Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín Científico. Centro de Museos. Museo de Historia Natural
Print version ISSN 0123-3068
Bol. Cient. Mus. Hist. Nat. Univ. Caldas vol.19 no.2 Manizales July/Dec. 2015
https://doi.org/10.17151/bccm.2015.19.2.9
DOI: 10.17151/bccm.2015.19.2.9
IN VITRO EFFECT OF Purpureocillium lilacinum (Thom) Luangsa-Ard et al. AND Pochonia chlamydosporia var. catenulata (Kamyschko ex Barron & Onions) Zare & Gams ON THE ROOT-KNOT NEMATODES [P. chlamydosporia (Kofoid & White) Chitwood AND Meloidogy nemayaguensis Rammh & Hirschmann]*
EFECTO IN VITRO DE Purpureocillium lilacinum (Thom) Luangsa-Ard et al. Y Pochonia chlamydosporia var. catenulata (Kamyschko ex Barron & Onions) Zare & Gams SOBRE EL NEMATODO DEL NUDO RADICAL [P. chlamydosporia (Kofoid & White) CHITWOOD Y Meloidogyne mayaguensis Rammh & Hirschmann]
* FR: 25-II-2015 - FA: 20-X-2015
1 Master of Science in Phytopathology, University of Caldas. Manizales, Colombia. E-mail: roalorpaz@hotmail.com
2 Assistant Professor, Master's degree program in Phytopathology, University of Caldas. Manizales, Colombia. E-mail: oscar.guzman@ucaldas.edu.co
3 Ph.D. in Plant Pathology. E-mail: jleg@une.net.co
CÓMO CITAR:
ORTIZ, R.A., GUZMÁN, Ó.A. & JAIRO LEGUIZAMÓN, J., 2015.- IN VITRO effect of Purpureocillium lilacinum (Thom) Luangsa-Ard et al. and Pochonia chlamydosporia var. catenulata (Kamyschko ex Barron & Onions) Zare & Gams on the root-knot nematodes [P. chlamydosporia (Kofoid & White) Chitwood and Meloidogy nemayaguensis Rammh & Hirschmann]. Bol. Cient. Mus. Hist. Nat. U. de Caldas, 19 (2): 154-172. DOI: 10.17151/bccm.2015.19.2.9
Purpureocillium lilacinum strain PL-11 and Pochonia chlamydosporia strain JL-1 fungal strains, are a biological alternative to reduce plant parasitic nematodes on the roots of plants. The objective of this research was to determine the most effective concentration of P. lilacinum strain PL-11 and P. chlamydosporia strain JL-1, for the management of root-knot nematode (Meloidogyne spp.). In the Plant Pathology laboratory, at University of Caldas, in a completely randomized design, 1 mL of P. lilacinum (1 x 109 spores / mL) were added to 32 bacteriological Petri dishes with agar; subsequently, 16 of them were inoculated with a 30 mL suspension containing 10 eggs of P. chlamydosporia and Meloidogyne mayaguensis and, the other 16, were inoculated with a 30 mL suspension containing 10 juveniles (J2) of the two Meloidogyne species. Fungal infection of eggs and mortality of juveniles (J2) of the two species of Meloidogyne were evaluated at 24, 72, 120 and 168 h. The same procedure was performed with P. chlamydosporia, the combination P. lilacinum and P. chlamydosporia, P. lilacinum and P. chlamydosporia in combination with Carbofuran at concentrations between 1 x 103 and 1 x 109 spores / mL. The positive and negative controls were Carbofuran and water, respectively. Results demonstrated that mixing P. lilacinum and P. chlamydosporia (1 x 106 spores / L) in combination with Carbofuran, and the mixture P. lilacinum and P. chlamydosporia (1 x 108 spores / mL), caused the highest infections on eggs with 85% and 80%, respectively, and caused the highest mortality of juvenile (J2) of M. incognita and M. mayaguensis with 93% and 75%, respectively, compared to the water treated group, at 168 h.
Key words: biological control, infection, Meloidogyne, Pochonia chlamydosporia, Purpureocillium lilacinum.
ResumenLos hongos Purpureocillium lilacinum, cepa Pl-11 y Pochonia chlamydosporia, cepa Jl-1, son una alternativa biológica para reducir los nematodos fitoparásitos en las raíces de las plantas. El objetivo de esta investigación fue determinar la concentración más efectiva de P. lilacinum, cepa Pl-11 y P. chlamydosporia, cepa Jl-1, para el manejo del nematodo del nudo radical (Meloidogyne spp.). En el Laboratorio de Fitopatología de la Universidad de Caldas, en un diseño completamente aleatorio, a 32 cajas de Petri con agar bacteriológico se les adicionó 1 mL de P. lilacinum (1 x 109 esporas/mL); posteriormente, a 16 de ellas se les agregó 30 uL de suspensión con 10 huevos de P. chlamydosporia y Meloidogyne mayaguensis y a las otras 16, se les agregó 30 uL de suspensión con 10 juveniles (J2) de las dos especies de Meloidogyne. La infección de los hongos sobre huevos y la mortalidad sobre juveniles (J2) de las dos especies de Meloidogyne, se evaluaron a las 24, 72, 120 y 168 h. El mismo procedimiento se realizó con P. chlamydosporia, la mezcla P. lilacinum y P. chlamydosporia y P. lilacinum o P. chlamydosporia en combinación con Carbofuran en concentraciones entre 1 x 103 y 1 x 109 esporas/mL. Los testigos fueron Carbofuran y agua. Se demostró que la mezcla de P. lilacinum y P. chlamydosporia (1 x 106 esporas/L) en combinación con Carbofuran, y la mezcla P. lilacinum y P. chlamydosporia (1 x 108 esporas/mL), causaron las mayores infecciones sobre los huevos con 85% y 80%, respectivamente, y produjeron las mayores mortalidades de juveniles (J2) de M. incognita y M. mayaguensis con 93% y 75%, respectivamente, en comparación con el testigo agua, a las 168 h.
Palabras clave: control biológico, infección, nematodos fitoparásitos.
The root-knot nematode, Meloidogyne spp. Goeldi, causes great losses to the agricultural economy, due to its high adaptability, reproduction, survival and wide host range (AGRIOS, 2005; PERRY et al., 2009). In Colombia, the losses caused by Meloidogyne spp. in the cultivation of guava (Psidium guajava L.) are above 60% (VILLOTA & VARÓN, 1997; BOLAÑOS et al., 2007).
Guava is essential in the Colombian economy, as commercial activities generated with its cultivation support 9,000 families, representing $ 40,000 million pesos annually 15,000 planted hectares with a production of 145,000 tons yield (14.9 tons / ha) established in 22 states; of which Valle del Cauca, Meta, Caldas, Risaralda, Santander and Quindío, with 17,926, 12,988, 6,756, 6,577, 6,147 and 4,839 tons, respectively, stand out (FAO, 2008; DANE, 2011; TAFUR, 2012).
Generally, control of Meloidogyne spp. is performed through the use of fumigant nematicides (Metam sodium and Dazomet) or non-fumigants (Cadusafos and Carbofuran) (BARRES et al., 2006; LIÑAN, 2009; ICA, 2014), perhaps by their effectiveness in reducing levels population and its availability on the market (ARAYA, 2003; PERRY et al., 2009). However, the ability to detect concentrations of nematicides in the atmosphere has increased environmental monitoring and growing concerns about its use (GOWEN, 1997; CHITWOOD, 2003; WADA & TOYOTA, 2008).
As an alternative to chemical management of Meloidogyne spp., biocontrol agents such as Pochonia chlamydosporia var. catenulata (Kamyschko ex Barron & Onions) Zare & Gams, Purpureocillium lilacinum (Thom) Luangsa-Ard, Houbraken, Hywel-Jones & Samso and Gams, Glomus clarum Nicol. & Shenck, Trichoderma harzianum Rifai, Cylindrocarpon destructans (Zinsmeister) Scholten and Athrobotrys oligospora, are being used; though, P. lilacinum and P. clamydosporia var. catenulata are considered the most promising in the management of populations of Meloidogyne spp. (CARDONA & LEGUIZAMÓN, 1997; KERRY & JAFFEE, 1997; MONTES DE OCA et al., 2005; PETEIRA et al., 2005; PUERTAS et al., 2006; SINGH et al., 2013).
The fungus P. lilacinum, which belongs to the phylum Ascomycota, Sordariomycetes class, order Hypocreales and family Ophiocordycipitaceae (HIBBETT et al., 2007; LUANGSA-ARD et al., 2011), infects Meloidogyne spp. by contact, with conidia that attach and germinate on the cuticle and then penetrate the body of the nematode through appresoria; then takes its nutrients and reproduce massively invading the nematode's body until its death (MONZÓN et al., 2009). In tomato (Solanum lycopersicum L.) P. lilacinum at a concentration of 2 x 106 spores / mL, infected 40% of females and 70% of eggs and juveniles of Meloidogyne spp. (KHAN & SAXENA, 1997, ESFAHANI & POUR, 2006). In American okra (Abelmoschus esculentus) P. lilacinum at a concentration of 2.3 x 108 spores / mL, reduced 78% of eggs and 81% of juveniles (J2) of Meloidogyne spp. (CRUZ, 2007). In limes (Citrus aurantifolia Christm. et Panz.) P. lilacinum at a concentration of 2 x 106 CFU / mL, mixed with P. chlamydosporia at a concentration of 2 x 106 CFU / mL, infected 49% of juveniles (J2) M. javanica and 54% of nematode eggs (RAO, 2005).
The fungus P. chlamydosporia var. catenulata, which belongs to the phylum Ascomycota, Sordariomycetes class, order Hypocreales and family Clavicipitaceae (HIBBETT et al., 2007; ZARE et al., 2001), acts by contact, infecting and parasitizing eggs of Meloidogyne spp., through appressoria developed from undifferentiated hyphae (MORGAN-JONES et al., 1983; MONTES DE OCA et al. (2005) and PETEIRA et al. (2005) demonstrated that the fungus P. chlamydosporia var. catenulata in concentration of 5 x 106 spores / mL, is a potential agent for biological control of root knot nematodes in crops of beans [Vigna unguiculata (L.) Walp.] and infect 80% of the eggs of Meloidogyne spp. In tomato (Solanum lycopersicum L.) P. chlamydosporia in concentration of 5 x 106 spores / mL, reduces 72.83% of M. javanica eggs (DALLEMOLE-GIARETTA et al., 2014). Also, P. chlamydosporia has great potential as a controller of Meloidogyne spp. by producing dictioclamidospores, which are resistant reproductive structures that allow it to survive, generate mycelium, colonize the rhizosphere and proliferate in the soil (GIRALDO & LEGUIZAMÓN, 1997; FLORES et al., 2008; HERNÁNDEZ & DÍAZ, 2008).
The biocontrol effect of P. lilacinum and P. chlamydosporia var. catenulata on the root-knot nematode, without producing harmful effects to humans, animals and the ecosystem, has led to interest in developing commercial inputs based on these fungi (ATKINS et al., 2003; GARCÍA et al., 2004). Moreover, it has been found that the mycelial growth and the production of conidia of these fungi increase with increasing concentration (HERNÁNDEZ & DÍAZ, 2008; CABRERA et al., 2011). Other research has reported that the effectiveness of P. lilacinum and P. chlamydosporia var. catenulata in handling Meloidogyne spp. varies depending on the strain, concentration of spores in the rhizosphere colonization and host specificity (STIRLING & WEST, 1991; KERRY & JAFFE, 1997; MORTON et al., 2004; DALLEMOLE-GIARETTA et al., 2014). Based on this information, the present research was conducted under conditions IN VITRO, in order to determine the most effective concentration of P. lilacinum strain PL-11 and P. chlamydosporia strain JL-1, alone or in combination, for use in future research in nurseries and established guava plantations in the management of root-knot nematode (Meloidogyne spp.).
MATERIALS AND METHODSLocation. The research was conducted in the laboratory of Plant Pathology Department of Agricultural Production, Faculty of Agricultural Sciences at the University of Caldas, Manizales, Caldas.
Preparation of inoculum of P. chlamydosporia and Meloidogyne mayaguensis. Samples of guava tree roots of Palmira ICA-1 variety, at 5 years of age, with presence of root knot, were collected. Trees were located in the Taparcal farm, in the town of La Manuela, municipality of Palestina, department of Caldas. Root samples were taken to the laboratory for nematode extraction procedure based on the principle of nematode flotation on sucrose gradient described by JENKINS (1964) and MEREDITH (1973).
Initially, the roots were washed with tap water, allowed to dry at room temperature, 30 g of which were weighed on a balance Analytical Plus, Shimadzu® mark, and 1 cm rootstock were cut transversely with scissors. Subsequently, the pieces were placed into a glass vase of blender, Osterizer, model 565-15, with 500 mL of water, liquefied three times at high speed for 10 s. The liquefied solution was deposited on a 250 micron mesh sieve followed by a 106 micron mesh and finally a 25 micron mesh screen. The sample was washed with water to cause detachment of nematodes and the material left on the 25 microns mesh sieve was placed in centrifuge tubes of 50 mL capacity. Then, the tubes were centrifuged at 3,750 rpm for 5 minutes on a Labnet® centrifuge. Following centrifugation there was sedimentation of heavy particles at the bottom of the tube, and the supernatant was removed. Next, the tubes were filled again with a sucrose solution 50% and subjected again to centrifugation at 3,750 rpm for 5 min so that the nematodes remain floating in the sucrose solution by differential density and were separated from the heavier particles. Then, the supernatant was transferred to a 25 microns mesh sieve to wash sucrose with tap water at low pressure and prevent any negative effect on the nematodes.
Subsequently, 20 mL of water with nematodes were collected in a petri dish, which is then mounted on a stereoscope Leica® brand increased 30 X to remove eggs and juveniles (J2) of Meloidogyne spp. using a micropipette brand Biohit® 100 uL. Immediately after, the quantification of eggs and juveniles (J2) was made Meloidogyne spp. in 100 g of roots, using a box counting 36 cells divided into (6 x 6) each of 1 cm2. Eggs and juveniles (J2) of Meloidogyne spp. were disinfected separately a solution of sodium hypochlorite 0.5% for 3 min, and then washed with sterile distilled water (ADE).
Additionally, P. chlamydosporia and Meloidogyne mayaguensis species were identified through the morphological characterization of perineal patterns of adult females and morpho-metrics tests of juveniles (J2), following taxonomic keys of TAYLOR & SASSER (1983), EISENBACK (1985), JEPSON (1987) and PERRY et al. (2009).
Biological and chemical actives used for control of M. incognita and M. mayaguensis. For the experiment, two biological products, formulated by Laverlam International Corp., Butte, Montana, USA, were used. The first was Purpureocillium lilacinum, strain PL-11 formulated as a wettable powder (WP) at a concentration of 4 x 109 spores / g, (trade name Biostat®), and the second was Pochonia chlamydosporia var. catenulata, strain JL-1, (provided by National Coffee Research Center - Cenicafé, strain Cenicafé Jl-1) formulated as wettable powder at a concentration 1.58 x 108 spores / g and 2 x 106 chlamydospores / g. The chemical active Carbofuran was used at concentration of 330 g / L (trade name Furadan® 3 SC). This input comes as a concentrated solution and toxicological category I.
Preparation of biological and chemical concentrations actives. The fungi were diluted in water to a solution containing 1 x 109 spores / mL determined by the following procedure: 1 g of P. lilacinum or P. chlamydosporia was weighed on a balance Analytical Plus brand Shimadzu® then deposited into a beaker containing 1 L of tap water and homogenized with a magnetic stirrer Arec® brand. Then the count of spores / mL was carried out through a hemocytometer Boeco® mark, following the procedure described by CASTAÑO-ZAPATA (1998). After performing various spore counts, the concentration of 1 x 109 spores / mL of P. lilacinum was achieved with 2.5 g / L of water and the concentration of 1 x 109 spores / mL of P. chlamydosporia was obtained with 2 g / L of water.
From the concentration of 1 x 109 spores / mL P. lilacinum or P. chlamydosporia, sequential concentrations were obtained up to 1 x 103 spores / mL, by applying the formula C1 = V2.V1/C2 suggested by CASTAÑO-ZAPATA (1998), where V1 = initial volume of the suspension; C1 = initial concentration of spores / mL; V2 = final volume of the suspension; C2 = final concentration of spores / mL.
The concentrations 125, 250 and 500 ppm of Carbofuran were achieved by adding 378.78, 757.56 and 1,137 uL, respectively, using a micropipette brand Biohit® 1000 uL into a liter of water of the chemical product Furadan®, to be used as the standard chemical positive control.
Preparation of culture medium. Bacteriological agar Oxoid® Brand No.1 1.5%, was used as culture medium. Prepared by weighing 7.5 g added to 1 L of distilled water in a beaker. The mixture was homogenized on a magnetic stirrer Arec® mark and then placed on a hot plate until boiling. Subsequently, the mouth of the beaker was covered with aluminum foil and autoclaved 60 120°C for 15 min at 18 psi. When the medium reached a temperature of 60°C, 5 mL of 25% lactic acid was added, under laminar flow hood, in order to inhibit the growth of any bacteria. Then, the mixture was homogenized using a sterile glass stirrer. Then, 6 mL of the prepared medium was poured into each Petri dish of 60 x 15 mm and then allowed to stand for 3 h until the medium became slurry.
Application of treatments. To thirty-two Petri dishes containing the semi bacteriological agar medium, 1 mL of P. lilacinum solution containing 1 x 109 spores / mL were added. Then, to 16 of these Petri dishes, 30 uL of a suspension containing 10 eggs of M. incognita and M. mayaguensis were added using a micropipette Biohit® capacity of 100 uL; and to the other 16 Petri dishes a 30 uL suspension containing 10 juveniles (J2) of M. incognita and M. mayaguensis were added. Subsequently, fungal infection on eggs and mortality (infection) of juveniles (J2) of Meloidogyne species was evaluated at 24, 72, 120 and 168 h. A water treated, negative control, group of 32 Petri dishes (16 boxes with eggs and 16 boxes with juveniles) was also tested.
The above procedure was performed with the other treatments: P. lilacinum (1 x 108 to 1 x 106 spores / mL), P. chlamydosporia (1 x 109 to 1 x 106 spores / mL), the mixture of P. lilacinum and P. chlamydosporia (1 x 105 to 1 x 108 spores / mL), P. lilacinum (1 x 107 to 1 x 104spores / mL) combined with Carbofuran (125 ppm), P. chlamydosporia (1 x 107 to 1 x 104 spores / mL) combined with Carbofuran (125 ppm), P. lilacinum and P. chlamydosporia (1 x 106 to 1 x 103 spores / mL) in combination with Carbofuran (125 ppm), and Carbofuran (125, 250 and 500 ppm). Regardless, for eggs and juveniles (J2) of M. incognita and M. mayaguensis 448 experimental units, determined by 28 treatments corresponding to concentrations of actives, four replicates and four exposure times were evaluated.
Statistical analysis. The experimental design used was completely random. The data obtained were subjected to normality test of Kolmogorov-Smirnoff, complying with the hypothesis of normality (p-value > 0.01), an analysis of variance was performed, and a comparison Tukey test at a 5% level of probability with the Statistical Analysis System (SAS, 2009).
Variables evaluated. Immediately after completion of exposure time of eggs and juveniles (J2) of M. incognita and M. mayaguensis to a given treatment, two drops of Lactophenol blue were added to each Petri dish; contents being deposited on a slide of 2.54 x 7.62 cm, using a micropipette Biohit ® brand of 100 uL, in order to evaluate the following variables:
Infection (%): defined as the invasion and multiplication of the fungus on eggs of M. incognita and M. mayaguensis and expressed in percentage as the number of infection per 100 eggs on the initial population. The corrected infection (I), was calculated using the following formula Schneider-Orelli (COSTA et al., 1974): I (%) = [(Infection in treatment (%) - Infection control (%) / (100 - Infection control)] x 100, where, Infection treatment = (Number of eggs infected x 100) / Initial population.
Mortality (%): defined as the rate of deaths in juveniles (J2) of M. incognita and M. mayaguensis, during the time of exposure to a particular treatment, and expressed in percentage as the number of J2 dead for each 100 individuals of the initial population. The corrected mortality (M), was calculated by the formula of Schneider-Orelli (COSTA et al., 1974) described below: M (%) = [Treatment Mortality (%) - Control Mortality (%) / 100 - Mortality in the control)] x 100; wherein Treatment Mortality = (Number of dead J2 x 100) / Initial population.
Lethal Concentration ninety (LC90) in spores / mL: defined as the concentration of a biological, physical or chemical agent which killed 90% of the organisms in a population (REPETTO, 1997). This variable was determined by relating the mortality data of juveniles (J2) of M. incognita and M. mayaguensis and their respective concentrations, through linear regressions in Microsoft Excel 2010 program. The criterion for a dead juvenile stage (J2) of Meloidogyne spp. was immobility, when stimulated with a bristle, after been placed in Petri dishes containing sterile distilled water for 24 h (PINKERTON & KITNER, 2006).
RESULTS AND DISCUSSIONEffect of P. lilacinum and P. chlamydosporia on eggs and juveniles (J2) of M. incognita and M. mayaguensis. Variance analysis for the variables infection of eggs and mortality of juvenile stages (J2) of M. incognita and M. mayaguensis, showed highly significant statistical differences between treatments at 168 h after application. The high coefficients of determination R2 = 0.93 and 0.95, respectively, as the low coefficients of variation CV = 11.78% and 12.75%, respectively, demonstrated the reliability of the results obtained (Table 1).
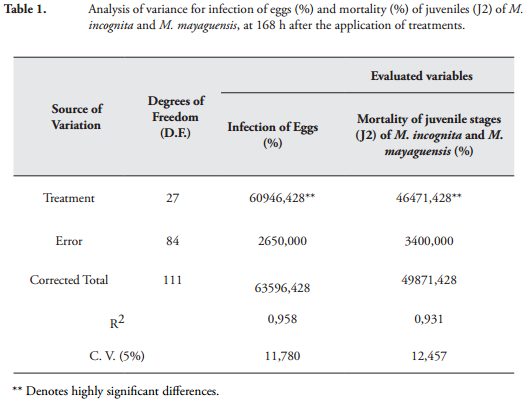
By performing a Tukey's test (p < 0.05) on the variables infection of eggs and mortality of juveniles (J2) of M. incognita and M. mayaguensis, it was determined that the mix P. lilacinum and P. chlamydosporia (1 x 106 spores / L) in combination with Carbofuran (125 ppm) was the best treatment, as it caused the highest infection of eggs with 85% and increased mortality of J2 of both species with 93% compared to the water treated group where no mortality occurred (Figures 1 and 2). The result of the mortality of juveniles (J2) of both species of Meloidogyne, obtained with such combination, had no statistical difference (p = 0.05) with that obtained with the Carbofuran at the highest concentration (500 ppm), but with the other treatments (Figures 1 and 2). The result of infection on eggs of M. incognita and M. mayaguensis obtained in this experiment with the P. lilacinum and P. chlamydosporia (1 x 106 spores / L) mixture in combination with Carbofuran (125 ppm) coincides with that reported by DHAWAN & SINGH (2009) who found that the mixture P. lilacinum, P. chlamydosporia and cake neem (Azadirachta indica A. Juss) in combination with Carbofuran in okra (Abelmoschus esculentus L.) infested with M. incognita caused 90% infection of eggs.
After the above treatment, the mixture of P. lilacinum and P. chlamydosporia (1 x 108 spores / mL) was the best treatment, being significantly different from other treatments (p = 0.05) since 80% infection was obtained on eggs and 75% mortality on J2 of M. incognita and M. mayaguensis in comparison to the water treated control (Figures 1 and 2). The result of infection of these fungi on the eggs of Meloidogyne species was greater than that reported by RAO (2005), who found that M. javanica eggs extracted from roots of limes (Citrus aurantifolia Christm. et Panz.), and exposed to P. lilacinum and P. chlamydosporia (2 x 106 CFU / mL) for 96 hours, showed an infection of 54% compared to that obtained in water treated groups. The results of infection of eggs and mortality of J2 of M. incognita and M. mayaguensis obtained in this study confirm what was stated by CANNAYANE & RAJENDRAN (2001), that the combination of P. lilacinum with other biocontrol agents such as P. chlamydosporia, a greater potential for the biological management of Meloidogyne spp.
Comparing the fungal infection caused by the fungi individually, it was found that P. chlamydosporia (1 x 109 spores / mL) was significantly greater (p = 0.05), as it caused a 68% infection of eggs on M. incognita and M. mayaguensis while P. lilacinum infected 63% at same spore concentration (Figures 1 and 2). The result of infection P. chlamydosporia on both eggs of Meloidogyne species obtained in this study was similar to that reported by ATKINS et al. (2003), who proved that the same fungus at the concentration of 1 x 106 spores / mL, under semi-controlled conditions, reached 68% infection on M. incognita eggs, after six months of application in a succession of crops of tomato (Solanum lycopersicum L.) and lettuce (Lactuca sativa L.).
However, in this study with P. chlamydosporia (1 x 109 spores / mL) 65% mortality of juveniles (J2) of M. incognita and M. mayaguensis was obtained, with no statistical difference with that obtained with P. lilacinum (1 x 109 spores / mL) (Figures 1 and 2). This result was similar to that obtained by VERGARA et al. (2012) with P. chlamydosporia (1.03 x 108 spores / mL) in conditions IN VITRO achieving 66% mortality of juveniles (J2) of R. simis after 120 h. In this study, it was also found that infection of P. chlamydosporia (1 x 109 spores / mL) on eggs of M. incognita and M. mayaguensis had no statistical difference (p = 0.05) with that obtained with P. lilacinum (1 x 109 spores / mL) and with treatments where P. chlamydosporia and P. lilacinum (1 x 104 or 1 x 105 spores / mL) were mixed in combination with Carbofuran (125 ppm). The mortality of J2 of the two species of Meloidogyne with P. chlamydosporia (1 x 109 spores / mL) showed no statistical difference (p = 0.05) with those achieved with treatments where P. lilacinum (1 x 106 or 1 x 107 spores / mL) were combined with Carbofuran (125 ppm) (Figures 1 and 2).
After the above treatments, median comparisons with a Tukey's test (p = 0.05), showed that P. lilacinum alone (1 x 109 spores / mL) caused 63% infection of eggs and 65% mortality of J2 of M. incognita and M. mayaguensis, at 168 h of exposure, showing above 30% in both variables compared with P. lilacinum alone and P. chlamydosporia alone, at the lowest concentration (1 x 106 spores / mL) (Figures 1 and 2). The result of infection of eggs on the two species of Meloidogyne obtained in this study was similar to that reported by AYATOLLAHY & FATEMY (2010), who indicate that P. lilacinum (isolate 8.1) at a concentration of 2.3 x 108 spores / mL, caused a 60% infection of Heterodera schachtii eggs at 48 h after application. Furthermore, the result of mortality of J2 of M. incognita and M. mayaguensis, seen with P. lilacinum (1 x 109 spores / mL), was similar to that reported by VERGARA et al. (2012), who obtained 62% mortality of J2 of R. similis with P. lilacinum (1.03 x 108 spores / mL) after 120 h of being applied in conditions IN VITRO.
In this research, 63% of infection caused by P. lilacinum (1 x 109 spores / mL) on eggs of M. incognita and M. mayaguensis had no statistical difference with that achieved by P. lilacinum or P. chlamydosporia (107 spores / mL) in combination with Carbofuran at low concentration (125 ppm). Similarly, 65% mortality of J2 of the two species of Meloidogyne achieved with P. lilacinum (1 x 10<9 spores / mL) was not statistically different from that obtained with P. chlamydosporia (1 x 109 spores / mL) and P. lilacinum (1 x 10<6 or 1 x 10<7 spores / mL) in combination with Carbofuran (125 ppm) (Figures 1 and 2).
Furthermore, the positive control Carbofuran at its maximum concentration (500 ppm) resulted in a 100% mortality of J2 of M. incognita and M. mayaguensis 168 h after application (Figures 1 and 2). However, Carbofuran had no effect on the eggs of both species of Meloidogyne in three concentrations (125, 250 and 500 ppm) and presented no statistical differences from the water treated control (Figures 1 and 2). A similar result was reported by VERGARA et al. (2012), with Carbofuran (330 g / L) in eggs of R. similis, were no effect of the chemical substance was found after 120 h of exposure. This result is due to the protective function of the egg cover, such as the vitellin, chitin and especially an internal glycolipid layer, which provides resistance to chemicals and prevents the entry of foreign substances (STIRLING & WEST, 1991; PERRY et al., 2009).
VERGARA et al. (2012) mention that the application of Carbofuran is not efficient to handle eggs of R. similis; contrary to what happens with the fungi P. lilacinum and P. chlamydosporia, which have high potential for biocontrol. However, the results of this study showed that Carbofuran (125 ppm) applied in combination with P. lilacinum and P. chlamydosporia (1 x 106 spores / mL) caused eggs of M. incognita and M. mayaguensis to be infected exceeding 5% of that produced when the two fungi were applied as a mixture, in concentration of 1 x 108 spores / mL (Figures 1 and 2). This happened due to the compatibility of the two fungi with the chemical input, which causes a synergistic effect, which allows for greater control of Meloidogyne spp. (MAHENDRA et al., 2009).
The results obtained in this investigation, allowed us to prove that the infection of the fungus on eggs and mortality of J2 of M. incognita and M. mayaguensis were higher when the concentration (spores / mL) of P. lilacinum and P. chlamydosporia also increased (Figures 1 and 2). In consequence, it was found that when fungi were applied (individually, in mixture or in combination with Carbofuran) with a greater concentration, the values of infection of eggs of the two Meloidogyne species are higher, which was equal or above 63%. Also the highest mortality values of both J2 of the Meloidogyne species, were obtained which were higher than 65%. However, when the same fungi, were applied in lower concentrations smaller infection values occurred, which were at or below 55%; similarly, mortality values of J2 were recorded at or below 58% (Figures 1 and 2).
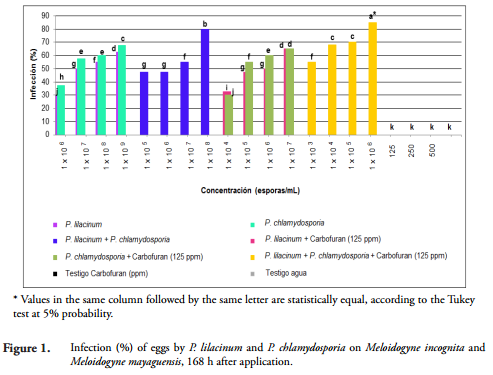
Similar to what happened with the effect of greater concentration of P. lilacinum and P. chlamydosporia on the variables infection of eggs and mortality of juveniles (J2) of Meloidogyne spp., it was also determined that these variables were directly related to the time of exposure to a particular treatment. Consequently, the infection of eggs and mortality of J2 of the Meloidogyne species caused by the fungal isolates was low after 24 hours (= 10%) but at 168 h it reached a range between 30 and 93% (Figures 1 and 2). This behavior was also reported by VERGARA et al. (2012), who found that in laboratory conditions P. lilacinum (1.03 x 108 spores / mL) caused an infection of11% on eggs of R. similis after 12 h of application, whereas after 120 h, this increased to 79%.
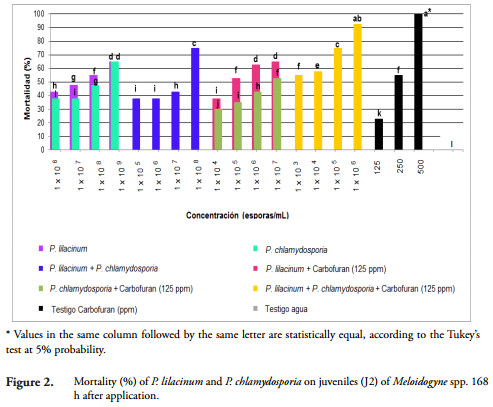
Infection with P. lilacinum and P. chlamydosporia on the egg vitellin layer and the outer layer of the cuticle of juveniles (J2) of M. incognita and M. mayaguensis was observed under a microscope with a 10X objective at 24, 72, 120 and 168 h after exposure (Figures 3 and 4).
At 24 h, both the vitelline layer of eggs and the cuticle of juveniles (J2) of M. incognita and M. mayaguensis, were surrounded by conidia of fungi. Subsequently, after 72 h of exposure, the same vitellin layers expressed fungal hyphae. After 120 h, the layers were partially covered by masses of hyphae and at 168 h these hyphae covered the whole eggs and juveniles (J2) of the species listed (Figures 3 and 4). Furthermore, at 168 h, a rupture of the egg cover, formed by the outer vitelline layer, median chitinase layder and the glycolipid inner layer, important for the development of the embryo, generally occurred (PERRY et al., 2009).
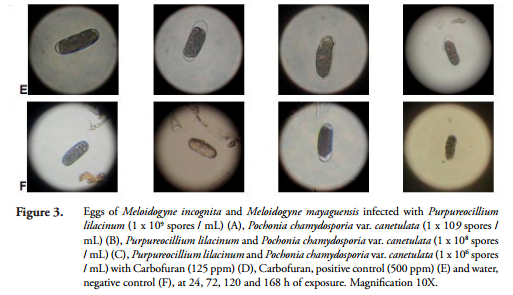
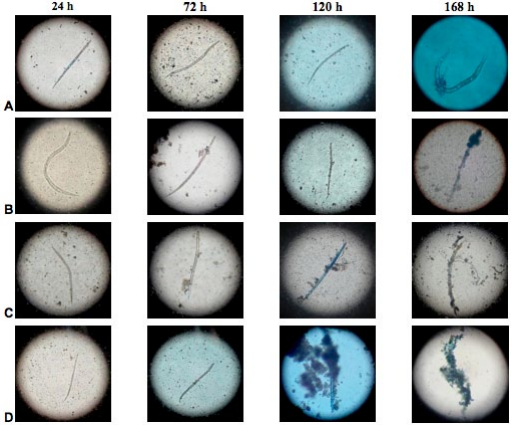
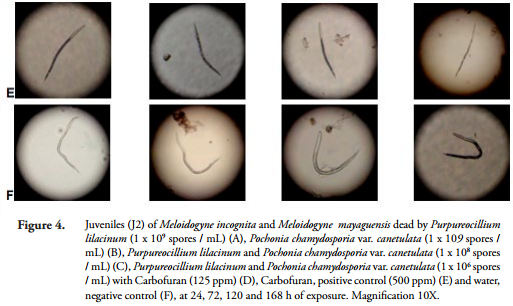
The results obtained in this research demonstrated that under IN VITRO conditions, the highest values of infection on mortality of eggs and juveniles (J2) of M. incognita and M. mayaguensis, which were between 63 and 93%, respectively, were achieved with the treatments listed below in descending order:
• Mixing P. lilacinum and P. chlamydosporia (1 x 106 spores / mL) in combination with Carbofuran at lower concentration (125 ppm) resulted in 85% egg infection and 93% mortality of juveniles (J2) of M. incognita and M. mayaguensis.
• Mixing P. lilacinum and P. chlamydosporia (1 x 108 spores / mL) resulted in 80% egg infection and 75% mortality of juveniles (J2) of the two Meloidogyne species.
• P. chlamydosporia (1 x 109 spores / mL) resulted in 68% infection of eggs and 65% mortality of juveniles (J2) of both species of Meloidogyne.
• P. lilacinum (1 x 109 spores / mL) resulted in 63% infection of eggs and 65% mortality of juveniles (J2) of M. incognita and M. mayaguensis.
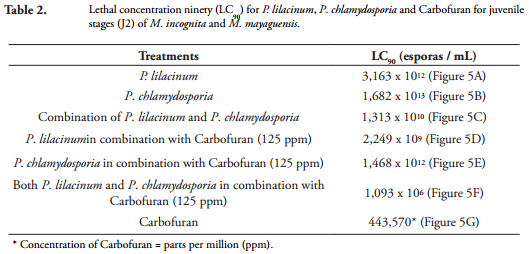
Lethal concentration ninety (LC90). The lethal concentrations (LC90), caused by P. lilacinum and P. chlamydosporia, alone, in mixture or in combination with Carbofuran, in the mortality of juveniles (J2) of M. incognita and M. mayaguensis were calculated by relating the values mortality of juveniles (J2) of Meloidogyne species, obtained in IN VITRO conditions at 168 h, in each of the concentrations described above. These values were adjusted to linear equations of the form Y = a + BX, where "Y" corresponded to the mortality of J2 (%); "X" to the concentration of each treatment; and "a" and "b", the coefficients calculated in the linear regression analysis (Figure 5).
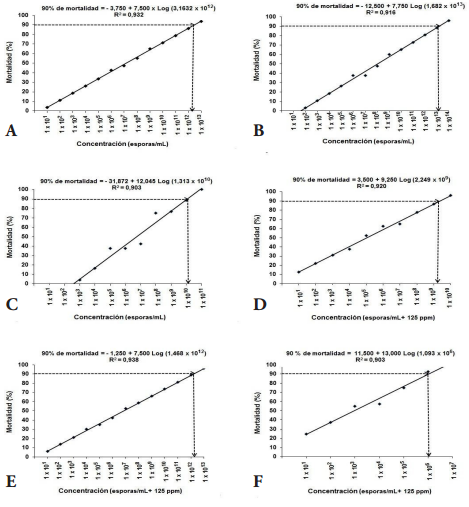
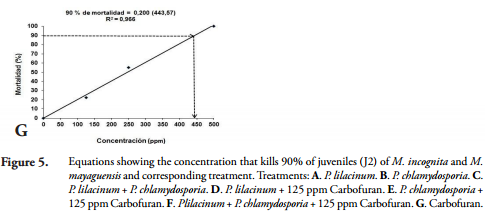
The high determination coefficients R2 = 0.90 to 0.96, obtained demonstrated the probabilistic reliability thereof; for this reason, we proceeded to clear the LC90 for each treatment. The concentrations of fungi alone, in mixture or in combination with the low concentration of Carbofuran (125 ppm), which caused 90% (LC90) of mortality of juveniles (J2) of M. incognita and M. mayaguensis is presented below (Table 2).
It was found that the equations obtained showed a positive correlation between the concentration and the percent mortality of juveniles (J2) of M. incognita and M. mayaguensis, and defined the lines that have a positive slope and are of increasing order, confirming that with increasing concentration of spores and ppm of Carbofuran, also increases mortality (%) of J2 nematodes.
The results showed that an increase of 1 x 101 spores / mL in P. lilacinum concentration causes an increase of at least 7.50% mortality in juveniles (J2) of Meloidogyne species; P. chlamydosporia an increase of 7.75%; P. lilacinumin mix with P. chlamydosporia 12.04%; P. chlamydosporia combined with 125 ppm Carbofuran 7.50%; P. lilacinum combined with 125 ppm of Carbofuran 9.25%; P. chlamydosporia combined with 125 ppm of Carbofuran 7.50%; and P. chlamydosporia mixed with P. lilacinum and combined with 125 ppm of Carbofuran 13%. In addition, an increase of 100 ppm Carbofuran, produces a 20% increase in mortality of J2 nematodes.
CONCLUSIONS• In IN VITRO conditions, the fungi P. lilacinum alone and P. chlamydosporia alone at a concentration of 1 x 109 spores / mL, as did the mixture of these fungi in the concentration 1 x 108 spores / mL, infected between 63 and 80% of eggs and juveniles (J2) of M. incognita and M. mayaguensis, at 168 h of exposure.
• The mixture of P. lilacinum and P. chlamydosporia in concentration of 1 x 106 spores / mL in combination with Carbofuran at a concentration of 125 ppm, caused 93% mortality of juveniles (J2) of M. incognita and M. mayaguensis under IN VITRO conditions at 168 h exposure.
• The evaluation of the Purpureocillium lilacinum strain PL-11 and Pochonia chlamydosporia var. catenulata strain JL-1, in IN VITRO conditions, confirmed their high potential in the control of M. incognita and M. mayaguensis, for promoting their use in conditions of nursery and field within an integrated management program for Meloidogyne spp. as an environmentally safe, affordable, accessible and user-friendly alternative.
ACKNOWLEDGEMENTSThe authors express sincere thanks to Laverlam International Corporation for the supply of formulations of fungi and financial support to conduct this research. Also thank the Vice Rectory of Research and Graduate Studies of the University of Caldas for co-funding this research.
AGRIOS, G.N., 2005.- Plant pathology. 5 ed. Elsevier Academic Press, Nueva York. [ Links ]
ARAYA, M., 2003.- Situación actual del manejo de nematodos en banana (Musa AAA) y plátano (Musa AAB) en el trópico americano (in) Actas /Manejo convencional y alternativo de la Sigatoka negra, nematodos y otras plagas asociadas al cultivo de Musáceas en los trópicos/. Guayaquil, Ecuador. [ Links ]
ATKINS, S.D., HIDALGO, L., KALISZ, H., MUCHLINE, T.H., HIRSCH, P.R. & KERRY, B.R., 2003.- Development of a new management strategy for the control froot-knot nematodes (Meloidogyne spp.) in organic vegetable production. Pest Manag. Sci., 59: 183-189. [ Links ]
AYATOLLAHY, E. & FATEMY, S., 2010.- IN VITRO assessment of pathogenicity and culture filtrates of fungi against Heterodera schachtii. Appl. Ent. Phytopath, 77 (2): 15-26. http://www.sid.ir/en/VEWSSID/J_pdf/877 [ Links ]
BARRES, M.T., BELLO, A., JORDÁ, C. & TELLO, J., 2006.- La eliminación del bromuro de metilo en protección de cultivos como modelo mundial para la conservación del medio ambiente. Universidad de Almería, MAPA, Madrid. [ Links ]
BOLAÑOS, M., GÓMEZ, J.M., MELO, J.P. & CAMPO, E.J., 2007.- Evaluación de prácticas de manejo de nematodos parásitos en cultivos de guayabo en el Valle del Cauca. Plegable divulgativo, noviembre de 2007. [ Links ]
CABRERA, R., THOMPSON-SOLER, R.A. & DÍAZ-GARCÍA, O.L., 2011.- Un medio simplificado y eficaz para la producción del hongo Pochonia chlamydosporia var. Chlamydosporia (Goddard) Zare & Gams en fase líquida. Revista Citri Frut, 28 (1): 19-22. [ Links ]
CANNAYANE, I. & RAJENDRAN, G., 2001.- Application of biocontrol agents and oil cakes for the management of P. chlamydosporia in brijal. Current Nematology, 12: 51-55. [ Links ]
CARDONA, N.L. & LEGUIZAMÓN, J.E., 1997.- Aislamiento y patogenicidad de hongos y bacterias al nematodo del nudo radical del café Meloidogyne spp. Goeldi. Fitopatología Colombiana, 21 (1): 39-52. [ Links ]
CASTAÑO-ZAPATA, J., 1998.- Prácticas de laboratorio de topatología. Práctica (2). Segunda edición. Universidad de Caldas, Manizales. [ Links ]
CHITWOOD, D.J., 2003.- Nematicides: 1104-1115 (en) PLIMER, J.R. (ed.) Encyclopedia of Agrochemicals. Vol. 3. New York. http://www.ars.usda.gov [ Links ]
COSTA, J.J., MARGHERITIS, A.E., MARSICO, O.J., 1974.- Introducción a la Terapéutica Vegetal. Primera Edición. Editorial Hemisferio Sur, Buenos Aires. [ Links ]
CRUZ, S.A., 2007.- Control del nematodo nodulador de raíz (Meloidogyne spp.) en el cultivo de okra americana (Abelmoschus esculentus) con Micorriza Vesiculo Arbuscular (VAM), Trichoderma harzianum, Paecilomyces lilacinus, Pochonia chlamydosporia y Marigold (Tagetes erecta). Proyecto especial presentado como requisito parcial para optar el título de Ingeniero Agrónomo en el grado Académico de Licenciatura. Zamorano-Honduras. [ Links ]
DALLEMOLE-GIARETTA, R., GRASSI, L., DEBORAH, M.X., FALCÃOZ, R., FERRAZ, S., LOPES, E.A., 2014.- Incorporação ao solo de substrato contendo micélio e conídios de Pochonia chlamydosporia para o manejo de Meloidogyne javanica. Cienc. Rural., 44 (4). http://www.scielo.org/ [ Links ]
DANE (DEPARTAMENTO ADMINISTRATIVO NACIONAL DE ESTADÍSTICA), 2011.- Resultados Encuesta Nacional Agropecuaria ENA. Bogotá [ Links ].
DHAWAN, S.C. & SINGH, S., 2009.- Compatibility of Pochonia chlamydosporia with nematicide and neem cake against root-knot nematode, P. chlamydosporia infesting okra. Indian Journal of Nematology, 39: 85-89. [ Links ]
EISENBACK, J.D., 1985.- Detailed morphology and anatomy of second-stage juveniles, males, and females of the genus Meloidogyne (root-knot nematodes) (in) SASSER, J.N. & CARTER, C.C. (eds.) An Advanced Treatise on Meloidogyne. Vol. I. Biology and Control. A cooperative publication of the Department of Plant Pathology and the United States Agency for International Development, North Carolina State University Graphics, Raleigh, North Carolina. [ Links ]
ESFAHANI, N.M. & POUR, A.B., 2006.- The effects of Paecilomyces lilacinus on the Pathogenesis of Meloidogyne javanica and Tomato Plant Growth. Parameters. Iran Agricultural Research, 24 (2): 67-76. [ Links ]
FAO (ORGANIZACIÓN DE LAS NACIONES UNIDAS PARA LA ALIMENTACIÓN Y LA AGRICULTURA), 2008.- Guayaba. Características generales. http://www.fao.org/ [ Links ]
FLORES, C.R., ATKINS, S.D., MANZANILLA-LÓPEZ, R. & PRADO-VERA, I.C., 2008.- Caracterización de aislamientos mexicanos de Pochonia chlamydosporia var. chlamydosporia (Goddard) Gams y Zare para el control biológico de Nacobbus aberrans (Thorne) Thorne y Allen. Revista Mexicana de Fitopatología, 26 (2): 93-104. [ Links ]
GARCÍA, L., BULNES, C., MELCHOR, G., VEGA, E., MONTES DE OCA, N., HIDALGO, L. & MARRERO, E., 2004.- Safety of Pochonia chlamydosporia var. catenulata on acute oral and dermal Toxicity/Pathogenicity evaluations in rats and rabbits. Vet. and Human Toxicology, 46 (5): 248-250. [ Links ]
GIRALDO, F.M. & LEGUIZAMÓN, C.J., 1997.- Aislamiento y evaluación IN VITRO de hongos a partir de estados de Meloidogyne spp. infectados naturalmente. Cenicafé, 48 (3): 195: 203. [ Links ]
GOWEN, S.R., 1997.- Alternate strategies for nematode control to wards sustainable agriculture (in) Plant nematode problems and their control in the Near East region. FAO Plant Production and Protection paper - 144. [ Links ]
GUZMÁN, P.O. & CASTAÑO, Z.J., 2010.- Identificación de nematodos fitoparásitos en guayabo (Psidium guajava L.), en el municipio de Manizales (Caldas), Colombia. Rev. Acad. Colomb. Cienc., 34 (130): 117-125. [ Links ]
HERNÁNDEZ, M.A. & DÍAZ, L.H., 2008.- KlamiC®: Bionematicida agrícola producido a partir del hongo Pochonia chlamydosporia var. catenulata. Revista Protección Vegetal, 23 (2): 131-134. [ Links ]
HIBBETT, D.S., BINDER, M., BISCHOFF, J.F., BLACKWELL, M., CANNON, P., ERIKSSON, O.E., ... ZHANG, N., 2007.- A higher-level phylogenetic classification of the Fungi. Mycological Research, 111 (5): 509-547. [ Links ]
ICA (INSTITUTO COLOMBIANO AGROPECUARIO), 2014.- Área de protección vegetal: Listado de Registro de Venta de plaguicidas químicos de uso agrícola. http://www.ica.gov.co [ Links ]
JENKINS, W.R., 1964.- A rapid centrifugal flotation technique for separating nematodes from soil. Plant Disease Reporter, 48 (9): 692. [ Links ]
JEPSON, S., 1987.- Identification of root-knot nematodes (Meloidogyne species). CAB International, United Kingdom. [ Links ]
KERRY, B.R. & JAFFEE, B.A., 1997.- Fungias biological control agents for plant parasitic nematodes. The Mycota, 4: 203-218. [ Links ]
KHAN, T.A. & SAXENA, S.K., 1997.- Integrated management of root-knot nematode Meloidogyne javanica infected tomato using organic materials and Paecilomyces lilacinus. Bioresourse Technology, 61: 247-250. [ Links ]
LIÑÁN, C., 2009.- Vademecum de Productos Fitosanitarios y Nutricionales 2009. Ed. 25. Edit. Agrotécnicas. [ Links ]
LUANGSA-ARD, J., HOUBRAKEN, J., VANDOORN, T., HONG, S.B., BORMAN, A.M., HYWEL-JONES, N.L. & SAMSON, R.A., 2011.- Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiology Letters, 321:141-149. [ Links ]
MAHENDRA, S., ANJU, J. & GUILL, J.S., 2009.- Dose optimization of egg parasitic fungus Paecilomyces lilacinus alone in combination with Carbofuran for control of P. chlamydosporia infecting tomato. International Journal of Nematology, Vol. 19 (2): 177-181. [ Links ]
MEREDITH, J., 1973.- Algunos métodos de campo y laboratorio para trabajar con nematodos. Maracaibo, Venezuela. [ Links ]
MONTES DE OCA, N., AREVALOS, J., A COSTA, N., PETEIRA, B., HIDALGO-DÍAZ, L. & KERRY, B.R., 2005.- Estabilidad de la cepa IMI SD 187 de P. chlamydosporia var. catenulata. Parte I. Indicadores morfológicos, productivos y patogénicos. Rev. Protección Veg., 20 (2): 93-100. [ Links ]
MONZÓN, A., HERRERA, I. & MÉNDEZ, E., 2009.- Guía uso y manejo de Paecilomyces lilacinus para el control de nematodos. Universidad Nacional Agraria, Managua, Nicaragua. 2009. Recuperado de http://es.scribd.co/doc/126840400/G [ Links ]
MORGAN-JONES, G., WHITE, J.F. & RODRÍGUEZ-KABANA, R., 1983.-Phytonematode Pathology: Ultrastructural Studies. Parasitism of Meloidogyne arenaria eggs by Verticillium chlamydosporium. Nematropica, 13 (2): 245-260. [ Links ]
MORTON, C.O., HIRSCH, P.R. & KERRY, B.R., 2004.- Infection of plant-parasitic nematodes by nematophagous fungi – A review of the application of molecular biology to understand infection processes and to improve biological control. Nematology, 6: 161-170. [ Links ]
PERRY, R., MOENS, M. & STARR, J., 2009.- Root knot nematodes. CAB International, London. [ Links ]
PETEIRA, B., PUERTAS, A., HIDALGO-DÍAZ, L., HIRSCH, P.R., KERRY, B.R. & ATKINS, S.D., 2005.- Real-time PCR to monitor and asses the efficacy of the nematophagous fungus Pochonia chlamydosporia var. catenulata against root-knot nematode populations in the field. Biotecnología Aplicada, 22 (4): 261-266. [ Links ]
PINKERTON, J.N. & KITNER, M.L.C., 2006.- Effects of biologically-derived products on mobility and reproduction of the root-lesion nematode, Pratylenchus penetrans, on strawberry. Nematrópica, 36 (2): 181-196. [ Links ]
PUERTAS, A., DE LANOVAL, B., MARTÍNEZ, B., MIRANDA, I., FERNÁNDEZ, F. & HIDALGO, L., 2006.- Interacción de Pochonia chlamydosporia var. catenulata con Rhizobium sp., Trichoderma harzianum y Glomus clarum en el control de P. chlamydosporia. Rev. Protección Veg., 21 (2): 80-89. [ Links ]
RAO, M.S., 2005.- Management of Meloidogyne javanica on acid lime nursey seedlings by using formulations of Pochonia chlamydosporia and Paecilomyces lilacinus. Nematol Medit, 33: 145-148. [ Links ]
REPETTO, M., 1997.- Toxicología fundamental. 3a ed. Díaz de Santos, Madrid. [ Links ]
SAS (Statistical Analysis System (SAS® 9.2). 2009. https://support.sas.com [ Links ]
SINGH, U.B., SAHU, A., SAHU, N., SINGH, R.K., RENU, S., SINGH, D.P., ... SINGH, K.P., 2013.- Arthrobotrys oligospora-mediated biological control of diseases of tomato (Lycopersicon esculentum Mill.) caused by P. chlamydosporia and Rhizoctonia solani. J. Appl Microbiol, 114 (1): 196-208. [ Links ]
STIRLING, G.R. & WEST, L.M., 1991.- Fungal parasites of root-knot nematode eggs from tropical and subtropical regions of Australia. Plant Pathology, 20: 149-154. [ Links ]
TAFUR, J.E., 2012.- Emprendimiento empresarial en tierras con vocación forestal: Evaluación financiera de la producción de guayaba en la Hacienda La María: Tesis, Universidad Nacional de Colombia, Palmira. [ Links ]
TAYLOR, A.L. & SASSER, J., 1983.- Biología, identificación y control de nematodos del nódulo de la raíz. Universidad del Estado de Carolina del Norte (ed.), Carolina del Norte, U.S.A. [ Links ]
VERGARA, D., GUZMÁN, Ó.A. & LEGUIZAMÓN, J. 2012.- Efecto IN VITRO de Purpureocillium lilacinum (Thom) Luangsa-Ard et al. y Pochonia chlamydosporia (Goddard) Zare y Gams sobre el nematodo barrenador Radopholus similis (Cobb) Thorne. Agron., 20 (2): 25-36. http://200.21.104.25/agronomia/downloads/Agronomia20(2)_4.pdf [ Links ]
VILLOTA, F. & VARÓN, F., 1997.- Evaluación de materiales de guayaba (Psidium guajava L.) por su comportamiento al ataque de P. chlamydosporia Raza 2. Fitopatología Colombiana, 21 (2): 31-37. [ Links ]
WADA, S. & TOYOTA, K., 2008.- Effect of three organophosphorous nematicides on non-target nematodes and soil microbial community. Microbes and Environments, 23 (4): 332-336. [ Links ]
ZARE, R., GAMS, W. & EVANS, H.C., 2001.- A revision of Verticillium section Prostrata. V. The genus Pochonia, with notes on Rotiferophthora. Nova Hedwigia, 73: 51-86. [ Links ]














