations were not significantly different. Females at Playa Chucheros averaged larger than those in the Isla Palma population, however this difference was significant for only six variables (MCCL, LV1, LCo3, ASL, SH, HEW). This pattern of variation indicates that Playa Chucheros females have a greater volume (greater height and width) than Isla Palma females. Males showed the greatest difference between localities, with 21 of the 35 variables assessed having the highest averages for individuals at Playa Chucheros (Table 1). Graphically, the principal components analysis shows no distinguishable grouping by demographic classes related to the sites (Figure 4).
DISCUSSION
We think that the difference in population densities in the two locations may be due to two factors, one is the island effect and the other is the difference in the degree of human disturbance. The theory of island biogeography predicts a decrease in species diversity depending on the size of the island and its distance from the mainland (MACARTHUR & WILSON, 1967); this decrease in diversity is often accompanied by high population densities of some species compared to those found in similar habitats on the mainland (MACARTHUR et al., 1972; GEORGE, 1987; PERES & DOLMAN, 2000). This phenomenon has been explained by many hypotheses, including: the reduction in interspecific competition and predation (MACARTHUR & WILSON, 1967; NILSSON et al., 1985; GEORGE, 1987); differences between island and mainland habitats (GEORGE, 1987), niche expansion (NILSSON, 1977); concentration of resources (CONNOR et al., 2000); reduced dispersal (MACARTHUR et al., 1972); and precise genetic adaptation to local conditions by reduced migration (EMLEN, 1979). In the specific case of R. nasuta its increase in population density on Isla Palma may be due to decreased predation since predators such as large mammals and crocodilians are absent; additionally there is less snake diversity than in continental locations (GIRALDO et al., 2012). This same explanation has been the factor used to hypothesize population increases of some species of turtles on islands (LANGTIMM et al., 1996; VOGT et al., 2009).
It is well documented that continental turtles are affected by habitat degradation due to loss of required resources for the species and human overexploitation (MITTERMEIER et al., 1992; GIBBONS et al., 2000). However, based on our observations, we consider the effect of overexploitation of this species to be insignificant for the Playa Chucheros locality because people in the community do not use traps and do not frequent the streams at night for fear of snake bite accidents; therefore the nocturnal habits of this species limit encounters with humans. This observation is supported by the lack of a difference in the proportion of each size class between the two populations for males and females, which represent the larger size classes that are most desirable for consumption (Figure 3) (Table 1). Therefore, habitat degradation is the most likely explanation for the difference in population density between these two study sites. The isolation of an environment such as Isla Palma, where habitat disturbance is minimal as compared to Playa Chucheros, which has increasing conversion of habitat that includes logging close to our sampled streams, may have generated the large difference in population size and density we observed.
The structure of chelonian populations responds to changes of intrinsic factors such as migration of individuals and differential predation (MARTINS & SOUZA, 2009); however, the factor involved to the greatest degree in changes of population structure is human exploitation, with individuals being extracted for consumption or used as pets (RAMO, 1982; FACHÍN-TERÁN & VOGT, 2004). Apparently, because there was relatively little removal of individuals from the impacted mainland locality, we found no significant differences in demographic and size classes between the two populations.
Body size is a life history characteristic of a species that may be associated with multiple factors (BLACKBURN & GASTON, 1997; BOBACK, 2003); considering that one of our localities is an insular site, the island effect should be taken into account. One of the most notable changes in the morphometry of populations on islands is body size as has been recorded in several groups of animals, e.g., large mammals (ROTH, 1993), rodents (PERGAMS & ASHLEY, 1999), snakes (BOBACK, 2003), turtles (GIBBONS et al., 1979), birds (CLEGG et al., 2002 and mammals (ADLER & LEVINS, 1994). Island populations may exhibit a trend toward increase or decrease in body size depending on the environmental characteristics of the island or the particular characteristics of life history of the species (CASE, 1978; ROTH, 1993; BOBACK, 2003; LOMOLINO, 2005). The effect of human exploitation on turtle populations is a decrease in average body size, as compared to communities without exploitation (CLOSE & SEIGEL, 1997; BERNAL et al., 2004; GAMBLE & SIMONS, 2004).
In this study we recorded a smaller average body size for males and females in the island population, with a more pronounced trend in males. This is the opposite of what would be expected if human overexploitation was the cause of the differences between the populations. The larger males and females in the population at Playa Chucheros may be a consequence of the relative lack of recruitment of juveniles into the male and female categories over a number of years. The aging adult population should exhibit a larger average size where there is little exploitation of the population. On the other hand, the cause of a decrease in body size of a species on an island can occur in species that are not territorial (CASE, 1978), as is the case of R. nasuta. This phenomenon can be explained by increased intraspecific competition in the island environment where the turtle population density was high (LANDA & SKOGLAND, 1995; NAVARRETE & MENGE, 1997). Many organisms have large body sizes at low population densities because each individual has plentiful resources available; however when population density increases resources are more scarce and the population may have a smaller body in order to reduce resource needs (SKOGLAND, 1983; ALUNNO-BRUSCIA et al., 2000).
In turtles, body proportion variation among populations that differ in habitat (ROWE, 1997), or with long geographical isolation (YASUKAWA et al., 1996) have been found. Morphometric differences between the two populations found in this study appear only in size, without a significant change in body proportions. The lack of such differences may be because there is no difference in the type of habitats between the sites. Another possibility is that the genetic drift caused by physical isolation over time has been insufficient to generate morphological differentiation (CLEGG et al., 2002).
With the current data it is impossible to resolve whether the island effect, through a reduction in interspecific competition and predation, or a reduction of human disturbance, or the synergy of these two factors has led to the differences in population density and body size detected between our mainland and island populations of R. nasuta. Sampling from other mainland locations that vary in degree of human disturbance may help resolve the important factors affecting population dynamics in this region. Despite this, the results obtained in this study emphasize the importance of Isla Palma as a site for conservation and research of the densest known population of R. nasuta.
ACKNOWLEDGMENTS
We thank all those who contributed to our many sampling campaigns; especially biologists Bellineth Valencia, Andrés Quintero and Nataly Calvo. We thank the Dirección General Marítima (DIMAR) for permission to work on Isla Palma and the residents of Playa Chucheros, who kindly cooperated within their means and made the working conditions more pleasant. The Universidad del Valle, the Grupo de Investigación en Ecología Animal of Univalle and COLCIENCIAS, as well as the Turtle Research Fund from Turtle Survival Alliance (TSA) and the University of Louisiana at Monroe, partially financed this work. Mario Garcés is a student of the Master of Science program in Biology of the Universidad del Valle and he was supported by the graduate program through a teaching assistantship during this research.
BIBLIOGRAPHY
ADLER, G.H. & LEVINS, R., 1994.- The island syndrome in rodent populations. Quarterly Review of Biology, 69 (4): 473-490. [ Links ]
ALUNNO-BRUSCIA, M., PETRAITIS, P.S., BOURGET, E. & FRÉCHETTE, M., 2000.- Body size-density relationship for Mytilus edulis in an experimental food-regulated situation. Oikos, 90 (1): 28-42. [ Links ]
ARESCO, M. & DOBIE, J., 2000.- Variation in shell arching and sexual size dimorphism of river cooters, Pseudemys concinna from two river systems in Alabama. Journal of Herpetology, 34: 313-317. [ Links ]
BERNAL, M., DAZA, J.M. & PÁEZ, V.P., 2004.- Ecología reproductiva y cacería de la tortuga Trachemys scripta (Testudinata: Emydidae), en el área de la Depresión Momposina, norte de Colombia. Revista de Biología Tropical, 52: 229-238. [ Links ]
BLACKBURN, T.M. & GASTON, K.J., 1997.- A critical assessment of the form of the interspecific relationship between abundance and body size in animals. Journal of Animal Ecology, 66 (2): 233-249. [ Links ]
BOBACK, S.M., 2003.- Body size evolution in snakes: evidence from island populations. COPEIA, : 81-94. [ Links ]
CAGLE, F.R., 1939.- A system of marking turtles for future identification. COPEIA, 1939: 170-173. [ Links ]
CANTERA-K., J.R., NEIRA-O., R. & RICAURTE, C., 1998.- Bioerosión en la Costa Pacífica Colombiana: Un estudio de la biodiversidad, la ecología y el impacto humano de los animales destructores de los acantilados rocosos. Fondo José Celestino Mutis, FEN, Bogotá [ Links ].
CARR, J.L. & GIRALDO, A., 2009.- Rhinoclemmys nasuta (Boulenger 1902), Large-Nosed Wood Turtle, Chocoan River Turtle: 034.1-034.6 (in) RHODIN, A.G.J., PRITCHARD, P.C.H., VAN DYKE, P.P., SAUMURE, R.A., BUHLMANN, K.A., IVERSON, J.B. & MITTERMEIER, R.A. (eds.) Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs. [ Links ]
CARR, J.L., GARCÉS, M.F., QUINTERO-ÁNGEL, A. & GIRALDO, A., 2010.- Rhinoclemmys nasuta (Chocoan River Turtle). Diet and feeding behavior. Herpetological Review, 41(3): 347-348. [ Links ]
CASE, T.J., 1978.- A general explanation for insular body size trends in terrestrial vertebrates. Ecology, 59: 1-18. [ Links ]
CASTAÑO, O.V., 2002.- Libro rojo de reptiles de Colombia. Instituto de Ciencias Naturales - Ministerio del Medio Ambiente - Conservación Internacional, Bogotá, Colombia. [ Links ]
CLEGG, S.M., DEGNAN, S.M., MORTZ, C., ESTOUP, A., KIKKAWA, J. & OWENS, I.P.F., 2002.- Microevolution in island forms: the roles of drift and directional selection in morphological divergence of a passerine bird. Evolution, 56: 2090-2099. [ Links ]
CLOSE, L.M. & SEIGEL, R.A., 1997.- Differences in body size among populations of Red-eared sliders (Trachemys scripta elegans) subjected to different levels of harvesting. Chelonian Conservation and Biology, 2: 563-566. [ Links ]
CONGDON, J.D., DUNHAM, A.E. & VAN LOBEN SELS, R.C., 1994.- Demographics of common snapping turtles (Chelydra serpentina): implications for conservation and management of long-lived organisms. American Zoologist, 34: 397-408. [ Links ]
CONNOR, E.F., COURTNEY, A.C. & YODER, J.M., 2000.- Individuals-area relationships: the relationship between animal population density and area. Ecology, 81: 734-748. [ Links ]
CONVERSE, S.J., IVERSON, J.B. & SAVIDGE, J.A., 2005.- Demographics of an ornate box turtle population experiencing minimal human-induced disturbances. Ecological Applications, 15: 2171-2179. [ Links ]
CORREDOR-LONDOÑO, G., AMOROCHO, D. & GALVIS-RIZO, C.A., 2006.- Plan de Acción para la Conservación de las Tortugas Continentales y Marinas del Departamento del Valle de Cauca. Corporación Autónoma Regional del Valle del Cauca (CVC), Santiago de Cali, Colombia. [ Links ]
CORREDOR-LONDOÑO, G., KATTAN, G., GALVIZ, C.A. & MOROCHO, D., 2007.- Tortugas del Valle del Cauca. Corporación Autónoma Regional del Valle del Cauca (CVC), Cali, Colombia. 74p. [ Links ]
EMLEN, J.T., 1979.- Land bird densities on Baja California islands. Auk, 96: 152-167. [ Links ]
FACHÍN-TERÁN, A. & VOGT, R.C., 2004.- Estrutura populacional, tamanho e razão sexual de Podocnemis unifilis (Testudines, Podocnemididae) no rio Guaporé (RO), norte do Brasil. Phyllomedusa, 3: 29-42. [ Links ]
GAMBLE, T. & SIMONS, A.M., 2004.- Comparison of harvested and nonharvested painted turtle populations. Wildlife Society Bulletin, 32: 1269-1277. [ Links ]
GEORGE, T.L., 1987.- Greater land bird densities on island vs. mainland: relation to nest predation level. Ecology, 68: 1393-1400. [ Links ]
GIBBONS, J.W., KEATON, G.H., SCHUBAUER, J.P., GREENE, J.L., BENNETT, D., MCAULIFFE, J. & SHARITZ, R.R., 1979.- Unusual population size structure in freshwater turtles on barrier islands. Georgia Journal of Science, 37: 155-159. [ Links ]
GIBBONS, J.W., SCOTT, D.E., RYAN, T.J., BUHLMANN, K.A., TUBERVILLE, T.D., METTS, B.S., GREENE, J.L., MILLS, T., LEIDEN, Y., POPPY, S. & WINNE, C.T., 2000.- The global decline of reptiles, déjà vu amphibians. BioScience, 50: 653-666. [ Links ]
GIRALDO, A., GARCÉS-RESTREPO, M.F., CARR, J.L. & LOAIZA, J., 2012.- Tamaño y estructura poblacional de la tortuga sabaletera (Rhinoclemmys nasuta, Testudines: Geoemydidae) en un ambiente insular del Pacífico colombiano. Caldasia, 34 (1): 109-125. [ Links ]
HEPPELL, S., 1998.- Application of life-history theory and population model analysis to turtle conservation. COPEIA, 1998: 367-375. [ Links ]
IUCN, 2010.- IUCN Red List of Threatened Species. Version 2010.4. http://www.iucnredlist.org/ (Downloaded on 05 February 2011). [ Links ]
LANDA, A. & SKOGLAND, T., 1995.- The relationship between population density and body size of wolverines in Scandinavia. Wildlife Biology, 1: 165-175. [ Links ]
LANGTIMM, C.A., DODD, C.K. & FRANZ, R., 1996.- Estimates of abundance of box turtles (Terrapene carolina bauri) on a Florida island. Herpetologica, 52: 496-504. [ Links ]
LESCANO, J.N., BONINO, M.F. & LEYNAUD, G.C., 2008.- Density, population structure and activity pattern of Hydromedusa tectifera (Testudines-Chelidae) in a mountain stream of Córdoba province, Argentina. Amphibia-Reptilia, 29: 505-512. [ Links ]
LOMOLINO, M.V., 2005.- Body size evolution in insular vertebrates: generality of the island rule. Journal of Biogeography, 32: 1683-1699. [ Links ]
MACARTHUR, R.H., & WILSON, E.O., 1967.- The Theory of Island Biogeography. Princeton Univ. Press, Princeton, New Jersey. [ Links ]
MACARTHUR, R.H., DIAMOND, J.M. & KARR, J.R., 1972.- Density compensation in island faunas. Ecology, 53: 330-342. [ Links ]
MARTINS, F.I. & SOUZA F.L., 2009.- Demographic parameters of the Neotropical freshwater turtle Hydromedusa maximiliani (Chelidae). Herpetologica, 65:82–91. [ Links ]
MEDEM, F., 1962.- La distribución geográfica y ecológica de los Crocodylia y Testudinata en el departamento del Chocó. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 11 (44): 279-342. [ Links ]
MEDEM, F.,1976.- Recomendaciones respecto a contar el escamado y tomar las dimensiones de nidos, huevos y ejemplares de los Crocodylia y Testudines. Lozanía, 20: 1-17. [ Links ]
MERCHÁN, M., 2003.- Contribución al conocimiento de la biología de la Tortuga Negra (Rhinoclemmys funerea) y la Tortuga Roja (R. pulcherrima manni) en Costa Rica. Tesis de Doctorado, Universidad Complutense de Madrid. [ Links ]
MERCHÁN, M., COLL, M. & FOURNIER, R., 2005.- Macromorfometría de juveniles de Geochelone sulcata (Testudines: Testudinidae) en Costa Rica. Revista de Biología Tropical, 53 (1-2): 213-225. [ Links ]
MILAM, J.C. & MELVIN, S.M., 2001.- Density, habitat use, movements, and conservation of spotted turtles (Clemmys guttata) in Massachusetts. Journal of Herpetology, 35 (3): 418-427. [ Links ]
MITCHELL, J.C. & KLEMENS, M.W., 2000.- Primary and secondary effects of habitat alteration: 5-32 (in) KLEMENS, M.W. (ed.) Turtle Conservation. Smithsonian Institution Press, Washington, D.C., USA. [ Links ]
MITTERMEIER, R.A., CARR, J.L., SWINGLAND, I.R., WERNER, T.B. & MAST, R.B., 1992.- Conservation of amphibians and reptiles: 59-80. (in) ADLER, K. (ed.), Herpetology; Current Research on the Biology of Amphibians and Reptiles. Society for the Study of Amphibians and Reptiles. [ Links ]
NAVARRETE, S.A. & MENGE, B.A., 1997.- The body size-population density relationship in tropical rocky intertidal communities. Journal of Animal Ecology, 66 (4): 557-566 [ Links ]
NILSSON, S.G., 1977.- Density compensation and competition among birds breeding on small islands in a South Swedish lake. Oikos, 28: 170-176. [ Links ]
NILSSON, S.G., BJORKMAN, C., FORSLUND, P. & HOGLUND, J., 1985.- Egg predation in forest bird communities on islands and mainland. Oecologia, 66: 511-515. [ Links ]
PERES, C.A. & DOLMAN, P.M., 2000.- Density compensation in neotropical primate communities: evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia, 122 (2): 175-189. [ Links ]
PERGAMS, O.R.W. & ASHLEY, M.V., 1999.- Rapid morphological change in Channel Island deer mice. Evolution, 53: 1573-1581. [ Links ]
RAMO, C., 1982.- Biología del galápago (Podocnemis vogli Muller, 1935) en el hato "El Frío" Llanos de Apure (Venezuela). Doñana, Acta Vertebrata, 9: 1-161. [ Links ]
RANGEL-CH., J.O. & ARELLANO-P., H., 2004.- El Chocó Biogeográfico: Ambiente Físico: 39-82. (in) RANGEL-CH., J. (ed.) Colombia Diversidad Biótica IV El Choco Biogeográfico/Costa Pacífica. Instituto de Ciencias Naturales, Bogotá D.C., Colombia. [ Links ]
ROTH, V.L., 1993.- Dwarfism and variability in the Santa Rosa Island mammoth (Mammuthus exilis): An interspecific comparison of limb-bone sizes and shapes in elephants: 433-442 (in) HOCHBERG, F.G. (ed.) Third California Islands Symposium. Santa Barbara Museum of Natural History. [ Links ]
ROWE, J., 1997.- Growth rate, body size, sexual dimorphism and morphometric variation in four populations of painted turtles (Chrysemys picta bellii) from Nebraska. American Midland Naturalist, 138 (1): 174-188. [ Links ]
RUEDA-ALMONACID, J.V., CARR, J.L., MITTERMEIER, R.A., RODRÍGUEZ-MAHECHA, J.V., MAST, R.B., VOGT, R.C., RHODIN, A.G.J., DE LA OSSA-VELÁSQUEZ, J., RUEDA, J.N. & MITTERMEIER, C.G., 2007.- Las tortugas y los cocodrilianos de los países andinos del trópico. Conservación Internacional, Bogotá, Colombia. [ Links ]
SKOGLAND, T., 1983.- The effect of density-dependent resource limitation on size of wild reindeer. Oecologia, 60: 156-168. [ Links ]
STATSOFT., 2004.- Statistica, Version 7. Statsoft, Inc., Tulsa, OK. [ Links ]
VOGT, R.C., PLATT, S.G. & RAINWATER, T.R., 2009.- Rhinoclemmys areolata (Duméril & Bibron 1851), Furrowed Wood Turtle, Black-bellied Turtle, Mojena: 022.1-022.7 (in) RHODIN, A.G.J., PRITCHARD, P.C.H., VAN DYKE, P.P., SAUMURE, R.A., BUHLMANN, K.A., IVERSON, J.B. & MITTERMEIER, R.A. (eds.) Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs. [ Links ]
WHITE, G.C. & BURNHAM, K.P., 1999.- Program MARK: Survival estimation from populations of marked animals. Bird Study, 46: 120-138. [ Links ]
WILLIAMS, E.C., Jr. & PARKER, W.S., 1987.- A long-term study of a box turtle (Terrapene carolina) population at Allee Memorial Woods, Indiana, with emphasis on survivorship. Herpetologica, 43: 328-335. [ Links ]
YASUKAWA, Y., OTA, H. & IVERSON, J.B., 1996.- Geographic variation and sexual size dimorphism in Mauremys mutica (Cantor, 1842) (Reptilia: Bataguridae), with description of a new subspecies from the Southern Ryukus, Japan. Zoological Science, 13: 303-317. [ Links ]
ZAR, J.H., 1999.- Biostatistical Analysis. Prentice Hall, New Jersey. 663p. [ Links ]













